Keywords
Autonomous gait entrainment; Movement disorders; Sensory feedback; Dopamine reward; Reward seeking behavior
Introduction
A seminal study [1] and subsequent clinical tests [2-4] have shown significant improvement in gait parameters of patients with Parkinson’s disease (PD) walking over transverse lines drawn on the ground. Early attempts to generate such visual cues by virtual means have resulted in open-loop systems, imposing, by a constantly moving visual cue [5-8], a constant walking speed on the patient. A comparison of open-loop visual cuing by virtual means to transverse line markings on the ground [9] has found the first to have a marginal effect and the second to have a significant positive effect on gait parameters in PD patients. Subject-stationary visual cues [10] represent another unnatural imposition, where the image moves in the same direction as the subject, which stands in contrast to the opposite motion of an earth-stationary image. External open-loop auditory entrainment of gait, where a rhythmic sound is imposed on the patient in a metronome-like fashion, have been studied [11-15], and reasoned on neurobiological grounds [16]. Yet, a need for constant vigilance and attention strategies to prevent reversion to impaired gait patterns caused by repetitive stimuli has been noted [3]. It has been further suggested that external rhythmic entrainment instigates dopamine reward [17,18], seemingly supporting open-loop intervention. However, open-loop control is known to be inherently unstable, with potentially disastrous consequences due to error accumulation [19]. In sharp contrast, closed-loop feedback systems, when correctly designed, can regulate and stabilize otherwise unstable dynamics [19]. Analysis has shown that walking over earthstationary visual markings constitutes a closed-loop feedback control system which stabilizes and regulates gait [20]. A wearable virtual realization of autonomous gait entrainment by sensory feedback, facilitated by the gradual miniaturization of sensing, computation and display technologies, has led to the development of a closed-loop sensory feedback device for gait improvement in the neurologically impaired [21]. While the effect created by the device is similar to that created by walking on a real floor with visual markings, the virtual earthstationary visual markings are location-independent, as the patients is, in principle, free to go anywhere, making such entrainment completely autonomous.
Here, we employ analytic and experimental findings in demonstrating and explaining the fundamental differences between external and autonomous entrainment. From a control-theoretic viewpoint, it is the difference between openloop and closed-loop control systems, which entails the difference between dynamic stability and instability. From the viewpoint of human cognition, it is the difference between dependence and independence, which is the difference between apathy and regression, on the one hand, and novelty and vigilance, on the other. Reviewing clinical studies of external and autonomous gait entrainment in neurological patients, we employ the control theoretic and the human cognition contexts to explain the differences between the resulting gait parameters. Significant gait improvement by autonomous visual and auditory entrainment in patients with PD, typically suffering from basal ganglia dopamine depletion, asserts previous findings that learning reward seeking behavior can replace actual reward. This effect is also expressed by residual gait improvement, lasting beyond actual entrainment. A change from one-dimensional (transverse lines) to two-dimensional (checkerboard tiles) geometry in autonomous visual entrainment, shown to produce higher novelty and vigilance, is found to result in a particularly high gait improvement in patients with MS. We close with a review of clinical studies on autonomous gait entrainment in patients with a variety of neurological disorders, specifically, PD, MS, CP, SG and PS. Albeit case-specific deviations, these studies show, on average, pronounced improvement in gait, demonstrating the transformative nature of autonomous gait entrainment.
Methods and Findings
Autonomous vs. external gait entrainment
An examination of the natural sensory-motor control system underlying human locomotion with respect to a visual scenery reveals that it is the physical motion of the body which generates the visual cue and not the other way around [20]. This observation is crucial to understanding the difference between external entrainment and autonomous entrainment of gait. The first represents a control system operating in openloop, the second a closed-loop feedback control system. The two control paradigms are illustrated in Figure 1. In external entrainment, a constant visual and/or an auditory cue is generated artificially and fed through the eyes and/or the ears to the brain, which activates the limbs so as to respond to the sensory cue. On the other hand, an artificial realization of the natural sensory-motor control system is a closed-loop feedback system, where the generation of the sensory feedback cue is controlled and regulated by the body movement caused by locomotion. The motion of the visual cue and the rhythm of the auditory cue are matched to the motion of the body, which is, normally, matched to the motion of the legs. When there is no motion of the body, there is no sensory cue.
