Keywords
Trans nasal evaporative cooling, Heart rate, Blood pressure
Introduction
Hypothermia decreases the cerebral metabolic rate for oxygen by about 6-7% per 1°C reduction in core body temperature [1]. Both experimental and clinical data indicate that mild hypothermia by several different mechanisms is neuroprotective and improves outcome after a period of global cerebral hypoxia-ischemia [2,3].
Therapeutic hypothermia may improve neurologic outcome in out-of-hospital cardiac arrest patients [4-6]. However the cooling method, optimal target temperature, duration of cooling and time of induction are still uncertain.
Experimental data suggest that hypothermia is beneficial in myocardial infarction ( i.e. reduced ischemic area and improved left systolic function) and in ischemic stroke (i.e. limited infarct size and functional outcome) [7-9]. However despite some promising results from smaller trials [10] the potential benefit of clinical use of therapeutic hypothermia in acute myocardial infarction has not yet been demonstrated [11]. In acute ischemic stroke, low temperature on admission has been associated with improved outcome [12]. Hypothermia in addition to thrombolysis has shown to be safe and feasible in small studies [13], but at present there is no evidence from larger randomised controlled trials that therapeutic hypothermia is beneficial in these patients [14,15]. In general, initiating hypothermia requires a high level of care and monitoring. The patient needs to be sedated or unconscious and therefore requires airway protection. There are several methods that have been shown effective in cooling unconscious patients [16,17]. It has also been demonstrated that the temperature can be lowered to <35 ºC in waking patients when sedative drugs are given [13]. However sedation of patients with ischemic stroke may be problematic as it complicates continuous neurological assessment.
trans nasal evaporative cooling is a safe and effective cooling method after cardiac arrest when patients are unconscious [18]. The method is appealing in ischemic stroke as it is designed primarily to cool the brain and subsequently the rest of the body. However, it has never been properly tested in waking individuals, such as patients with ischemic stroke and myocardial infarction.
The aim of this study is to study if trans nasal evaporative cooling is safe, tolerable and effective in inducing hypothermia in healthy awake volunteers without giving any sedative medication.
Methods
Study setting and population
The study was performed between February 1st and March 30th, 2012. The study has ethical approval from the ethical committee in Stockholm, Sweden (registration number 2012/1594-31/4). All volunteers received oral and written information before participation in the study. The volunteers completed a written medical history and underwent medical examination prior to study start including physical status and 12-lead ECG. The study population consisted of 9 medical students and one person from the armed forces. Written informed consent was received from all volunteers.
The study was performed at the Medical Intensive Care Unit (MICU) at Sodersjukhuset, which is one of the major hospitals in Stockholm, Sweden. All necessary equipment for advanced care and resuscitation was available at the MICU.
The method of trans nasal evaporative cooling
The method of trans nasal evaporative cooling with Rhinochill™ (Benechill, USA) consists of a control unit, a nasal catheter and cooling fluid (Figure 1). The control unit is electric and controls the flow of cooling liquid and oxygen. The liquid is perfluorohexane, a volatile, inert liquid. The cooling liquid is sprayed into the nasal cavity by either air or oxygen at a rate of 40 or 20 litres/ min via the nasal catheter. The 10 cm long nasal catheters are inserted fully into the nostrils. The catheters have spray ports on the dorsal side to distribute the coolant in the nasal cavity. The coolant is nebulised by close contact with oxygen at the spray ports. Evaporation of the coolant absorbs heat from the tissue and rapidly cools the nasal cavity to approximately 2°C. The air or oxygen is only used as a transporter for the liquid and has no medical purpose. The air/oxygen flow may be increased from 10 litres/min to 40 litres/min. The coolant comes in 1-litre bottles that last for 30 minutes. The method has been described previously [19].
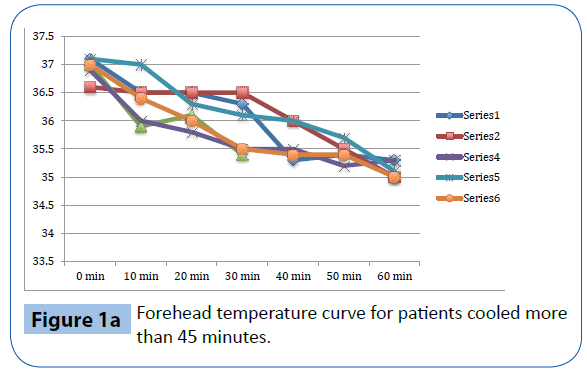
Figure 1a: Forehead temperature curve for patients cooled more than 45 minutes.
