Keywords
Chemotherapy; Posterior fossa tumors; Sequelae; Cranial radiotherapy;
Introduction
Central nervous system (CNS) tumors are the most common solid neoplasms in children [1-3]. These tumors also represent the second most common malignancy during childhood, preceded by leukemia [1,4] and the most common cause of death from solid tumors [1]. 60-70% of CNS tumors in children occur in the posterior fossa [3]. These posterior fossa tumors cover a heterogeneous group of tumor types, localized in the infratentorial compartment of the brain, more specifically the cerebellum and the brain stem [5]. The most prevalent types of posterior fossa tumors in children are medulloblastoma, cerebellar pilocytic astrocytoma, ependymoma, atypical teratoid rhabdoid tumor (ATRT) and brainstem glioma [1,3,6].
Acute symptoms of posterior fossa tumors in children are mainly the result of increased intracranial pressure, due to the mass effect of the tumor and obstructive hydrocephalus. Therefore, patients often present with headaches, nausea, emesis and cranial neuropathies causing extra-ocular muscle disorders. Signs of cerebellar derangement, such as ataxia and dysmetria are also frequent [1,7]. Brain stem involvement, caused by brainstem gliomas or other posterior fossa tumors invading the brain stem, induces specific brainstem symptoms. These include pyramidal tract signs such as hemi- or quadriparesis, ataxia, dysmetria, swallowing problems and impairment of multiple cranial nerves such as the oculomotor, trochlear, abducens and the facial nerve [1,8].
When such alarming symptoms occur, imaging is the next step to define the diagnosis. Although CT-scans can be helpful in emergency situations to make a quick preoperative evaluation, MRI is the preferred imaging technique to determine the tumor extent, to provide arguments for the most suitable treatment plan and to evaluate treatment response later [1]. Furthermore, MRI-modalities such as diffusion weighted imaging, MR spectroscopy and MR perfusion have been shown valuable in discriminating between the histologic tumor types before pathological confirmation is accomplished [6,9]. Craniospinal MRI is the golden standard to detect dissemination in the cerebrospinal fluid [1]. Histological typing of the tumor is performed during pathological examination of the resection species. A biopsy is only acquired if surgery is impossible [3]. For instance, in diffuse intrinsic pontine glioma, stereotactic biopsy is currently recommended. These findings provide a gateway to developing targeted therapies in this aggressive tumor type [10].
Once the diagnosis is determined, an individualized treatment plan is to be established. Currently, three important treatment modalities are applied for posterior fossa tumors: surgery, radiotherapy and chemotherapy. A combination of these three modalities is administered according to the tumor type, localization and age at diagnosis. First, surgical resection is included in all treatments. However, precarious tumor localization (e.g. in the brain stem) can make a resection impossible [8]. Other specific surgical treatments (an external ventricular drain, a ventriculoperitoneal shunt or a third ventriculostomy) are often performed to reduce intracranial pressure in case of hydrocephalus [8,11,12]. Second, radiotherapy is indispensable for some tumor types. Medulloblastoma patients receive craniospinal radiotherapy with posterior fossa boost [11,13], whereas in astrocytoma radiotherapy is exceptionally added in children with progressive and inaccessible tumors [12]. Ependymoma patients receive postoperative 3D conformal radiation therapy to the tumor bed [14,15]. Although radiotherapy is very effective, age at diagnosis is an important factor to consider. Radiotherapy is usually avoided in patients younger than three years, due to the deleterious impact on the developing brain [3]. Finally, the alternative treatment option for fossa posterior tumors is adjuvant chemotherapy, for which administration mainly depends on the tumor histology. In medulloblastoma, postoperative chemotherapy is the standard of care [13], while in astrocytoma it is only first line therapy if resection is impossible [12]. In ependymoma, only patients younger than three years of age are treated with chemotherapy, albeit not always effective [14].
Cancer treatment can lead to acute as well as long-term symptoms. A well-known acute consequence of cerebellar surgery is the posterior fossa syndrome (PFS). This syndrome appears in 25% of children after tumor resection in the posterior fossa. The syndrome comprises distressing symptoms [5], postoperative mutism [16], ataxia, hypotonia, emotional lability and behavioral symptoms. Recovery of PFS is slow and often incomplete. As a result, the syndrome is associated with potential long-term symptoms including reading deficits, lower intellectual ability, poor academic and cognitive outcomes, psychosocial complaints, neurologic deficits and lower quality of life (Qol) [5].
Due to the heterogeneity of fossa posterior tumors and their treatment, prognosis is also very variable. The prognoses for medulloblastoma and ependymoma patients are moderate, with 5-year survival rates of approximately 70% and 60%, respectively. Astrocytoma patients have the best prognosis, with a 90% 5-year survival rate [3]. This contrasts strongly with the dismal outlook of ATRT patients, with only 29.9% 5 year survival [17]. Finally, DIPG patients suffer the worst prognosis: the mean overall survival is 11 months and the 5-year overall survival is 2% [8,18-21].
Since survival rates for medulloblastoma, ependymoma and astrocytoma have significantly increased during the last decades [3], long-term therapy-related sequelae receive more and more attention. These sequelae could include somatic (e.g. endocrine problems [2,11,21], second neoplasms [11,21], kyphosis and vertebral demineralization [11]), neuropsychological (e.g. motor speech deficits [22], intellectual impairment, attention deficits, memory difficulties and executive dysfunction [3,4,11]) and psychosocial issues (e.g. unemployment, incapacity to drive and lower Qol [21]).
bulk of research exists for the different domains of longterm deficits of posterior fossa tumor survivors. To our knowledge, a systematic review of long-term symptoms from which these children can suffer throughout life, is missing. Therefore, this study aims to give a comprehensive and an integrated overview of all long-term sequelae (including somatic, neuropsychological and psychosocial outcomes) related to posterior fossa tumor therapy in children. A second aim is to link the various treatment types to specific long-term sequelae.
Methods
The PubMed/Medline database was screened using the following search algorithm: ("Infratentorial Neoplasms"(Mesh) OR cerebellar tumor OR brain stem tumor) AND ("Child"(Mesh) OR "Infant"(Mesh) OR "Adolescent"(Mesh)) AND treatment AND (long term sequelae OR outcome). Two additional filters were applied including: date of publication in 1990-2017 and English as publication language. Records were included if long-term somatic, long-term neuropsychological and long-term psychosocial effects of posterior fossa tumor survivors were depicted. Studies were excluded in case of in vitro or animal studies, also studies with patients >18 years who did not receive treatment during childhood, studies including current patients (i.e. if >50% of study population was followed <2 years after cancer diagnosis), studies covering other diagnoses (i.e. non-cancer or other types of cancer), non-original-research articles (case reports, expert opinions, conference summaries) and articles not reporting long-term effects. Duplicates were removed from the dataset. Studies were divided into three categories (medical, neuropsychological and psychosocial effects) and related to the treatment modality (surgery, chemotherapy and radiotherapy).
Results
The literature search returned 1369 articles, which were screened systematically. Stepwise amounts of included/ excluded articles are shown in the flowchart below (Figure 1), according to PRISMA guidelines. After removal of duplicates (n=2), the first selection was based on screening of the titles, eliminating 857 titles. Next, articles were screened for eligibility based on the abstracts, which resulted in 75 remaining studies for full-text screening. 7 studies were excluded, since full text was not available. Subsequently, each included record was classified into one of the categories mentioned above. 16 of the included articles described somatic symptoms, 39 articles reported on neuropsychological long-term effects, 3 articles covered psychosocial issues and 9 studies reported on all these effects. Two articles were excluded after analysis of the full text. Characteristics of the included studies are represented in Table 1, as well the number of articles describing long-term effects of each treatment modality.