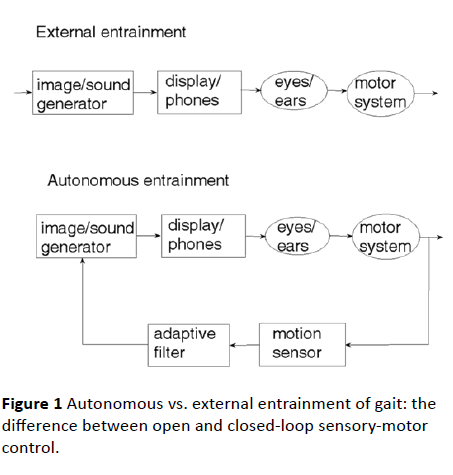
Figure 1 Autonomous vs. external entrainment of gait: the difference between open and closed-loop sensory-motor control.
Depicted in Figure 2, a wearable sensory feedback device, employing inertial sensors, an adaptive filter and a microprocessor contained in a belt-mounted cellphone-size box, is connected to a see-through micro-display, generating an earth-stationary visual feedback cue in response to the body motion. In addition to the visual feedback cue delivered by the display, the device also produces an auditory feedback cue in the form of a clicking sound delivered through earphones in response to every step taken by the patient. In contrast to open-loop, metronome-like devices, which attempt to impose a walking pace on the patient by a constant auditory cue, the feedback device produces an auditory cue matched to the walking pattern. A balanced steady walk will generate a rhythmic auditory cue. Any deviation from such a gait pattern will result in a deviation from the auditory rhythm and will be corrected by a change of gait in a feedback fashion. The headmounted display and earphones bring the sensory feedback signals closer to the sensors – the eyes and the ears, making the sensory effect more pronounced, easier to follow and to learn. An open-loop capability, producing constant movement of the visual cue and a constant rhythmic auditory cue, was also added to an early version of the device for experimental comparison purposes.
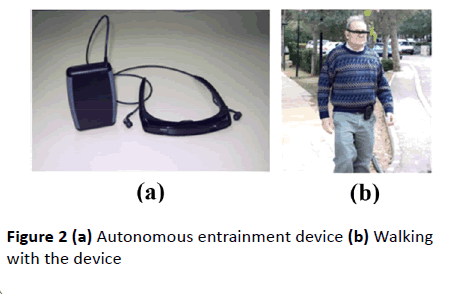
Figure 2 (a) Autonomous entrainment device (b) Walking with the device
The safety of autonomous gait entrainment is ratified by control theoretic considerations: closed-loop feedback systems, correctly designed, are, due to error correction, inherently stable, while open-loop, externally driven, systems are, due to error accumulation, inherently unstable, hence, unsafe [19]. As can be seen from Figure 1, while error correction (hence, stabilization) is made possible by the sensory feedback path of autonomous entrainment, it cannot be implemented by the open loop (no feedback) system of external entrainment.
Dopamine reward and reward seeking: The edge of autonomy
It has been hypothesized that dopamine neurotransmission, highly evidenced in limbic areas of the basal ganglia [22], elicits exhilaration or excitement in response to novel stimuli [23]. The dopamine receptor gene D4DR has been linked to novelty seeking behavior [24,25]. In the context of cognition, novelty has been associated with "the ability to think and act independently" [26]. While it has been suggested that novelty seeking, representing intellectual curiosity, aesthetic sensitivity and risk taking, is low in dopamine-deficient patients with PD [27], highly pronounced novelty seeking behavior has been found in patients with PD, regardless of medication status [28]. Dopamine reward has also been associated with vigilance [29,30], defined as "alert watchfulness" [26]. A highly insightful study [31] suggests that the frequency level of beta-range neuronal activity in the basal ganglia is modulated by the level of dopamine discharge at sites of cortical input, which are, in turn, modulated by salient internal and external cues. Moreover, it suggests that the level of the beta-range frequency provides a measure of the likelihood that a new voluntary action will need to be actuated. Put in our context, this insight suggests that autonomous entrainment of gait is more highly rewarded by dopamine discharge than rhythmic external cues. A case study has found coordinated beta-range activity in the occipital, parietal, and motor lobes of a patient with PD responding to visual autonomous entrainment [32]. Yet, dopamine depletion in basal ganglia neurons of patients with PD suggests that it may be reward seeking, rather than actual dopamine reward, which comes into play in gait entrainment of patients with PD.