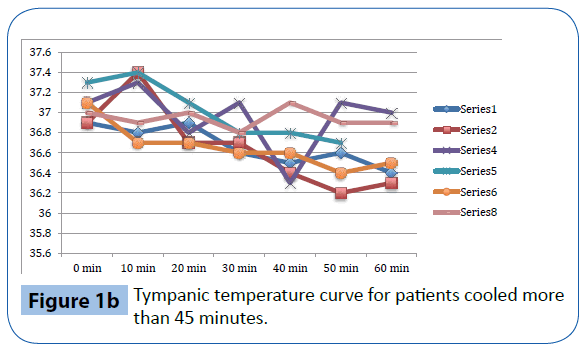
Figure 1b: Tympanic temperature curve for patients cooled more than 45 minutes.
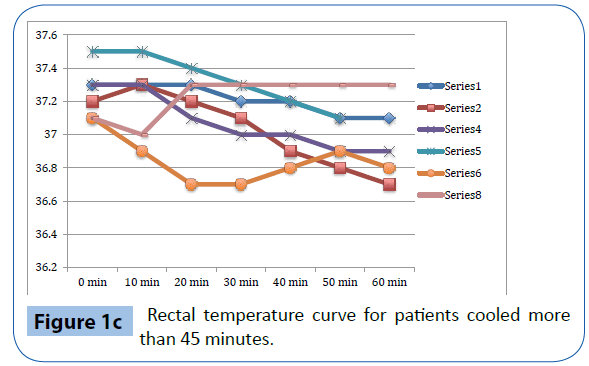
Figure 1c: Rectal temperature curve for patients cooled more than 45 minutes.
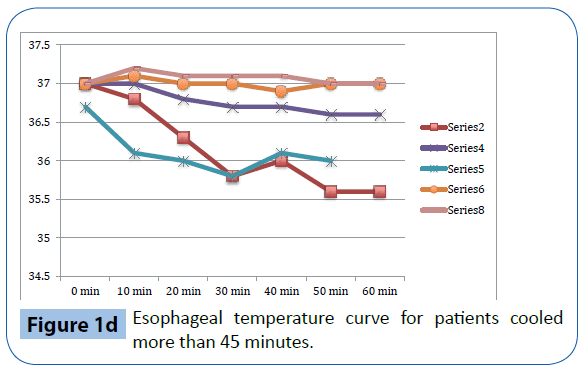
Figure 1d: Esophageal temperature curve for patients cooled more than 45 minutes.
Study protocol
This prospective, non-randomized, single centre study had the following endpoints:
Efficacy
• Cool the volunteers by -1.5°C from baseline temperature (tympanic or esophageal) or
• Cool the volunteers for 60 minutes, whichever was reached first.
Safety and tolerability
• Adverse events during cooling
• To tolerate cooling for 60 minutes without sedative drugs. The volunteers received monitoring in the following areas: non-invasive blood pressure (NIBP), oxygen saturation SaO2, electrocardiogram (ECG), pulse rate, respiratory rate and temperature. Temperature was measured using four different methods: tympanic, esophageal, rectal and forehead thermometers. The tympanic thermometer was a Braun™ (Melsungen, Germany) thermometer. The esophageal and rectal temperatures were continuously measured via probe attached to a Philips monitoring system. The forehead temperature was measured with a crystal line™-moving line™ sensor (Sharn anaesthesia inc Florida, USA) that has previously been used to detect temperature trends [20]. The measurements were documented every tenth minute and when any special deviations were observed. If the volunteer experienced anything adverse, additional measurements were performed. The trial was monitored by at least two nurse anaesthetists and one intensive care specialist.
Visual analogue scales (VAS) were used to evaluate pain and discomfort during cooling, and volunteers were instructed to point on a scale from 0-10 where 10 indicated the worst possible pain and 0 indicated no pain at all. On the discomfort scale, 10 indicated the worst possible discomfort and 0 indicated no discomfort at all. No pharmacological treatment was used in this trial except for local anaesthetics (Lidocain spray 10 mg/ml) that were administered into the nostrils and throat before applying the nasal catheters and the esophageal temperature probe. All volunteers received intravenous lines prior to start of cooling.
Cooling began with a low flow of oxygen at a rate of 10-20 litres/ min, which then was increased to 40 litres/min for a total period of 60 minutes of cooling.
After the cooling period, the volunteers were monitored until they were normothermic without any associated symptoms and could eat and drink without problems swallowing. After 1-2 weeks the volunteers were instructed to give summaries of their experiences.
Statistical analysis
Continuous variables are reported as median and ranges. Categorical variables are reported as counts and percentages.