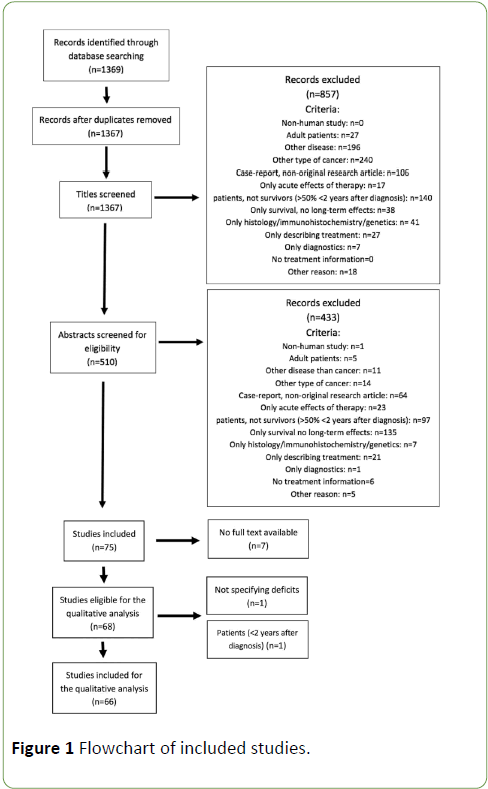
Figure 1: Flowchart of included studies.
| Treatment |
# Articles |
# Patients (ranges) |
Somatic |
Neuropsychology |
Psychosocial |
MB |
Ependymoma |
Astrocytoma |
Multiple |
| Surgery |
17 |
4-203 |
5 |
10 |
2 |
1 |
1 |
9 |
6 |
| RT |
33 |
7-151 |
15 |
18 |
2 |
27 |
0 |
0 |
6 |
| Chemo |
1 |
21-35 |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
| Surgery +RT+Chemo |
15 |
16-137 |
4 |
15 |
2 |
7 |
2 |
0 |
6 |
| Total |
66 |
4-203 |
16 |
38 |
3 |
36 |
3 |
9 |
18 |
Table 1: Included articles. Note: The included articles were classified into the different treatment modalities, categories of longterm sequelae and tumor types. Several articles described multiple types of long-term effects. RT: Radiotherapy, Chemo: Chemotherapy, MB: Medulloblastoma.
Surgery-induced symptoms
Long-term effects related merely to surgery, have only recently gained interest. The most frequently investigated patient population to study these effects are cerebellar pilocytic astrocytoma patients, because their treatment often consists of surgery only.
Surgery-related somatic effects: Nagib et al. reported permanent lower cranial nerve dysfunction in 2 of the 4 included patients after surgery for posterior fossa lateral ependymoma, a rare type of ependymoma [23]. The effects of surgery on general brainstem functions in ependymoma patients were studied by Merchant et al. They conducted a long-term neurological follow-up in 64 ependymoma patients treated with surgery and conformal radiotherapy (54–59.4 Gy). 23 patients experienced incomplete brain stem recovery after 5 years, defined as impaired function of cranial nerves V-VII and IX-XII, motor weakness and dysmetria.
These neurological outcomes depended on surgical variables such as CSF shunting, the number of interventions (resection and shunting), the extent of resection and tumor volume [24]. Variations in brainstem radiation dose (range 54– 59.4 Gy) and volume did not impact the outcome. Another study of Schoch et al. depicted the balance function in 22 patients treated for benign and malign posterior fossa tumors in childhood. More specifically, this study compared patients with and without affected cerebellar nuclei and patients with and without adjuvant therapy (chemotherapy and/or radiotherapy). The authors concluded that surgical damage of the deep cerebellar nuclei, especially the fastigial nucleus was responsible for balance impairment in these patients [25]. Zuzak et al. reviewed multiple long-term effects in 21 cerebellar astrocytoma survivors. Neurological deficits (limb ataxia, truncal ataxia, dysarthria, and ocular movement disorders) occurred frequently (41%, n=9) [26]. Similarly, Villarejo et al. retrospectively reviewed 203 astrocytoma survivors. Most of these patients exhibited no neurological deficits (55%). Some of them (27%) had mild neurological deficits (strabismus, nystagmus or ataxia) without psychosocial implications, while a few survivors (4%) were severely disabled and incapable of leading a normal life [27].
Surgery-related neuropsychological effects: Four records covered the neuropsychological consequences of pilocytic astrocytoma surgery in childhood. 201 adolescents and adults were included. Although most of these patients obtained a normal intelligence quotient and close to normal academic achievements, milder neuropsychological deficits were reported in 57-100% of them. These deficits included: dysarthria, difficulties in sustained attention, visuospatial capacities, executive functioning, memory, processing speed, behavioral problems, psychiatric symptoms [28-32]. Special education was needed in 11-24% of patients [28,29]. Beebe et al. (n=103) found little effect of tumor localization on these deficits [30], whereas Steinlin et al. claimed that vermis involvement was associated with more neuropsychological problems (n=24) [31].
In another study of Grill et al., perioperative risk factors of intellectual impairment were reviewed in 76 patients treated for malignant posterior fossa tumors. Average verbal intelligence quotient (VIQ) was in the lower average range (86.9 ± 18.9), whereas performance intelligence quotient (PIQ) was subnormal (75.8 ± 17.5). Preoperative hydrocephalus, vermis incision and neuropsychological the amount of postoperative cerebellar damage was significantly related to lower IQ scores [33]. Puget et al. also highlighted the effect of surgical cerebellar nuclei damage in 61 children treated for malignant posterior fossa tumors. Damage of the dentate nuclei and the inferior vermis was related to lower intellectual outcome than patients without this damage, after correction for other risk factors (radiation, hydrocephalus, surgical complications…) [34]. Not only cognitive and affective symptoms define the spectrum of long-term neuropsychological outcome. Also, speech can be affected. Four studies explored long-term speech deficits in 56 patients (astrocytoma n=25, medulloblastoma n=29, ependymoma n=2) following surgery. Most patients who experienced postoperative cerebellar mutism also encounter cerebellar dysarthria, slower speech and dysfluency in the long-term [35,36], but results are somewhat inconclusive for patients without postoperative mutism. Huber et al. found no difference in speech capacities between patients without postoperative mutism and healthy controls [35]. On the contrary, De Smet et al. and Morgan et al. both found higher percentages of long-term speech deficits (e.g. distorted vowels, slow rate, voice tremor) even in patients without postoperative cerebellar mutism [37,38].
Surgery-related psychosocial effects: Psychosocial effects associated with surgery were investigated by Zuzak et al. [26]. The authors evaluated 21 cerebellar astrocytoma survivors, of whom 33% (n=7) had behavioral problems and 19% (n=4) required special education due to cognitive deficits. However, most of them were still capable to perform normal daily life activities and their health-related Qol was rated like or even higher than controls. Apparently, these objective deficits had only minor influence on the subjective Qol [26]. These findings were nuanced by Pompili et al. who described Qol in 20 adults treated for cerebellar astrocytoma in childhood compared to 20 matched controls. They used two Qol questionnaires: the KPS and a detailed QoL questionnaire. These adult survivors had normal global functioning (as indicated by the KPS) and can lead a normal life, consistently with the findings of Zuzak et al. However, using the more profound Qol questionnaire, they had significant lower satisfaction than the control group when it came to social contacts, cognition, memory, well-being and adolescence [39].
