Shih-Yi Kao1,Jia-Fwu Shyu2,Hwai-Shi Wang3,Chen-Yuan Hsiao4,5,Cheng-Hsi Su6,Tien-Hua Chen3,7,Zen-Chung Weng8,9 and Pei-Jiun Tsai10,11
1Ten-Chan General Hospital Zhongli, Taoyuan, Taiwan, R.O.C.
2Department of Biology and Anatomy, National Defense Medical Center, Taipei, Taiwan, R.O.C.
3Institute of Anatomy and Cell Biology, School of Medicine, National Yang Ming University, Taipei, Taiwan, R.O.C.
4Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan, R.O.C.
5Department of Surgery, National Yang Ming University Hospital, Yilan, Taiwan, R.O.C.
6Department of Surgery, Cheng Hsin General Hospital, Taipei, Taiwan, R.O.C.
7Department of Surgery, Veteran General Hospital, Taipei, Taiwan, R.O.C.
8Division of Cardiovascular Surgery, Department of Surgery, Taipei Medical University Hospital, Taipei, Taiwan, R.O.C.
9Department of Surgery, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, R.O.C.
10Department of Critical Care Medicine, Veteran General Hospital, Taipei, Taiwan, R.O.C.
11Institute of Clinical Medicine, National Yang Ming University, Taipei, Taiwan, R.O.C.
Corresponding Author:
Dr. Pei-Jiun Tsai, Ph.D.
Department of Critical Care MedicineVeteran General Hospital
201 Shih-Pai Road ,Section 2, Taipei, Taiwan 112, R.O.C.
Tel: +(886)-2-28712121
Fax: +(886)-2-28757890
E-mail: pjtsai@vghtpe.gov.tw
Citation: Yi Kao S, Fwu Shyu J, Shi Wang H, et al. Transplantation of Hepatocytelike Cells Derived from Umbilical Cord Stromal Mesenchymal Stem Cells to Treat Acute Liver Failure Rat. J Biomedical Sci. 2015, 4:1. doi:10.4172/2254-609X.10002
Copyright: © 2015 Yi Kao S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Wharton’s jelly, mesenchymal stem cells, hepatocytes, liver failure, transplantation
Introduction
Liver failure occurs when the liver is damaged beyond repair. It can be induced by a variety of factors causing severe impairment in synthesis, detoxification, excretion, biotransformation, and other hepatic physiological functions. Medications are the main cause of acute liver failure, whereas alcohol and viral hepatitis are common causes of chronic liver failure. The mortality rate of liver failure is high, especially in patients who have coagulation disorders, jaundice, hepatic encephalopathy, and ascites [1]. If detected early enough, acute liver failure may resolve spontaneously or can be treated successfully with supportive care. Unfortunately, most liver failure is the result of long-term decompensation, which does not resolve with simple measures. In those cases, intensive medical care may permit some regeneration of liver function, but liver transplantation may ultimately be needed for treatment of the end-stage disease. However, the shortage of donor organs and the complex technical, medical, and socioeconomic challenges of liver transplantation make it an inefficient and expensive method of treating liver failure, and many patients die awaiting transplantation. Thus, alternative methods for treating liver failure are continually being considered. Regeneration therapy with mesenchymal stem cell is one of these that we find attractive.
Mesenchymal stem cells express several surface antigens, such as CD29, CD44, CD90, SH2 (CD105), SH3 (CD166), SH4 (CD73) and STRO-1 [2] in contrast to the markers CD38, CD45 of hematopoietic lineage cells and the hematopoietic stem cell marker CD34 [3]. Mesenchymal stem cells are believed present in all human tissues and may play an integral role in renewal of aging cells and replenishment after apoptosis. Mesenchymal stem cells may maintain tissue homeostasis and organ stability [4,5]. In addition to being obtained through purification from bone marrow, mesenchymal stem cells can be obtained from other tissues, including the umbilical cord [6], umbilical cord blood [7], and amniotic fluid [8], and fat [9]. We [6] have found that fibroblast-like cells from Wharton’s jelly of human umbilical cord are similar to bone marrow mesenchymal stem and can be induced to differentiate into adipogenic, osteogenic, and cardiomyogenic cells. Also, Wharton’s jelly mesenchymal stem cells (WJ-MSCs) are capable of differentiating into many different types of mature cells and suppressing immune responses induced by T lymphocytes. Since mesenchymal stem cells from the umbilical cord can be easily isolated and expanded in vitro culture, we have considered using these cells in treatment of hepatic failure.