While movement has been widely associated with reward, specifically, dopamine discharge by basal ganglia neurons, movement improvement in PD patients suggests the existence of a bypass mechanism. Prior reward learning, possibly facilitated by earlier normal preconditioning or medication, provides response incentive without actual dopamine signaling [33]. In other words, learning to select responses that lead to reward results in reward seeking behavior [34,35]. As novelty and vigilance have been widely recognized as key proponents of dopamine reward, the level of dopamine reward or reward seeking should correspond to the levels of novelty and vigilance. While neither novelty, nor vigilance, have generally accepted measurable manifestations, both seem to be intuitively well understood notions. In a cognitive perspective, novelty has been characterized as the ability to think and act independently [26]. In this respect, novelty does not seem to apply to external entrainment, which enforces a rhythmic auditory cue, or a constantly moving visual cue, on the patient. Indeed, it has been noted that the failure of external entrainment to produce satisfactory gait control in most patients is caused by the perpetual motion of the image, which, being unaffected by the patient, is eventually neglected [3]. In sharp contrast, autonomous entrainment, allowing the patient to decide if and when to take a step and at what stride length and speed of walk, is an embodiment of independent, free-willed, motion. Yet, vigilance appears to play a role in both external and autonomous entrainment, as both motivate a certain correspondence between step preparation and execution, on the one hand, and the presentation of a sound or an image, on the other. The unequal balance between novelty and vigilance in the two cases suggests that while both external and autonomous entrainments present cases for dopamine reward, or reward seeking, autonomous entrainment, instigating both novelty and vigilance, may present a much stronger case than external entrainment, which instigates vigilance only.
The effects of geometric dimension
Figure 3 shows the earth-stationary transverse lines geometry (a) and the checkerboard tiles geometry (b), with shoe markings indicating that, following a few corrective steps, the patient, by controlling stride-length, has reached a steadystate in which each of the steps ends, essentially, with a foot placed either between two consecutive transverse lines (in case (a)) or on a tile of a given color (white in case (b)).
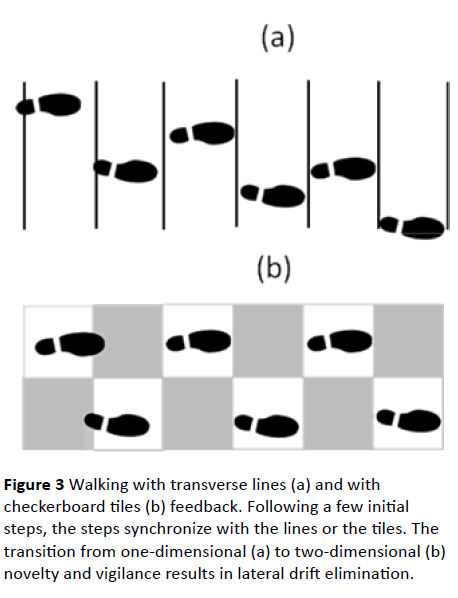
Figure 3 Walking with transverse lines (a) and with checkerboard tiles (b) feedback. Following a few initial steps, the steps synchronize with the lines or the tiles. The transition from one-dimensional (a) to two-dimensional (b) novelty and vigilance results in lateral drift elimination.