Radiotherapy-related somatic effects
All articles describing long-term somatic effects related to radiotherapy, considered medulloblastoma survivors only. Eight of these studies explored long-term endocrine sequelae in 339 patients treated with postoperative craniospinal radiotherapy (doses ranging from 18 to 39 Gy craniospinal and 18-60 Gy posterior fossa boost). The most frequent endocrine long-term effects found in these studies included growth hormone (GH) deficiency and both primary and secondary thyroid dysfunction. GH deficiency leads together with spinal radiation to diminished adult height [40-46]. According to Kennedy et al. hyperfractionated radiotherapy (n=74) resulted in worse growth compared to conventional radiotherapy (n=77) [47]. Also, regarding GH deficiency, starting GH hormone administration at younger age resulted in better growth outcomes [44]. Helseth et al. studied the long-term outcome of 34 medulloblastoma survivors, treated with 35 Gy craniospinal radiation and 20 Gy posterior fossa boost. They found diminished height in most patients and the need of hormonal therapy in 50% of patients [48]. Also, Christopherson et al. followed up 53 medulloblastoma patients treated with a median craniospinal dose of 28.8 Gy and a posterior fossa or tumor bed boost of 25 Gy. The most important long-term effect of craniospinal radiotherapy was growth suppression (61.5% of patients). Less frequent endocrine sequelae were adrenal insufficiency, hypogonadism and precocious puberty [46]. These long-term effects could manifest up to 15 years post-treatment and were more prevalent in patients treated with chemotherapy (cisplatin, vincristine and lomustine) [45]. Symptoms also appeared to be dose-dependent [46].
A second important long-term somatic effect related to radiotherapy is the development of secondary neoplasms. This was illustrated in several studies with a total of 167 included patients, who were all treated with craniospinal radiotherapy for medulloblastoma. Malign neoplasms included breast cancer, thyroid cancer, lung cancer, stomach cancer, basal cell carcinoma and glioblastoma multiforme. Benign neoplasms described were meningiomas and cavernomas [49-52]. Helseth et al. showed second neoplasms in 14% of of 34 medulloblastoma survivors.
Intensity modulated proton therapy (IMPT), one of the more recent radiotherapy techniques, was associated with a lower risk of secondary neoplasms compared to 3D conformal radiotherapy (CRT) [49]. One study made a risk estimation of developing secondary malignancies after conventional craniospinal radiotherapy, IMPT and another technique called inversely-optimized arc therapy (RA). They made hypothetical treatment plans for the three techniques in 10 medulloblastoma survivors who already received craniospinal radiotherapy. The life time risk of a solid secondary neoplasm was estimated the highest in RA, the lowest in IMPT and increased with higher radiation dose. However, these estimations were merely based on predictive modeling. Exact data were missing [52].
Sporadically, hearing loss was observed in medulloblastoma patients, due to radiation-induced serous otitis media and sensorineural damage, skull radio necrosis, and cerebral ischemia or aneurysm development [53]. According to Kiltie et al. children treated with craniospinal radiotherapy under three years of age experienced similar problems, albeit to a higher degree than older patients. Furthermore, 75% of these younger patients suffered from severe neurologic late effects as ataxia, epilepsy, cranial nerve deficits and blindness [54].
Radiotherapy-related neuropsychological effects: Neuropsychological dysfunction is the most extensively investigated long-term effect in posterior fossa tumor survivors. Most patients participating in these studies are medulloblastoma survivors treated with craniospinal radiotherapy. Nevertheless, ependymoma and astrocytoma patients were also occasionally included. Both high (range 30.6-41.4 Gy) and reduced doses (range 18-25.2 Gy) of craniospinal radiotherapy were applied. Posterior fossa boost dose was ranged between 12.6 and 60 Gy, tumor bed boost between 16 and 86 Gy. The most frequently assessed outcome was intelligence, objectified by the Wechsler scale of intelligence (population mean=100, SD=15). George et al. studied long-term general cognitive sequelae for medulloblastoma and astrocytoma survivors (n=15). The mean full-scale intelligence quotient (FSIQ), VIQ, and PIQ were all significantly lower than the normal population, as were verbal, visual and general memory. There was no significant difference between VIQ and PIQ. Verbal and visual memory were also equally affected. Younger age at diagnosis (<6 years) resulted in lower long-term IQ scores than older age [55]. A study of Mabbott et al. specifically compared patients treated with surgery and cranial radiation and patients who received surgery only. Both cranial radiation and postsurgical complications were related to lower IQ and information processing speed. Age at diagnosis was not a predictor for these problems [56]. According to Abd El-aal et al., the addition of postoperative chemotherapy (vincristine, etoposide and cisplatin) had no effect on cognitive outcome [57].
The evolution and determinants of this intellectual decline were investigated in three studies, including 191 medulloblastoma and 9 ependymoma survivors treated with 23.4 or 35-40 Gy craniospinal radiation [58-60]. IQ declined year after year (1.70-2.05 points per year) [58,60], as well as academic achievement [60]. Higher baseline intelligence was associated with steeper intellectual decline [58-60]. Younger age at diagnosis also resulted in steeper decline in two of the three records [59,60]. This effect of age at diagnosis was confirmed by Rutkowski et al. These researchers observed 29 children with medulloblastoma diagnosed younger than three years old. 12 of them relapsed during chemotherapy and were administered 24 Gy craniospinal radiotherapy with a boost on the metastases. These patients had very low intellectual outcome 6.1 years after diagnosis (IQ 77.7 ± 7.2) [61].
The mechanism of radiation-induced intellectual deterioration could be found in both volume reduction and microscopic injury of white matter. Three studies investigated brain damage after craniospinal and posterior fossa boost radiotherapy with diffusion tensor MRI imaging (DTI). In a record of Riggs et al. irradiated medulloblastoma and astrocytoma survivors (n=20) exhibited reduced white matter volume compared to healthy controls (n=13). The fasciculus uncinatus and the right hippocampus were the most affected areas. Volume reductions resulted in lower memory scores [62]. Additionally, Mabbott et al. (n=8) and Law et al. (n=29) focused on apparent diffusion coefficient (ADC) and fractional anisotropy (FA). Craniospinal radiotherapy was associated with increased ADC and decreased FA of white matter. If the cerebello-thalamo-cerebral connections were affected by these changes, this was associated with poor intellectual outcome and working memory [63,64]. Similarly, a study of Wilburn confirmed reduced white matter in pediatric brain tumor survivors to be associated with lower IQ and academic achievements [65].
Extensively analyzed characteristics of intellectual decline are the influence of radiation volume and dose-dependency. This was illustrated in four studies comparing posterior fossa or tumor boost radiotherapy (45-55.4 Gy) without craniospinal radiotherapy, with reduced dose (15-25 Gy) and with standard dose craniospinal radiotherapy (30.6-39 Gy) in 184 patients. All these studies concluded that local radiotherapy resulted in smaller decrease in FSIQ than craniospinal radiotherapy. Furthermore, higher craniospinal doses were associated with greater decrease in FSIQ [59,66-68]. Moxon-Emre et al. also added that higher boost volumes (posterior fossa boost vs. tumor bed boost), hydrocephalus and postoperative mutism resulted in poorer intellectual outcome [67].