In this study, we attempted to stimulate the WJ-MSCs of human umbilical cord with conditioned medium and growth factors to differentiate into fully functioning hepatocyte-like cells in vitro. We then transplanted the cells into rats with carbon tetrachlorideinduced liver failure [10] to determine if the cells could reverse or ameliorate the failure.
Methods
Culture of umbilical cord mesenchymal stem cells
Institutional Review Board approval was obtained for all procedures. With the written informed consent of parents, fresh human umbilical cords were obtained after birth and stored in Hank’s balanced salt solution (Biological Industries, Israel) for 1 to 24 h before tissues were processed to obtain mesenchymal stem cells (MSCs). The isolation of MSCs followed the methods of Wang, et al. [6]. Briefly, blood vessels were removed, the mesenchymal tissue was scraped off the Wharton's jelly with a scalpel and centrifuged at 1800 rpm for 5 min at room temperature. The pellet was washed with serum-free Dulbecco's modified Eagle's medium (DMEM, Gibco, Grand Island, NY) and centrifuged at 1800 rpm for 5 min at room temperature. The pellet was suspended in 15 ml of DMEM containing 0.2 g/ml of collagenase I and incubated for 18-24 h at 37°C. The cells were washed, resuspended in 10 ml of DMEM containing 2.5% trypsin, and incubated for 30 min at 37°C with agitation. The cells were washed, cultured in DMEM supplemented with 10% fetal bovine serum (Sigma St. Louis, MO), and incubated in 5% CO2 in a 37°C incubator prior for use in experiments.
Preparation of liver tissue culture solution
With the written informed consent of parents, fresh human liver tissues were obtained. The healthy liver parenchyma tissue was resected from the normal portion of patients who were younger than 65-years-old. The average size of the liver specimens was 0.5 * 1 * 2 cm, weighing 0.5-2 g. The liver tissues were washed with Hank’s balanced salt solution (Biological Industries), cut into pieces, and centrifuged at 1200 rpm for 10 min at 25°C. The cells were cultured in 20 ml of DMEM/F12 containing 5% fetal bovine serum and incubated in 5% CO2 . Two days later, the supernatants were collected, filtered (0.22 μm), and stored at -80°C [11].
In vitro hepatocyte-like cell differentiation
WJ-MSCs between fourth and sixth passage were used to induce differentiation into hepatocyte-like cells. 70% confluence were washed with PBS and cultured with liver-tissue culture solution for three days. The cells were washed with PBS and cultured with Iscove’s Modified Dulbecco’s Medium containing hepatocyte growth factor (20 ng/ml), beta-fibroblast growth factor (10 ng/ ml), nicotinamide (0.61 g/L), dexamethasone (10-6M), and insulintransferrin- selenium. The culture medium was changed every three days, and the cells were cultured for nine days [8,12,13].
Immunofluorescence staining and microscopic analysis
The cells, treated or not treated for hepatocyte-like cell differentiation, were fixed on cover slide with 4% paraformaldehyde for 15 min at room temperature. The cells were permeabilized with 0.1% triton X-100 for 15 min and blocked with 5% goat serum for 15 min. Mouse anti-human serum albumin monoclonal antibody (Sigma, 1:100) were incubated with the cells overnight at 4°C. Anti-mouse cy2 antibody (Chemicon, 1:200) was incubated with the cells for 1 h at room temperature. The nuclei were stained with Hoechst (1:5000) for 15 min. The samples were mounted with an anti-photobleaching medium containing 20 mM n-propylgallate (Sigma) in 80% glycerol/20% PBS and observed under a microscope equipped with phase contrast and epifluorescence light paths (Leica DMIRE2, Heidelberg, Germany). For confocal imaging, a Zeiss confocal microscope LSM 510 (Zeiss Göttingen, Germany) was used for data acquisition.