As the patient, employing either geometry for autonomous visual feedback, has the freedom to move on the floor in any direction, novelty will be visually expressed by edge-crossing, or its avoidance. In the case of the transverse lines, edgecrossing can occur in the longitudinal direction only – perpendicularly to the direction of the transverse lines. On the other hand, in the case of the checkerboard tiles, edge- crossing can occur in two orthogonal directions: longitudinal and lateral. Novelty in the first case is, then, one-dimensional, while in the second case it is two-dimensional. The vigilance required in the case of transverse lines is similarly onedimensional, while the vigilance required in the case of checkerboard tiles is two-dimensional. A dopamine reward, if available, can be expected to be higher, then, in the case of checkerboard tiles than in the case of transverse lines. Simply compare the perceived pleasure of drawing in one dimension to the one of drawing in two dimensions.
Clinical Results
Autonomous vs. external entrainment
It has been suggested [31] that the level of beta frequency activity in basal ganglia neurons provides a measure of the likelihood that a new voluntary action will need to be actuated. The frequency level of this activity is modulated by the level of dopamine discharge at sites of cortical input, which are, in turn, modulated by salient internal and external cues. These insights suggest that autonomous entrainment of gait is more highly rewarded by dopamine discharge than rhythmic external cues. It is highly interesting, then, to compare clinical findings on the effects of external rhythmic entrainment and autonomous entrainment of gait in patients with PD, suffering from dopamine depletion in basal ganglia neurons.
A study of visual entrainment of gait in patients with PD [36] has compared the effects of external (open-loop) constant entrainment and autonomous (closed-loop) feedback entrainment. Patients were off their regular medication (hence, without dopamine enhancement drugs) for twelve hours. The study has found that patients who used external entrainment improved their gait, on average by 13.8% in walking speed and by 15.0% in stride length (for comparison purposes, we present only first-order statistics). Two of the patients went into freezing midway when using external entrainment. Patients who used autonomous entrainment improved their gait on average by 25.7% in walking speed and by 30.8% in stride length. In addition to doubling the level of improvement in gait parameters with respect to external entrainment, none of the patients employing autonomous entrainment experienced freezing. A subsequent study of autonomous visual gait entrainment in patients with PD on their regular medication [27] has found on-line improvement of 21.85% in walking speed and 13.50% in stride length. The same study has found short-term (15 min) residual improvement of 12.68% in walking speed and 13.05% in stride length. Residual effects of visual feedback entrainment by markings on the ground have been reported as well [3].
Application of external, open-loop, rhythmic (metronomelike) auditory entrainment [15] was found to increase or decrease walking speed in patients with PD on their regular medication schedule according to changes in the rhythm frequency above or below their preferred pace, respectively. At the same time, stride length has not been found to be significantly affected by such changes, or by external auditory cuing altogether. At the preferred pace, open-loop rhythmic auditory cuing has been reported to result in 4.2% average improvement in the PG score, combining walking speed and stride length among a variety of posture and gait measures [15]. A study of the effects of autonomous auditory entrainment on gait in patients with PD on their regular medication schedule [37] has found, on average 12.37% improvement in walking speed and 4.30% improvement in stride length with respect to baseline performance. This study has also found a pronounced short-term residual effect of auditory autonomous entrainment on both walking speed (9.1%) and stride length (6.5%). In contrast, external open-loop rhythmic auditory entrainment has been found to have no functional carry-over effects [15].
It can be seen that, regardless of medication, autonomous entrainment, visual or auditory, has produced, on-line and residually, significantly higher improvement in gait parameters of patients with PD than external entrainment, as suggested by reward seeking considerations. It has been suggested that external open-loop entrainment instigates dopamine reward [17,18], seemingly supporting open-loop intervention. However, as shown by the above-noted clinical results, such entrainment, having some positive effects on some of the patients, also results in adverse effects, such as freezing of gait in patients with PD. These mixed results may be characterized as “the double-edged sword” of external entrainment.
Effects of visual geometry dimension
Following the positive effects found in patients with MS subject to autonomous entrainment [38,39], a clinical study has compared the effects of walking over transverse lines to the effects of walking on checkerboard tiles in such patients [40]. Two groups, each consisting of ten randomly selected patients on their regular medication were tested, one employing transverse lines, the other checkerboard tiles. The study has found that, while the average improvement with respect to baseline performance in the group employing transverse lines was 7.79% in walking speed and 7.20% in stride length, the average improvement in the group employing checkerboard tiles was 21.09% in walking speed and 12.99% in stride length.