Primary analyses for the efficacy end points (i.e. temperature change) were conducted with Pearson _2 tests for comparison of binomial proportions. Other analyses were performed with 2-group t tests or Wilcoxon signed rank sum tests for continuous variables and Pearson _2 tests for categorical variables. All probability values were 2-sided, with values less than 0.05 regarded as statistically significant. Statistical analyses were performed with IBM SPSS version 20.0.
Results
Among the ten healthy volunteers that were assessed in the previous medical examination, nine could participate in the study. The volunteer that was excluded had undergone nasal surgery due to a fracture, so the nasal catheter could not be placed at the time for the intervention. In the nine persons where cooling was initiated, five of them underwent the entire cooling period of 60 minutes.
Baseline characteristics
The volunteers had a median age of 27 years. Table 1 shows the baseline characteristics of the 9 participating volunteers before cooling was initiated.
| Baseline characteristics prior to cooling,(n=9) |
Median (range) |
| Age (years) |
27 (23-32) |
| Sex, female, n (%) |
5 (44) |
| Heart rate -1 |
74 (57-100) |
| Blood pressure, systolic (mmHg) |
126 (95-150) |
| Blood pressure, diastolic, mmHg |
68 (45-80) |
| Respiratory rate-1 |
15 (12-20) |
| Peripheral oxygen saturation (%) |
99,9 (99-100) |
| Tympanic temperature (°C) |
37.1 (36.7-37.5) |
| Esophageal temperature (°C) |
37.0 (36.7-37.2) |
| Rectal temperature (°C) |
37.2 (37,1-37.5) |
| Forehead temperature (°C) |
36.4 (35,9-37.0) |
Table 1: Baseline characteristics.
Parameters during cooling
During cooling there was a significant increase in mean heart rate compared to before cooling was started. It increased from 70 to 81 beats /minutes (p=0.009), Table 2. There was a significant increase in mean systolic (140 to 163 mmHg, p=0.001) and diastolic blood pressure (77 to 93, p=0.01) during cooling compared to before cooling started. A significant difference in respiratory rate (14 to 18 breaths/ minute, p=0.04) was also observed. The oxygen saturation was not significantly changed during cooling.
| |
Baseline |
Cooling |
p-value |
| Heart rate, minute-1 |
70 |
81 |
0,009 |
| MMR* |
57-100 |
65-100 |
|
| Peripheral oxygen saturation % |
100 |
100 |
Ns |
| MMR* |
99-100 |
97-100 |
|
| Respiratory rate, minute-1 |
14 |
18 |
0,04 |
| MMR* |
12-20 |
12-20 |
|
| Systolic blood pressure, mmHg |
140 |
163 |
0,001 |
| MMR* |
115-150 |
128-179 |
|
| Diastolic blood pressure, mmHg |
77 |
93 |
0,01 |
| MMR* |
60-80 |
75-107 |
|
*Minimum-maximum range
Table 2: Physiological parameters (median) during cooling among patients cooled >45 minutes, n=6.
Cooling efficacy
As presented in Table 3 and Figure 1a-d, there was a significant decrease in tympanic temperature (37.1 to 36.2, p=0.04) and forehead temperature (36.5 to 35.0, p=0.04) in the six volunteers that were cooled for more than 45 minutes. No significant changes were seen in rectal and esophageal temperatures.
| |
Baseline |
Cooling** |
p-value |
| Tympanic temperature (°C) |
37.1 |
36,2 |
0,04 |
| MMR* |
36.1-37.5 |
36.0-37.0 |
|
| Esophageal temperature (°C) |
37.0 |
36.6 |
ns |
| MMR* |
36.7-37.2 |
35.5-39.9 |
|
| Rectal temperature (°C) |
37.3 |
36.9 |
ns |
| MMR* |
37.1-37.5 |
36.6-37.3 |
|
| Forehead temperature |
36.5 |
35.0 |
0,043 |
| MMR* |
36.0-37.0 |
34.5-35.3 |
|
*Minimum-maximum range
Table 3: Median temperature change during cooling among patients cooled >45 minutes, n=6.
Physical experience, tolerability and safety
All of the nine participants experienced some discomfort and pain during cooling (Table 4). The pain experienced was somewhat less pronounced. Other registered side effects were headache, shivering, deep voice and hearing sensations.
| Physical experience/adverse events |
Median (range) |
| Pain (VAS) |
6(3-8) |
Discomfort (VAS)
Coolant not fully evaporated, n |
7(6-9)
9 |
| Headache, n |
4 |
| Deep voice/vocal changes, n |
2 |
ECG changes*, n
Periorbital emphysema |
2
1 |
| Shivering**, n |
1 |
Hearing affection, n
Dizziness, n |
1
1 |
* One pat asymptomatic bradycardia and one patient with ventricular extrasystoles in bigeminy for <10 seconds
**Intermittent, not-continuous shivering.
Table 4: Physical experience/adverse events among all patients that received cooling (n=9).