Besides radiation dose, genetics could also influence the risk of radiation induced brain injury, as postulated by Bracket et al. The childhood cancer survivor study neurocognitive questionnaire and the Brief Symptom Inventory of 109 medulloblastoma patients were compared to 143 healthy siblings. Polymorphisms in multiple antioxidant enzymes (SOD2, GPX1, GSTP1, GSTM1, GSTT1) were determined in both groups. Medulloblastoma survivors scored significantly worse on memory and task efficiency, the latter even more impaired in children <7 years at diagnosis. Globally these findings were independent of the investigated polymorphisms. However, a subgroup of survivors with homozygous GTMS 1 experienced significantly more anxiety, depression and global distress [69].
Finally, modern radiotherapy methods have received attention only more recently. Jain et al. showed that there was no difference in cognitive decline between IMRT and conventional radiotherapy in 25 medulloblastoma patients [70]. Similarly, FSIQ in standard risk medulloblastoma patients treated with HFRT (n=71) did not significantly differ from patients treated with conventional radiotherapy (n=66) [71]. Additionally, Kennedy et. al even showed improved executive functioning in patients treated with hyperfractionated radiotherapy, compared to conventional radiotherapy [47].
Radiotherapy-related psychosocial effects: Radiotherapy for posterior fossa tumors can lead to serious psychosocial problems. Helseth et al. found psychosocial impairments (learning abilities, sociability, hobbies, relations) in 62% of the 34 medulloblastoma survivors [48]. This was also previously investigated in a study of Mabbott et al. This record included 53 posterior fossa tumor survivors (46 medulloblastoma patients and 7 ependymoma patients). 48 patients were treated by craniospinal radiation (23.4-41.6 Gy) with a posterior fossa boost (45.0-55.8 Gy). They were evaluated with standardized achievement tests as well as parent and teacher questionnaires. Three years post-diagnosis, school skills and academic achievement were about one standard deviation below the population average. Hydrocephalus and younger age at diagnosis were associated with poorer academic outcome, whereas radiation dose, extent of resection and use of chemotherapy were not. Behavioral problems were only mild. A longitudinal analysis (median follow-up after diagnosis 4.84 years for academic achievement and 4.17 years for behavioral function) revealed that academic skills continued to decline over time. Behavior remained rather stable according to parent questionnaires. However, some showed social withdrawal and attention problems after a few years [72].
Chemotherapy-induced effects
Chemotherapy-induced somatic effects: This review only returned one article describing a well-known consequence of chemotherapy in medulloblastoma surivors, being hearing loss. Lafay-Cousin et al. acquired audiograms in 35 children during and after cisplatin treatment for average risk (AR) and high risk (HR) medulloblastoma patients. AR patients received higher doses of cisplatin (412.5 mg/m2 vs 270 mg/m2), whereas HR patients received higher doses of craniospinal radiotherapy (36-39 Gy vs 23.4 Gy). Both AR and HR patients often required hearing support five years after treatment. However, the AR group more frequently required dose reduction of cisplatin during treatment due to hearing loss. This finding suggests that the effect of higher cumulative dose of cisplatin exceeds the damage of a higher radiation dose in the short term. However, this difference disappears over time [73].
Combined therapies
Somatic effects: Multiple included studies (n=145 patients in total, mostly medulloblastoma patients) depicted different types of long-term effects of different treatment regiments (surgery, craniospinal radiotherapy with or without chemotherapy). All of them could confirm the abovementioned results. Endocrine deficits, neurologic sequelae, hearing loss and academic difficulties were very frequent in all of these studies [21,74-76].
Neuropsychological effects: As could be inferred from abovementioned studies, each treatment constituent might cause long-term effects in childhood posterior fossa tumors. Many additional studies investigated the effects of combined treatments. Von Hoff et al. and Chapman et al. investigated the influence of radiotherapy and surgery on neuropsychological outcome [77,78]. They evaluated intelligence scores in 23 ependymoma survivors treated with surgery and posterior fossa boost radiotherapy (mean 54 Gy). PIQ was significantly lower than normal, whereas FSIQ, VIQ were only moderately impaired. All patients experienced reading difficulties. However, no decline in IQ scores was notified in a longitudinal analysis. Factors associated with lower IQ included the posterior fossa syndrome, preoperative hydrocephalus and radiation volume. Again, younger age at irradiation resulted in lower neuropsychological outcome [77]. In the record of Chapman et al. 13 medulloblastoma and 2 ependymoma patients were included. The radiotherapy protocols consisted of high radiation doses, also in children under three years of age. Younger age at diagnosis and perioperative issues (preoperative hydrocephalus, resection of neocerebellum tissue) were once more associated with worse outcome, but also with a higher incidence of obtundation at diagnosis [78]. This effect of age at diagnosis was once more confirmed by Edelstein et al. [74] and Johnson et al. [79].
The above-mentioned general studies considering somatic long-term effects, also reported on neuropsychological effects. Not surprisingly, neuropsychological issues were a major problem in most medulloblastoma survivors [21,74-76].
Huber et al. studied long-term speech deficits in both astrocytoma and medulloblastoma survivors. All experienced dysfluent speech, but atactic dysarthria and slow speech were only frequent in medulloblastoma survivors [22]. In a record of Szathmari et al. speech deficits in medulloblastoma survivors could be predicted by tonsillar herniation and a larger tumor volume on preoperative MRI [80]. Other neuropsychological differences between medulloblastoma and astrocytoma survivors were described by Roncadin et al. and Ronning et al. As expected, medulloblastoma survivors clearly scored worse on several outcome scales (memory, intelligence, attention) than astrocytoma survivors [81,82]. In both studies, younger age at diagnosis predicted lower cognitive functioning in medulloblastoma survivors. The important contribution of craniospinal radiotherapy on cognitive decline was confirmed in a prospective study of 35 posterior fossa tumor survivors by Stargatt et al. The previously described detrimental effect of hydrocephalus was replicated in several studies [81,83,84]. This was less clear in astrocytoma survivors [81,82]. Finally, Schreiber et al. added the presence of the posterior fossa syndrome, hearing loss, and high-risk status as significant risk factors for intellectual and academic decline in 165 medulloblastoma survivors, next to younger age at diagnosis [85].
Psychosocial effects: Kulkarni et al. investigated long-term Qol in 62 survivors of different types of posterior fossa tumors (medulloblastoma, astrocytoma and ependymoma). Consistent with previous findings, Qol in those survivors was not significantly lower than the general population. Risk factors for lower scores included hydrocephalus and socioeconomic status [86]. While Kulkarni et al. stated that there was no difference in general Qol between the tumor types, Ribi et al. noted that social functioning was lowest in medulloblastoma survivors. Notably, parents rated Qol for their children was lower than patients themselves [76].
Discussion
Treatment for posterior fossa tumors evolved impressively over the last decades. This had led to improved survival rates for children diagnosed with medulloblastoma, astrocytoma and ependymoma. However, this positive trend also involves the apparition of higher treatment-related symptoms. This was extensively illustrated in the 66 articles included in this review. First, surgery appears as responsible for neurologic symptoms (ataxia, impaired balance and cranial nerve deficits) and mild cognitive and psychosocial issues. Second, radiotherapy (especially craniospinal radiotherapy) is related to a much wider range of long-term sequelae. These include endocrine deficits, secondary neoplasms, neurological sequelae such as hearing loss, intellectual decline and severe psychosocial problems (lower academic achievement and social functioning). Third, chemotherapy was only associated with hearing loss in this review.