It can be seen that the level of improvement in gait parameters due to higher-dimensional visual feedback geometry, found in patients with MS, increased significantly when the transverse lines geometry was replaced by the checkerboard tiles geometry, which can be attributed to the higher dimensionality of the novelty and the vigilance associated with the checkerboard tiles geometry. These findings were sufficiently conclusive for us to drop any further employment of the transverse lines geometry and adopt the checkerboard tiles geometry in all subsequent trials.
Two independent studies on autonomous entrainment of gait in patients with PD, off medication for twelve hours, were performed under similar condition, differing, however, in two fundamental ways: while the first employed transverse lines drawn on the ground, the second employed earth-stationary checkerboard tiles produced by the autonomous entrainment device [21]. The first study [4], participated by 16 patients, has shown, on average, 7.895% ± 5.26% improvement in walking speed and 4.30 ± 0% improvement in stride length with respect to baseline performance without line markings. The second study [36], participated by 16 patients (with one stopping midway and excluded from the calculations) has found 19.76% ± 14.96% improvement in walking speed and 21.79% ± 17.40% improvement in stride length compared to baseline performance. It might be noted that while the margin in the improvement results in walking speed obtained for patients with PD corresponding to the two geometries are similar to those obtained for patients with MS, the improvement results in stride length obtained for patients with PD are markedly higher than those obtained for patients with MS.
It can be seen that, regardless of medication, the level of improvement in gait parameters due to higher-dimensional visual feedback geometry, found in patients with PD and in patients with MS, were essentially similar. The considerable advantage for PD patients in stride length improvement further emphasizes that it is reward seeking, rather than actual dopamine reward, which comes into play in such patients, suffering from impaired dopamine discharge in basal ganglia.
Transformative nature of autonomous entrainment across neurological disorders
Further studies on autonomous entrainment in patients with PD have found on-line and residual therapeutic effects following training with combined visual and auditory feedback [41,42], Effects of "on" predominant freezing of gait [43], online [44,45] and short-term residual [45] improvement by separate visual feedback, freezing predictability by gait initiation with visual feedback [46], and gait improvement by combined visual and auditory feedback in patients subject to deep brain stimulation [47]. Studies of the effects of visual [38] and auditory [39] autonomous entrainment on patients with multiple sclerosis (MS) have found significant on-line and residual improvement in gait parameters.
Our exploratory studies in PD and MS, suggesting the transformative nature of autonomous entrainment, have lead us to studies of autonomous entrainment in other neurological disorders, which do not appear to have been approached by gait entrainment before, apparently because they present a considerably larger variety of behaviors than patients with PD, and even patients with MS. Due to behavioral complexities, these disorders could not be addressed by external rhythmic entrainment, but seemed to present a case for autonomous entrainment which allows for individual self-generated entrainment. The results of our clinical findings for autonomous entrainment in these disorders are now briefly presented.
Cerebral palsy (CP) has predominantly pre-natal causes and is symptomatically addressed at a young age. A study of patients with gait disorders due to CP [48] has found that, for patients training with visual feedback, the short-term residual improvement was 21.70% in the walking speed and 8.72%in the stride length. For CP patients training with auditory feedback, the short-term residual improvement was 25.43% in the walking speed and 13.58% in the stride length. Agematched controls who trained with either visual or auditory feedback showed no improvement in gait.
A study of randomly selected old-age home residents suffering from senile gait (SG) without PD [49] showed that, in patients with baseline performance above the median, the average on-Line improvement when using visual feedback was considerably higher (6.31% in walking speed and 6.41% in stride length) than in patients with baseline performance below the median (−0.72% in walking speed and 3.39% ± 11.26% in stride length). Improvement in gait parameters was further shown to increase with the number of years of schooling. Patients with SG also suffering from PS [49], using on-line visual feedback, improved their walking speed or stride length or both by more than 10%. In patients with baseline performance above the median, improvement was considerably higher (13.2% in walking speed and 16.6% in stride length) than in patients with baseline performance below the median (−9.9% in walking speed and −7.7% in stride length).