In one volunteer cooling was interrupted by the investigators after 24 minutes due to an instance of mild periorbital emphysema. This spontaneously resolved within 12 hours without any sequel. In three persons the cooling was terminated due to discomfort.
One person had a short period of dizziness and bradycardia to 40 beats/minutes after 60 minutes of cooling. The same person had a few sequences of ventricular extrasystoles in bigeminy for less than 10 seconds. These symptoms occurred after the cooling had been stopped.
Discussion
Hypothermia is an effective neuroprotectant, and hyperthermia in certain conditions is associated with worsening of neurological outcomes [21]. The emphasis of clinical studies has been on the potential reduction of the injuries associated with global cerebral ischemia/reperfusion response in comatose patients.
There might be other conditions-such as ischemic stroke and myocardial infarction-where mild hypothermia could perhaps be beneficial. A compelling approach would be to find a method that is easy to apply and that is accepted by waking patients without any sedation. Trans nasal evaporative cooling is a potential method to induce cooling among these patients. It cools effectively without giving the patients any volume load such as cold fluids that have proven to be unfavourable in cardiac arrest patients [22].
This study evaluates trans nasal evaporative cooling in waking patients without sedation. Our main finding was that despite discomfort the cooling was tolerated by the majority of those studied. Significant but modest temperature reductions were seen in tympanic and forehead measurements but not in rectal and esophageal temperatures.
One of the obstacles with cooling waking patients is the mechanism to resist temperature reduction, such as shivering. In other trials sedation with synthetic drugs, opioids and anxiolytics have been given to enhance cooling [23,24]. However in patients with ischemic stroke where continuous neurologic evaluation is important it is preferable to avoid sedative drugs that may hide neurological symptoms. In this study, only one person experienced intermittent shivering which might have been a limitation for lowering temperature.
The aim was to cool the healthy volunteers by -1.5°C (tympanic or esophageal) or to continue cooling for 60 minutes. None of the patients reached the temperature goal. This differs from the cooling efficacy seen in unconscious patients with trans nasal evaporative cooling where continuous cooling has shown to lower the tympanic temperature by 2.2 °C per hour and invasive brain temperature by 1.4 °C per hour [25]. Most likely, some type of sedative agent is needed to limit the associated defence mechanisms to resist hypothermia [23].
Besides temperature reduction this study investigated the tolerability and safety of trans nasal evaporative cooling in healthy awake volunteers. Although the majority of the participants could tolerate 60 minutes of cooling, they all experienced discomfort by the method. The most obvious problems were residual liquid in the nasopharynx. Some experienced voice changes. Although local anaesthetics were given, the participants experienced pain to various extents. The combination of pain and discomfort was the main reason for interruption of cooling. We also believe that this combination of pain and discomfort was the main reason for the significant rise in heart rate, respiratory rate, and systolic and diastolic blood pressure. These effects could probably have negative impact on patients with acute ischemic stroke. An increase in blood pressure has also been noted in previous studies in both waking and sedated patients [26,27].
One subject of the study had a local adverse event with discrete periorbital emphysema, which resolved spontaneously within 24 hours. No further investigation regarding this finding was done. This is known as a side effect from a previous clinical study in cardiac arrest patients [18]. One person had a short period of sinus bradycardia and ventricular extrasystoles. We do not know the mechanisms behind these findings. One common side effect to trans nasal hypothermia is epistaxis, but in this study there were no cases of that.
Limitations
In this study we used healthy volunteers that may not be comparable in terms of age and co-morbidity to the target patient population with acute ischemic events.
A small number of subjects were studied.
A probable limitation to achieve temperature reduction was that subjects were not given sedatives or analgesics.
Conclusions
Trans nasal evaporative cooling in waking, healthy volunteers without sedatives is safe and may be tolerated. Cooling was associated with a modest but significant lowering of tympanic and forehead temperatures but not in rectal and esophageal temperatures. The volunteers experienced pain and discomfort and a significant increase in heart rate, blood pressure and respiratory rate was observed.
Acknowledgements
We thank the volunteers for their participation and all the staff at the Medical ICU, Sodersjukhuset, Stockholm, Sweden.