As treatment modalities result in different sequelae, it is evident that the long-term symptoms a survivor can experience, highly depend on the treatment that was administered. This in turn is determined by the tumor type. However, tumor type possibly also influences baseline performance (before initiation of therapy). Unfortunately, this was insufficiently investigated sofar. Astrocytoma survivors treated with surgery only can experience severe neurological sequelae, but cognitive and psychosocial impairment seem rather mild. On the contrary, medulloblastoma survivors suffer the greatest from treatment-related morbidity due to the burdensome combination of surgery, craniospinal radiotherapy and chemotherapy. Logically, the results of ependymoma survivors are situated somewhere in between.
When it comes to somatic long-term sequelae, surgeryinduced brain damage was most frequently related to neurological symptoms include ataxia, impaired balance and several cranial nerve deficits. The latter can cause ocular movement disorders (n. III, IV, VI) [23,25-27]. Such symptoms are probably due to damage to specific cerebellar nuclei, particularly balance dysfunction [25].
Radiotherapy can also give rise to neurological symptoms such as hearing loss (n. VIII) and even blindness (n. II) in severe cases. Hearing loss can be induced both by this radiationinduced serous otitis media or sensorineural damage and by platinum-based chemotherapy [53,73]. Irradiation is known to induce brain damage through multiple toxic mechanisms, including vascular abnormalities, inflammation, gliosis, demyelination and ultimately white matter necrosis [2,10,11] and decreased neurogenesis [12]. From animal models we know that oligodendrocytes decrease, leading to lower myelination levels [13]. Also, vascular endothelial cells at the blood-brain barrier are affected [14,15]. Another highly relevant problem is endocrine impairment. This is caused by radiation to the hypothalamic–pituitary axis and the thyroid gland. The most common clinical manifestations are GH deficiency, hypothyroidism (both primary and secondary), adrenal insufficiency, hypogonadism and precocious puberty [40-45,87]. Consequently, posterior fossa tumor survivors are smaller than their peers, because of GH deficiency and spinal radiation. These deficits are clearly dose-dependent [43]. While endocrine defects are very frequent (up to 50%), albeit not life-threatening, the development of second neoplasms is rarer (14%) but can be fatal [48]. Second neoplasms can occur within the radiated area (meningiomas, cavernomas, glioblastomas, thyroid cancer, basal cell carcinoma) or even in areas not strictly localized in the radiation field (breast cancer, lung cancer). These remote neoplasms were mostly seen in older radiotherapy regiments with higher doses and larger radiation fields [49-52]. Mortality is due either to the inherent malignant character of the neoplasm, either to local complications of the lesion (for instance bleeding of a cavernoma) [49,50].
All these somatic inferences might affect daily life and neuropsychological functioning of the patient. This is present in all types of posterior fossa tumors, but obviously more prominent in the medulloblastoma group. These children exhibit clearly worse FSIQ, PIQ, VIQ, memory, nonverbal intellectual functioning, processing speed and academic skills than the normal population [55,69]. Furthermore, IQ continues to decline [58,60]. Astrocytoma and ependymoma survivors mostly encounter more subtle problems like reading difficulties and slower processing speed, whereas IQ and academic achievement remain most often normal [28-32,77]. Younger age at diagnosis results in lower cognitive outcome in medulloblastoma and ependymoma survivors [77,78]. The effect of younger age at diagnosis was less clear for astrocytoma patients, with even better outcome in younger patients [88]. This could be explained either by great neuronal plasticity in a young brain unexposed to radiotherapy, or by lack of testing sensitivity in younger children [88]. Preoperative hydrocephalus and postoperative surgical complications (e.g. mutism, the posterior fossa syndrome, damage of the dentate nuclei or vermis) also holds a risk of greater intellectual disabilities and poorer academic outcome in all posterior fossa tumors [34,67,81,85]. Important causes of neuropsychological sequelae are underlying neuroanatomical changes, especially radiation-induced neural atrophy and microscopic damage of cerebral white matter [62-64]. This could explain why somatic risk factors described above result in greater intellectual decline, since they either directly induce more white matter injury (higher radiation volume and dose, hydrocephalus) or increase the susceptibility to radiationinduced damage (younger age at diagnosis).
Another disabling long-term related to neurological damage, is speech impairment. Medulloblastoma as well as astrocytoma survivors experience speech dysfluency. However, atactic dysarthria and slow speech are only evident in children treated for medulloblastoma. This indicates that irradiation also has detrimental effects on the cerebellum, frontocerebellar tracts, language and motor coordination [22,35-38].
It is not surprising that all these sequelae have a major impact on the future lives of these patients. They all must deal with multiple psychosocial issues, especially medulloblastoma survivors. These patients frequently have a decline in school skills (reading, mathematics, spelling), need special education, and are unemployed. Hydrocephalus and younger age at diagnosis result in poorer academic outcome. In astrocytoma patients, these psychosocial issues are clearly less pronounced. Considering Qol, social functioning was recurrently lower. Despite difficulties in establishing and sustaining relationships, global Qol of medulloblastoma, astrocytoma and ependymoma survivors is often not reported as lower than normal. This might signify that patients eventually learn to cope with their disabilities, or might be unaware of their symptoms [26,39,48,72,86].
Limitations
This study has several limitations. First, a quantitative analysis could not be established due to great heterogeneity in the included studies. These studies all included different patient populations of different ages, often depicted multiple risk factors and tumor subtypes (low risk and high risk). Also, the exact radiotherapy doses of the cranial and the spinal component of craniospinal radiation were usually not specified. This impedes valuable conclusions in a meta-analysis about dose dependency of symptoms and about distinctions between cranial or spinal radiation doses. Secondly, most of the included articles were older than 2010, given that recent research focuses new therapies of which long-term effects cannot be judged yet. Thirdly, the search algorithm did not return many articles about chemotherapy-induced sequelae only. The reason for this could be that chemotherapy was added only quite recently and therefore it might still be too early to evaluate its long-term effects. Finally, this literature search covers a wide extent of symptoms. Given the large number of screened articles through the entire procedure, screening was only performed by one author. Due to limited timing, no inter-observer reliability could be determined.
Future Directions
As somatic symptoms, and neurocognitive dysfunction highly affects quality of life of posterior fossa tumor patients, patients will require treatment adaptations, and early motor and cognitive rehabilitation. Recently, the diagnostic approach for brain tumors is dramatically changing. While the former WHO classification of CNS tumors (2007) primarily categorized the neoplasms according to histology, the most recent classification (2016) is based on the combination of both histological and genetics findings. As a result, formerly recognized histological entities are subdivided into different genetic subtypes. Medulloblastomas can now be assigned to one of five genetic subgroups (WNT activated, SHH activated TP53 mutated, SHH activated TP53 Wild Type, group 3 and group 4) next to the pre-existing histological classification. These strictly defined subgroups will most likely contain greater prognostic value and will certainly be a guide line for personalized CNS tumor therapy in the future [89,90]. Advanced MRI technique (dynamic contrast enhanced, susceptibility weighted imaging, diffusion tensor imaging and functional MRI) will be implemented more and more often for maximal preoperative tumor characterization. Additionally, 11C-methionine PET has recently been shown useful in discriminating between high grade and low-grade tumors. On the other hand, intraoperative MRI (iMRI) is a promising modality for evaluating the extent of resection during surgery [7]. These new technologies will hopefully also contribute to a more tailored treatment plan.