Discussion
The findings reviewed and compared in this study unambiguously show that autonomous entrainment of gait by visual and/or auditory feedback is not only decisively safer, but also significantly more effective than external entrainment. More specifically, while external gait entrainment is a “doubleedged sword” producing adverse effects such as freezing in patients with PD, autonomous entrainment comes without adverse effects. While the motivation underlying the effectiveness of autonomous entrainment is dopamine reward, it is materialized in patients with PD, lacking in dopamine reward, by preconditioned, or learned, reward seeking. Underlying reward and reward seeking are the cognitive attributes of novelty and vigilance. Novelty, cognitively characterized as the ability to think and act independently, can only materialize under autonomous entrainment and not under externally enforced entrainment. Geometrically defined with respect to edge crossing under visual feedback, both novelty and vigilance are more highly expressed in movement with respect to two dimensional visual objects, notably, checkerboard tiles, than in movement with respect to onedimensional visual objects, notably, one dimensional transverse lines.
While external entrainment represents an open-loop control system which is, due to error accumulation, inherently unstable, hence, unsafe for patient use, autonomous entrainment constitutes a closed-loop feedback control system which is, by error correction, inherently stable, hence, safe for patient use. Autonomous entrainment of gait by visual or auditory feedback produces significantly higher improvement in gait parameters than external entrainment. The level of improvement in gait parameters due to autonomous entrainment found in PD patients without dopamine supplement was higher than that achieved with dopamine supplement, indicating that the effect of learned reward seeking can be at least as high as that of actual dopamine reward. While external auditory entrainment of gait in patients with PD produced no residual effects, autonomous auditory entrainment in such patients has resulted in residual improvement, indicating that autonomous entrainment is more effective in learning reward seeking behavior than external entrainment. The checkerboard tiles geometry, being two-dimensional, produces in autonomous entrainment significantly higher levels of novelty and vigilance, resulting in significantly higher improvement in gait parameters than the one-dimensional transverse lines geometry. The transformative nature of autonomous gait entrainment across neurological disorders, predicted by stability, learnability, reward and reward seeking considerations, was ratified by gait improvement results obtained in independent clinical studies of patients with different disorders, specifically, PD, MS, CP, SG and PS.
Conclusion
The highly transformative nature of autonomous entrainment implies that patients with different disorders can benefit from it. While PD patients are largely deprived of basal ganglia dopamine production, there does not seem to be any known reason to presume such deprivation in other disorders. Yet, the level of gait improvement can vary significantly, not only with respect to the nature of the disorder, but also with respect to disorder severity and patient’s own biological and biographical attributes, such as age, education, mental state and cognitive abilities. While the association with certain attributes, such as education, has been noted, attempting to predict patient’s response to autonomous entrainment according to the various attributes would present a painstaking task. Yet, certain categories not addressed by the studies reviewed herewith, such as, e.g., PD accompanied by dementia, seem to be particularly interesting. This, and other relevant categorization, such as cognitive abilities, are suggested for future research. Finally, it might be noted that hundreds of publications related to gait entrainment, while making reference to autonomous (or closed-loop) entrainment, have, in fact, addressed external entrainment without making a distinction between external and autonomous entrainment. We find such presentations to be highly, although not necessarily intentionally, misleading. In order to make the distinction between external and autonomous entrainment unambiguously clear, we have only noted works that have made a clear case for one or the other.
Acknowledgement
This study was supported by the Technion’s Roy Matas/ Winnipeg Chair in Biomedical Engineering. The author wishes to thank the anonymous reviewers, whose comments and suggestions helped improve the presentation.
Conflicts of Interest
The author is the developer of the autonomous entrainment device used in some of the reviewed clinical tests.
18562
References
- Martin JP (1967) Locomotion and the basal ganglia. In Martin JP, (ed). The basal ganglia and posture. London: Pitman medical: 20-35.