7720
References
- McCullough JN, Zhang N, Reich DL, JuvonenTS, Klein JJ, et al. (1999) Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann ThoracSurg 67: 1895-1899.
- Gunn AJ,Thoresen M (2006) Hypothermic neuroprotection. NeuroRx 3: 154-169.
- Froehler MT,GeocadinRG (2007) Hypothermia for neuroprotection after cardiac arrest: mechanisms, clinical trials and patient care. J Neurol Sci 261: 118-126.
- Hypothermia after Cardiac Arrest Study Group (2002) Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 346: 549-556.
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, et al. (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346: 557-563.
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, et al. (2013) Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. The New England journal of medicine369:2197-2206.
- Nolan JP, Soar J, Zideman DA, Biarent D, Bossaert LL, et al. (2010) European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation 81: 1219-1276.
- Yannopoulos D, Zviman M, Castro V, Kolandaivelu A, Ranjan R, et al. (2009) Intra-cardiopulmonary resuscitation hypothermia with and without volume loading in an ischemic model of cardiac arrest. Circulation120:1426-1435.
- van der WorpHB,SenaES, Donnan GA, Howells DW, Macleod MR (2007) Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 130: 3063-3074.
- Gotberg M, OlivecronaGK, Koul S, Carlsson M, Engblom H, et al. (2010) A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circulation Cardiovascular interventions 3:400-407.
- Erlinge D, Gotberg M, Lang I, Holzer M, Noc M, et al. (2014) Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: a randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. Journal of the American College of Cardiology 63:1857-1865.
- Kammersgaard LP,Jørgensen HS, Rungby JA, Reith J, Nakayama H, et al. (2002) Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke 33: 1759-1762.
- Hemmen TM, Raman R, GulumaKZ, Meyer BC, Gomes JA, et al. (2010) Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke 41: 2265-2270.
- Wan YH, Nie C, Wang HL, Huang CY (2014) Therapeutic Hypothermia (Different Depths, Durations, and Rewarming Speeds) for Acute Ischemic Stroke: A Meta-analysis. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association.
- LydenPD, Hemmen TM, Grotta J, Rapp K, Raman R (2014) Endovascular therapeutic hypothermia for acute ischemic stroke: ICTuS 2/3 protocol. IntJ stroke 9: 117-125.
- NeumarRW, Nolan JP, Adrie C, Aibiki M, Berg RA, et al. (2008)Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation79: 350-379.
- PoldermanKH,Herold I (2009) Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 37: 1101-1120.
- Castren M, Nordberg P, Svensson L, Taccone F, Vincent JL, et al. (2010) Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSCIntraNasal Cooling Effectiveness). Circulation 122:729-736.
- Nordberg P, TacconeFS, Castren M, Truhlar A, Desruelles D, et al. (2013) Design of the PRINCESS trial: pre-hospital resuscitation intra-nasal cooling effectiveness survival study (PRINCESS). BMC emergency medicine 13:21.
- Allen GC,HorrowJC, Rosenberg H (1990) Does forehead liquid crystal temperature accurately reflect "core" temperature? Can J Anaesth 37: 659-662.
- Maher J,Hachinski V (1993) Hypothermia as a potential treatment for cerebral ischemia. Cerebrovasc Brain Metab Rev 5: 277-300.
- Kim F, Nichol G, Maynard C, Hallstrom A, KudenchukPJ, et al. (2013) Effect of Prehospital Induction of Mild Hypothermia on Survival and Neurological Status Among Adults With Cardiac Arrest: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association.
- LydenPD, AllgrenRL, Ng K, Akins P, Meyer B, Al-Sanani F, et al. (2005) Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. J strokecerebrovasc14:107-114
- Testori C,Sterz F, Behringer W, Spiel A, Firbas C, et al. (2011) Surface cooling for induction of mild hypothermia in conscious healthy volunteers - a feasibility trial. Crit Care 15: R248.
- Abou-CheblA, Sung G, Barbut D and Torbey M (2011) Local brain temperature reduction through intranasal cooling with the RhinoChill device: preliminary safety data in brain-injured patients. Stroke 42:2164-2169.
- Koehn J,Kollmar R, Cimpianu CL, Kallmünzer B, Moeller S, et al. (2012) Head and neck cooling decreases tympanic and skin temperature, but significantly increases blood pressure. Stroke 43: 2142-2148.
- Poli S,Purrucker J, Priglinger M, Sykora M, Diedler J, et al. (2014) Safety evaluation of nasopharyngeal cooling (RhinoChill®) in stroke patients: an observational study. Neurocrit Care 20: 98-105.