Treatment regiments are improving in order to reduce neurotoxicity as well. Advances in radiation therapy, such as intensity modulated radiation therapy (IMRT), intensity modulated proton therapy (IMPT) and hyper fractionated radiation therapy (HFRT), have recently been introduced in posterior fossa tumor therapy. In HFRT, overall survival is like standard craniospinal radiotherapy [91]. First results about therapy-related sequelae were promising. HFRT was associated with better long-term executive functioning compared to conventional radiotherapy [47] but these results remain limited and inconsistent [70,71]. IMRT could also result in less hearing loss [92] and IMPT in lower risk of second neoplasms [93]. Another recent development in treatment is the use of myeloablative chemotherapy with autologous stem cell transplantation in high risk or recurrent medulloblastoma, especially in children younger than three years [94,95]. This therapeutic strategy might also reduce the need of craniospinal radiation in this high-risk group and may in that way decrease the risk of intellectual decline [94]. Finally, the age limit for craniospinal therapy remains an important issue, since intellectual decline is clearly more pronounced in patients with younger age at diagnosis [55,58,59,74,77,79,81,85]. Chemotherapy regiments are indeed evolving in this group. Delaying craniospinal radiotherapy until the age of six might become more and more feasible. Large international clinical studies will be necessary to demonstrate the efficacy, safety and impact on long-term effects of this approach.
Finally, although motor rehabilitation received a lot of attention in the past, standardized cognitive trainings and rehabilitation is currently lacking. Nevertheless, multiple methods (training processing speed, attention, memory etc.) recently seemed beneficial [96,97]. Also, more and more attention is given to computerized trainings, including recent developments in virtual reality applications [98]. However, results of these methods are still to be waited for.
Conclusion
Posterior fossa tumors in children constitute a heterogeneous group of neoplasms, with medulloblastoma, astrocytoma and ependymoma as the predominant tumor types. Advances in treatment over the last decades have led towards better survival, but also gave rise to the development of long-term treatment-related sequelae. This study gave an overview of different somatic, neuropsychological and psychosocial effects linked to the different treatment regiments. Medulloblastoma survivors suffered the highest treatment-related morbidity, with increased risk due to craniospinal radiotherapy, higher radiation dose, younger age at diagnosis and preoperative hydrocephalus. As a conclusion, long-term treatment-related sequelae should receive sufficient attention of all specialists involved (neurosurgeons, radiotherapists and oncologists). Treatments highly require advancements to preventively lower neurotoxicity, as well as rehabilitation in patients after treatment.
Acknowledgement
The authors are grateful to their colleagues from pediatric oncology and radiology for sharing their insights and successful collaboration. They also wish to thank Kinderkankerfonds Leuven for their financial support of the authors’ current neurocognitive research which is in progress.
Funding
Kinderkankerfonds Leuven provided financial support.
Conflicts of Interest
None.
22443
References
- Poussaint TY, Panigrahy A, Huisman TAGM (2015) Pediatric brain tumors. Pediatr Radiol 45: 443-453.
- Bereket A (2015) Endocrinologic consequences of pediatric posterior fossa tumours. J Clin Res Pediatr Endocrinol 7: 253-259.
- Hanzlik E, Woodrome SE, Abdel-Baki M, Geller TJ, Elbabaa SK (2015) A systematic review of neuropsychological outcomes following posterior fossa tumor surgery in children. Childs Nerv Syst 31: 1869-1875.
- Wolfe KR, Madan-Swain A, Kana RK (2012) Executive dysfunction in pediatric posterior fossa tumor survivors: A systematic literature review of neurocognitive deficits and interventions. Dev Neuropsychol 37: 153-175.
- Lanier JC, Abrams AN (2017) Posterior fossa syndrome: Review of the behavioral and emotional aspects in pediatric cancer patients. Cancer 123: 551-559.
- Brandão LA, Young Poussaint T (2017) Posterior Fossa Tumors. Neuroimaging Clin N Am 27: 1-37.
- Choudhri AF, Siddiqui A, Klimo P (2016) Pediatric cerebellar tumors: emerging imaging techniques and advances in understanding of genetic features. Magn Reson Imaging Clin N Am 24: 811-821.
- Grimm SA, Chamberlain MC (2013) Brainstem glioma: A review. Curr Neurol Neurosci Rep 13: 346.
- Raybaud C, Ramaswamy V, Taylor MD, Laughlin S (2015) Posterior fossa tumors in children: developmental anatomy and diagnostic imaging. Child’s Nerv Syst 31: 1661-1676.
- Hamisch C, Kickingereder P, Fischer M, Simon T, Ruge MI (2017) Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: a systematic review and meta-analysis of 735 cases. J Neurosurg Pediatr 20: 1-8.
- Massimino M, Giangaspero F, Garrè ML, Gandola L, Poggi G, et al. (2011) Childhood medulloblastoma. Crit Rev Oncol Hematol 79: 65-83.
- Bonfield CM, Steinbok P (2015) Pediatric cerebellar astrocytoma: a review. Child’s Nervous System 31: 1677-1685.
- Coluccia D, Figuereido C, Isik S, Smith C, Rutka JT (2016) Medulloblastoma: Tumor biology and relevance to treatment and prognosis paradigm. Curr Neurol Neurosci Rep 16: 43.
- Thompson YY, Ramaswamy V, Diamandis P, Daniels C, Taylor MD (2015) Posterior fossa ependymoma: current insights. Child’s Nerv Syst 31: 1699-1706.
- Ramaswamy V, Taylor MD (2016) Treatment implications of posterior fossa ependymoma subgroups. Chin J Cancer 35: 93.
- Fischer-Valuck BW, Chen I, Srivastava AJ, Floberg JM, Rao YJ, et al. (2017) Assessment of the treatment approach and survival outcomes in a modern cohort of patients with atypical teratoid rhabdoid tumors using the National Cancer Database. Cancer 123: 682-687.
- Sun T, Wan W, Wu Z, Zhang J, Zhang L (2013) Clinical outcomes and natural history of pediatric brainstem tumors: With 33 cases follow-ups. Neurosurg Rev 36: 311-319.
- Hassan H, Pinches A, Picton SV, Phillips RS (2017) Survival rates and prognostic predictors of high grade brain stem gliomas in childhood: a systematic review and meta-analysis. J Neurooncol 0: 1-8.
- Veldhuijzen van Zanten SEM, Baugh J, Chaney B, De Jongh D, Sanchez Aliaga E, et al. (2017) Development of the SIOPE DIPG network, registry and imaging repository: a collaborative effort to optimize research into a rare and lethal disease. J Neurooncol 132: 255-266.
- Frange P, Alapetite C, Gaboriaud G, Bours D, Zucker JM, et al. (2009) From childhood to adulthood: Long-term outcome of medulloblastoma patients. The Institut Curie experience (1980-2000). J Neurooncol 95: 271-279.
- Huber JF, Bradley K, Spiegler B, Dennis M (2007) Long-term neuromotor speech deficits in survivors of childhood posterior fossa tumors: effects of tumor type, radiation, age at diagnosis, and survival years. J Child Neurol 22: 848-854.
- Nagib MG, O’fallon MT (1996) Posterior fossa lateral ependymoma in childhood. Pediatr Neurosurg 24: 299-305.
- Merchant TE, Chitti RM, Li C, Xiong X, Sanford RA, et al. (2010) Factors associated with neurological recovery of brainstem function following postoperative conformal radiation therapy for infratentorial ependymoma. Int J Radiat Oncol Biol Phys 76: 496-503.
- Schoch B, Konczak J, Dimitrova A, Gizewski ER, Wieland R, et al. (2006) Impact of surgery and adjuvant therapy on balance function in children and adolescents with cerebellar tumors. Neuropediatrics 37: 350-358.
- Zuzak TJ, Poretti A, Drexel B, Zehnder D, Boltshauser E, et al. (2008) Outcome of children with low-grade cerebellar astrocytoma: long-term complications and quality of life. Childs Nerv Syst 24: 1447-1455.