- Bagley S, Kelly B, Tunniclife N, Turnbull GI, Walker JM (1991) The effect of visual cues on the gait of independently mobile Parkinson’s disease patients. Physiotherapy 77: 415–420.
- Morris ME, Iansek R, Matyas TA, Summers JJ (1996) Sride length regulation in Parkinson's disease: normalizations strategies and underlying mechanisms. Brain 119: 551-568.
- Azulay JP, Mesure S, Amblard B, Blin O, Sangla I, et al. (1999) Visual control of locomotion in Parkinson’s disease. Brain 122: 111-120.
- Prothero J (1993) The treatment of akinesia using virtual images. M.S. thesis, Human Interface Technol Lab, Univ Washington, Seattle.
- Riess T, Weghorst S (1995) Augmented reality in the treatment of parkinson’s disease. In Proc Medicine Meets Virtual Reality III. Morgan K, Satava RM, Sieburg HB, R. Mattheus R, Christensen JP, (Eds.) Amsterdam, The Netherlands, IOS Press 298–302.
- Weghorst S, Prothero J, Furness T (1994) Virtual images in the treatment of Parkinson’s disease akinesia. In Proc Medicine Meets Virtual Reality II, San Diego, CA: 242–243.
- Kaminsky TA, Dudgeon BJ, Billingsley FF, Mitchell PH, Weghorst SJ (2007) Virtual cues and functional mobility of people with Parkinson's disease: A single-subject pilot study. J Rehabil Res & Devel (JRRD) 44: 437-448.
- Griffin HJ, Greenlaw R, Limousin P, Bhatia K, Quinn NP, et al. (2011) The effect of real and virtual visual cues on walking in Parkinson’s disease. J Neurol 258: 991-1000.
- Lewis GN, Byblow WD, Walt SE (2000) Stride length regulation in Parkinson's disease: the use of extrinsic, visual cues. Brain 123: 2077-2090.
- Kritikos A, Leahy C, Bradshaw JL, Iansek R, Phillips JG, et al. (1995) Contingent and non-contingent auditory cueing in Parkinson’s disease. Neuropsych 33: 1193–1203.
- Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, et al. (1996) Rhythmic auditory stimulation in gait training for Parkinson's disease patients. Move Disord 11: 193–200.
- McIntosh GC, Brown SH, Rice RR, Thaut MH (1997) Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson's disease. J Neurol Neurosurg Psych 62: 22-26.
- Howe TE, Lovgreen B, Cody FWJ, Ashton VJ, Oldham JA (2003) Auditory cues can modify the gait of persons with early-stage Parkinson’s disease: a method for enhancing parkinsonian walking performance? Clin Rehab 17: 363-367.
- Nieuwboer A, Kwakkel G, Rochester L, Jones D, Van Wegen E, et al. (2007) Cueing training in the home improves gait-related mobility in Parkinson’s disease. The RESCUE trial. J Neurol Neurosurg & Psych 78: 134–140.
- Thaut MH, McIntosh GC, Hoemberg V (2014) Neurobiological foundations of neurologic music therapy: rhythmic entrainment and the motor system. Front Psychol 5: 1185.
- Miendlarzewska WJ, Trost EA (2014) How musical training affects cognitive development: rhythm, reward and other modulating variables. Front Neurosci: 20.
- Salimpoor VN, Zald DH, Zatorre RJ, Dagher A, Mc Intosh AR (2015) Predictions and the brain: how musical sounds become rewarding. Trends Cogn Sci 19: 86-91.
- Kuo BC (1962) Automatic Control Systems. Prentice-Hall, Englewood Cliffs, N.J.
- Baram Y (2004) Closed-loop augmented reality apparatus. US Patent No. 6,734,834-B1.
- Di Chiara G, Morelli M, Acquas E, Carboni E (1992) Functions of dopamine in the extrapyramidal and limbic systems. Clues for the mechanism of drug actions. Arzneimittelforschung 42: 231-237.
- Cloninger CR (1987) A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry 44: 573-588.
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, et al. (1996) Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of Novelty Seeking. Nature Genet 12: 78-80.
- Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, et al. (1996) Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nature Genet 12: 81-84.
- Menza MA, Golbe LI, Cody RA, Forman NE (1993) Dopamine-related personality traits in Parkinsons disease. Neurology 43: 505−508.
- Djamshidian A, O'Sullivan SS, Wittmann BC, Lees AJ, Averbeck BB (2011) Novelty seeking behaviour in Parkinson's disease. Neuropsychologia 49: 2483-2488.
- Aston JG, Rajkowski J, Kubiak P, Alexinsky T (1994) Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci 14: 4467–4480.
- Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56: 27– 78.
- Jenkinson N, Brown P (2011) New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci 34: 611–618.
- Velu PD, Mullen T, Noh E, Valdivia M, Poizner H, et al. (2009) Effect of visual feedback on the occipito-parietal-motor network in Parkinsons disease patients with freezing of gait. Frontiers in Neurology 4: 209.
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT (2011) Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem 18: 475-483.
- Graef S, Biele G, Krugel LK, Marzinzik F, Wahl M, et al. (2010) Differential Influence of Levodopa on Reward-Based Learning in Parkinson's Disease Front Hum Neurosci 4: 169.
- Moustafa A (2010) Levodopa enhances reward learning but impairs reversal learning in Parkinson's disease patients. Front Hum Neurosci 4: 240.
- Baram Y, Aharon J, Simionotici Y, Ron L (2002) Walking on virtual tiles. Neur Proc Lett 16: 227-233.
- Baram Y, Aharon J, Badarny S, Susel Z, Schlesinger I (2016) Closed-loop auditory feedback for the improvement of gait in patients with Parkinson's disease. J Neurol Sci 363: 104–106.
- Baram Y, Miller A (2006) Effects of Virtual Reality Cues on Gait in Multiple Sclerosis Patients. Neurology 66: 178-181.
- Baram Y, Miller A (2007) Auditory feedback for improvement of gait in multiple sclerosis patients. J Neurol Sci 254: 90-94.
- Baram Y, Miller A (2010) Glide-Symmetric locomotion reinforcement in patients with multiple sclerosis by visual feedback. Disab & Rehab: Assis Techn 5: 323-326.
- Espay J, Baram Y, Dwivedi AK, Shukla R, Gartner M, et al. (2010) At-home training with closed-loop augmented-reality cueing device for improvement of gait in patients with Parkinson's disease. J Rehab Res & Devel (JRRD) 47: 573-582.
- Espay AJ (2010) Management of motor complications in Parkinson disease: Current and emerging therapies. Neurol Clin 28: 913–925.
- Chong R, Lee KH, Morgan J, Mehta S, Griffin J, et al. (2011) Closed-loop VR-based interaction to improve walking in Parkinson’s disease. J Nov Physiother 1: 1-7.
- Espay AJ, Gaines L, Gupta R (2013) Sensory feedback in Parkinson's disease patients with “on”-predominant freezing of gait. Front Neur 4: 14.
- Badarny S, Aharon J, Susel Z, Habib G, Baram Y (2014) Virtual reality feedback cues for improvement of gait in patients with Parkinson’s disease. Tremor & Other Hyperkinet Mov: 4.
- Chong R, Lee KH, Morgan J, Wakade C (2015) Duration of step initiation predicts freezing in Parkinson’s disease. Acta Neurologica Scandinavica.
- De Oliveira Souza C, Voos MC, Chien HF, Barbosa AF, Rodrigues RB, et al. (2015) Combined auditory and visual cueing provided by eyeglasses influence gait performance in Parkinson Disease patients submitted to deep brain stimulation: a pilot study. International Archives of Medicine 8.
- Baram Y, Lenger R (2012) Gait improvement in patients with cerebral palsy by visual and auditory feedback. Neuromodul 15: 48-52.
- Baram Y, Aharon J, Lenger R (2010) Virtual reality feedback for gait improvement in patients with idiopathic senile gait disorders and in patient with history of strokes. J Amer Geriat Soc 58: 191-192.