- Villarejo F, Belinchón de Diego JM, Gómez de la Riva Á (2008) Prognosis of cerebellar astrocytomas in children. Child’s Nerv Syst 24: 203-210.
- Aarsen F, Van Dongen H, Paquier P, Van Mourik M, Catsman-Berrevoets C (2004) Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology 62: 1311-1316.
- Ait Khelifa-Gallois N, Laroussinie F, Puget S, Sainte-Rose C, Dellatolas G (2015) Long-term functional outcome of patients with cerebellar pilocytic astrocytoma surgically treated in childhood. Brain Inj 29: 366-373.
- Beebe DW, Ris MD, Armstrong FD, Fontanesi J, Mulhern R, et al. (2005) Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies. J Clin Oncol 23: 5198-5204.
- Steinlin M, Imfeld S, Zulauf P, Boltshauser E, Lövblad KO, et al. (2003) Neuropsychological long-term sequelae after posterior fossa tumour resection during childhood. Brain 126: 1998-2008.
- Aarsen FK, Paquier PF, Arts WF, Van Veelen ML, Michiels E, et al. (2009) Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol 27: 3526-3532.
- Grill J, Viguier D, Kieffer V, Bulteau C, Sainte-Rose C, et al. (2004) Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. J Neurosurg 101: 152-158.
- Puget S, Boddaert N, Viguier D, Kieffer V, Bulteau C, et al. (2009) Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer 115: 1338-1347.
- Huber JF, Bradley K, Spiegler BJ, Dennis M (2006) Long-term effects of transient cerebellar mutism after cerebellar astrocytoma or medulloblastoma tumor resection in childhood. Child’s Nerv Syst 22: 132-138.
- Steinbok P, Cochrane DD, Perrin R, Price A (2003) Mutism after posterior fossa tumour resection in children: Incomplete recovery on long-term follow-up. Pediatr Neurosurg 39: 179-183.
- De Smet HJ, Catsman-Berrevoets C, Aarsen F, Verhoeven J, Mariën P, et al. (2012) Auditory-perceptual speech analysis in children with cerebellar tumours: A long-term follow-up study. Eur J Paediatr Neurol 16: 434-442.
- Morgan AT, Liégeois F, Liederkerke C, Vogel AP, Hayward R, et al. (2011) Role of cerebellum in fine speech control in childhood: Persistent dysarthria after surgical treatment for posterior fossa tumour. Brain Lang 117: 69-76.
- Pompili A, Caperle M, Pace A, Ramazzotti V, Raus L, et al. (2002) Quality-of-life assessment in patients who had been surgically treated for cerebellar pilocytic astrocytoma in childhood. J Neurosurg 96: 229-234.
- Ricardi U, Corrias A, Einaudi S, Genitori L, Sandri A, et al. (2001) Thyroid dysfunction as a late effect in childhood medulloblastoma: A comparison of hyperfractionated versus conventionally fractionated craniospinal radiotherapy. Int J Radiat Oncol Biol Phys 50: 1287-1294.
- Karadağ O, Demiröz-Abakay C, Özkan L, Sağlam H, Demirkaya M (2015) Evaluation of late effects of postoperative radiotherapy in patients with medulloblastoma. Turk J Pediatr 57: 167-171.
- Heikens J, Michiels EMC, Behrendt H, Endert E, Bakker PJM, et al. (1998) Long-term neuro-endocrine sequelae after treatment for childhood medulloblastoma. Eur J Cancer 34: 1592-1597.
- Xu W, Janss A, Moshang T (2003) Adult height and adult sitting height in childhood medulloblastoma survivors. J Clin Endocrinol Metab 88: 4677-4681.
- Chae HW, Park YS, Kim DS, Kwon AR, Kim HS, et al. (2013) Final height and insulin-like growth factor-1 in children with medulloblastoma treated with growth hormone. Child’s Nerv Syst 29: 1859-1863.
- Uday S, Murray RD, Picton S, Chumas P, Raju M, et al. (2015) Endocrine sequelae beyond 10 years in survivors of medulloblastoma. Clin Endocrinol (Oxf) 83: 663-670.
- Xu W, Janss A, Packer RJ, Phillips P, Goldwein J, et al. (2004) Endocrine outcome in children with medulloblastoma treated with 18 Gy of craniospinal radiation therapy. Neuro Oncol 6: 21-27.
- Kennedy C, Bull K, Chevignard M, Culliford D, Dörr HG, et al. (2014) Quality of survival and growth in children and young adults in the PNET4 European controlled trial of hyperfractionated versus conventional radiation therapy for standard-risk medulloblastoma. Int J Radiat Oncol Biol Phys 88: 292-300.
- Helseth E, Due-Tønnessen B, Wesenberg F, Lote K, Lundar T (1999) Posterior fossa medulloblastoma in children and young adults (0-19 years): Survival and performance. Child’s Nerv Syst 15: 451-456.
- Brodin NP, Vogelius IR, Maraldo M V, Munck Af Rosenschöld P, Aznar MC, et al. (2012) Life years lost-comparing potentially fatal late complications after radiotherapy for pediatric medulloblastoma on a common scale. Cancer 118: 5432–5440.
- Lew SM, Morgan JN, Psaty E, Lefton DR, Allen JC, et al. (2006) Cumulative incidence of radiation-induced cavernomas in long-term survivors of medulloblastoma. J Neurosurg 104: 103-107.
- Stavrou T, Bromley CM, Nicholson HS, Byrne J, Packer RJ, et al. (2001) Prognostic Factors and Secondary Malignancies in Childhood Medulloblastoma. J Pediatr Hematol Oncol 23: 431-436.
- Brodin NP, Munck Af Rosenschöld P, Aznar MC, Kiil-Berthelsen A, Vogelius IR, Nilsson P, et al. Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol. 2011;50(6):806-816.
- Christopherson KM, Rotondo RL, Bradley JA, Pincus DW, Wynn TT, et al. (2014) Late toxicity following craniospinal radiation for early-stage medulloblastoma. Acta Oncol (Madr) 53: 471-480.
- Kiltie AE, Lashford LS, Gattamaneni HR (1997) Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol 28: 348-354.
- George AP, Kuehn SM, Vassilyadi M, Richards PMP, Parlow SE, et al. (2003) Cognitive sequelae in children with posterior fossa tumors. Pediatr Neurol 28: 42-47.
- Mabbott D, Penkman L, Witol A, Strother D, Bouffet E (2008) Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology 22: 159-168.
- Abd El-aal HH, Mokhtar MM, Habib E, El-kashef AT, Fahmy ES (2005) Medulloblastoma: Conventional radiation therapy in comparison to chemo radiation therapy in the post-operative treatment of high-risk patients. J Egypt Nat Cancer Inst 17: 301-307.
- Palmer SL, Gajjar A, Reddick WE, Glass JO, Kun LE, et al. (2003) Predicting intellectual outcome among children treated with 35-40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology 17: 548-555.
- Kieffer-Renaux V, Viguier D, Raquin MA, Laurent-Vannier A, Habrand JL, et al. (2005) Therapeutic schedules influence the pattern of intellectual decline after irradiation of posterior fossa tumors. Pediatr Blood Cancer 45: 814-819.
- Ris MD, Walsh K, Wallace D, Armstrong FD, Holmes E, et al. (2013) Intellectual and academic outcome following two chemotherapy regimens and radiotherapy for average-risk medulloblastoma: COG A9961. Pediatr Blood Cancer 60: 1350–1357.
- Rutkowski S, Gerber NU, von Hoff K, Gnekow A, Bode U, et al. (2008) Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol 11: 201-210.
- Riggs L, Bouffet E, Laughlin S, Laperriere N, Liu F, et al. (2014) Changes to memory structures in children treated for posterior fossa tumors. J Int Neuropsychol Soc 20: 168-180.
- Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, Laughlin S (2006) Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro Oncol 8: 244-252.
- Law N, Bouffet E, Laughlin S, Laperriere N, Brière ME, et al. (2011) Cerebello–thalamo–cerebral connections in pediatric brain tumor patients: Impact on working memory. Neuroimage 56: 2238-2248.
- Reddick WE, White HA, Glass JO, Wheeler GC, Thompson SJ, et al. (2003) Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer 97: 2512-2519.
- Grill J, Renaux VK, Bulteau C, Viguier D, Levy-Piebois C, et al. (1999) Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys 45: 137-145.
- Moxon-Emre I, Bouffet E, Taylor MD, Laperriere N, Scantlebury N, et al. (2014) Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol 32: 1760-1768.
- Wahba HA, Abu-Hegazy M, Wasel Y, Ismail EI, Zidan AS (2013) Adjuvant chemotherapy after reduced craniospinal irradiation dose in children with average-risk medulloblastoma: a 5-year follow-up study. J BUON 18: 425-429.
- Brackett J, Krull K, Scheurer M, Liu W, Srivastava D, et al. (2012) Antioxidant enzyme polymorphisms and neuropsychological outcomes in medulloblastoma survivors: A report from the Childhood Cancer Survivor Study. Neuro Oncol 14: 1018–1025.
- Kushner BH, LaQuaglia MP, Wollner N, Meyers PA, Lindsley KL, et al. (1996) Desmoplastic small round-cell tumor: Prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol 14: 1526-1531.
- Câmara-Costa H, Resch A, Kieffer V, Lalande C, Poggi G, et al. (2015) Neuropsychological outcome of children treated for standard risk medulloblastoma in the PNET4 European randomized controlled trial of hyperfractionated versus standard radiation therapy and maintenance chemotherapy. Int J Radiat Oncol Biol Phys 92: 978-985.
- Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, et al. (2005) Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol 23: 2256-2263.
- Lafay-Cousin L, Purdy E, Huang A, Cushing SL, Papaioannou V, et al. (2013) Early cisplatin induced ototoxicity profile may predict the need for hearing support in children with medulloblastoma. Pediatr Blood Cancer 60: 287-292.
- Edelstein K, Spiegler BJ, Fung S, Panzarella T, Mabbott DJ, et al. (2011) Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neuro Oncol 13: 536–545.
- Massimino M, Gandola L, Cefalo G, Lasio G, Riva D, et al. (2000) Management of medulloblastoma and ependymoma in infants: a single-institution long-term retrospective report. Child’s Nerv Syst 16: 15-20.
- Ribi K, Relly C, Landolt MA, Alber FD, Boltshauser E, et al. (2005) Outcome of medulloblastoma in children: Long-term complications and quality of life. Neuropediatrics 36: 357-365.
- von Hoff K, Kieffer V, Habrand JL, Kalifa C, Dellatolas G, et al. (2008) Impairment of intellectual functions after surgery and posterior fossa irradiation in children with ependymoma is related to age and neurologic complications. BMC Cancer 8: 15.
- Chapman CA, Waber DP, Bernstein JH, Pomeroy SL, LaVally B, et al. (1995) Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: Role of age and perioperative factors. J Child Neurol 10: 209-212.
- Johnson DL, McCabe M, Nicholson HS, Joseph L, Getson PR, et al. (1994) Quality of long-term survival in young children with medulloblastoma. J Neurosurg 80: 1004-1010.
- Szathmari A, Thiesse P, Galand-desmé S, Mottolese C, Bret P, et al. (2010) Correlation between pre- or postoperative MRI findings and cerebellar sequelae in patients with medulloblastomas. Pediatr Blood Cancer 55: 1310-1316.
- Roncadin C, Dennis M, Greenberg ML, Spiegler BJ (2008) Adverse medical events associated with childhood cerebellar astrocytomas and medulloblastomas: natural history and relation to very long-term neurobehavioral outcome. Childs Nerv Syst 24: 995-1002.
- Ronning C, Sundet K, Due-Tonnessen B, Lundar T, Helseth E (2005) Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg 41: 15-21.
- Stargatt R, Rosenfeld J V, Maixner W, Ashley D (2007) Multiple factors contribute to neuropsychological outcome in children with posterior fossa tumors. Dev Neuropsychol 32: 729-748.
- Hardy KK, Bonner MJ, Willard VW, Watral MA, Gururangan S (2008) Hydrocephalus as a possible additional contributor to cognitive outcome in survivors of pediatric medulloblastoma. Psychooncology 17: 1157-1161.
- Schreiber JE, Gurney JG, Palmer SL, Bass JK, Wang M, et al. (2014) Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol 16: 1129-1136.
- Kulkarni AV, Piscione J, Shams I, Bouffet E (2013) Long-term quality of life in children treated for posterior fossa brain tumors. J Neurosurg Pediatr 12: 235-240.
- Weizhen X, Janss A, Packer RJ, Phillips P, Goldwein J, et al. (2004) Endocrine outcome in children with medulloblastoma treated with 18 Gy of craniospinal radiation therapy. Neuro Oncol 6: 21-27.
- Levisohn L, Cronin-Golomb A, Schmahmann JD (2000) Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain 123: 1041-1050.
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al. (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131: 803-820.
- Glod J, Rahme GJ, Kaur H, Raabe E, Hwang EI, et al. (2016) Pediatric brain tumors: Current knowledge and therapeutic opportunities. J Pediatr Hematol Oncol 38: 249-260.
- Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, et al. (2012) Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: Results from the randomized multicenter HIT-SIOP PNET 4 Trial. J Clin Oncol 30: 3187-3193.
- Polkinghorn WR, Dunkel IJ, Souweidane MM, Khakoo Y, Lyden DC, et al. (2011) Disease control and ototoxicity using intensity-modulated radiation therapy tumor-bed boost for medulloblastoma. Int J Radiat Oncol Biol Phys 81: 15-20.
- Stokkevåg CH, Engeseth GM, Ytre-Hauge KS, Röhrich D, Odland OH, et al. (2014) Estimated risk of radiation-induced cancer following paediatric cranio-spinal irradiation with electron, photon and proton therapy. Acta Oncol (Madr) 53: 1048-1057.
- Dhall G, Grodman H, Ji L, Sands S, Gardner S, et al. (2008) Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer 50: 1169-1175.
- Chi SN, Gardner SL, Levy AS, Knopp EA, Miller DC, et al. (2004) Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol 22: 4881-4887.
- Han EY, Chun MH, Kim BR, Kim HJ (2015) Functional improvement after 4-week rehabilitation therapy and effects of attention deficit in brain tumor patients: Comparison with subacute stroke patients. Ann Rehabil Med 39: 560-569.
- Maschio M, Dinapoli L, Fabi A, Giannarelli D, Cantelmi T (2015) Cognitive rehabilitation training in patients with brain tumor-related epilepsy and cognitive deficits: a pilot study. J Neurooncol 125: 419-426.
- Salisbury DB, Dahdah M, Driver S, Parsons TD, Richter KM (2016) Virtual reality and brain computer interface in neurorehabilitation. Proc (Bayl Univ Med Cent) [Internet]. 2016;29(2):124–127.