Reverse transcription polymerase chain reaction (RT-PCR)
Reagent (Qiagen, Valencia, CA). A 4-μg sample was reverse transcribed with Mmlv reverse transcriptase (Amersham Life Science, Uppsala, Sweden) for 30 min at 42°C in the presence of an oligo-dT primer. The PCR reaction mixture consisted of 38.5 μl sterile distilled water, 5 μl 10X PCR buffer, 1 μl dNTP, 1.5 μl of each primer, 2 μl cDNA (4 μg), and 0.5 μl polymerase (5 U/μl) (Amersham Life Science). cDNA was amplified by use of the following primer sequences: albumin forward CCTTGGTGTTGATTGCCTTTGCTC, reverse CATCACATCAACCTCTGGTCTCACC; alpha 1-antitrypsin forward TCGCTACAGCCTTTGCAATG, reverse TTGAGGGTACGGAGGAGTTCC; AFP forward TGAAATGACTCCAGTAAACCC, reverse AATGAGAAACTCTTGCTTCATC; glucose-6-phosphatase forward TCAGCTCAGGTGGTCCTCTT, reverse CCTCCTTAGGCAGCCTTCTT; CK18 forward ATGGGAGGCATCCAGAACGAGAA, reverse GGGCATTGTCCACAGTATTTGCGA; tryptophan 2,3-dioxygenase forward AGTCAAACCTCCGTGCTT, reverse TCGGTGCATCCGAGAAACA; Cytochrome P450 3A4 forward TCACCCTGATGTCCAGCAGAAACT, reverse TACTTTGGGTCACGGTGAAGAGCA; GAPDH forward CACCATCTTCCAGGAGCGAG, reverse TCACGCCACAGTTTCCCGGA (Mission Biotech, Taiwan). PCR was performed for 30 cycles of denaturation at 95°C for 30 s, annealing at 55-63°C for 30 s, and elongation at 72°C for 1 min, with a final 10 min extension at 72°C. To exclude the possibility of contaminating genomic DNA, PCRs were also run without reverse transcriptase. The amplified cDNA was separated by electrophoresis on a 1% agarose gel, stained, and photographed under ultraviolet light.
Real-time PCR
cDNA was prepared from 4 μg of total RNA as above, and 200 ng RNA equivalents were used for PCR, with specific primers in the presence of SYBR Green I (Light CyclerTM-FastStart DNA Master SYBR Green I; Roche, Basel, Switzerland). A LightCycler ® 480 (Roche, Indianapolis, IN) was used for real-time PCR.
Ammonia clearance test
The cells grown in differentiation medium until 80% confluence were used for testing of ammonia clearance. The cells were cultured in Iscove’s Modified Dulbecco’s Medium (no phenol red) with insulintransferrin- selenium and 3.24 mM NH4Cl in 5%CO2 for 36 hours. The culture medium was collected and analyzed for ammonia by use of Ammonia Assay Kit (Sigma) and a spectrophotometer (DU 800 UV/Visible, Beckman Coulter).
Glycogen storage test
The cells were fixed with 4% paraformaldehyde for 15 minutes at 4°C, then incubated with periodic-acid solution (Sigma) at room temperature for 5 min. After washes with PBS, Schiff’s reagent was added to the cells at room temperature for 15 minutes. After the cells had been washed with distilled water for 5 minutes, cell nuclei were stained with hematoxylin (Sigma) for 90 s, and the cells were washed with distilled water for 15~30 s. The cells were mounted with mounting medium (Assistant 1025/500) and examined under a microscope.
Acute hepatic-injury animals
Twenty four male, 350-400 g Sprague-Dawley rats (Laboratory Animal Center, Yang-Ming University, Taiwan) were fed and housed on a 12-h light and 12-h dark cycle. The experiments followed institutional animal welfare guidelines. Sixteen rats were given intraperitoneal injections of 2 ml/kg of carbon tetrachloride (CCl4) solution in olive oil (1:1) on days 1, 3, 8, and 10 to induce acute hepatic injury. Eight rats received intraperitoneal 2 ml/kg of olive oil injection on the same days [14,15].
Cell transplantation
The rats were divided into a control group (CCl4 induction only), study group (CCl4 induction and hepatocyte-like cells transplantation), and sham group (olive oil injection only). For the study group, on day 9 of induction of acute hepatic injury, the rats were restrained, and 1*106 hepatocyte-like cells, suspended in 0.2 ml PBS, were injected into the catheter, followed by a volume of normal saline equivalent to the volume of the Port-A catheter (0.35 ml) to push the grafts into the portal vein. The control group and the sham group underwent the same procedure, but the animals were injected with normal saline only.
Serum liver function tests
Blood was collected from the rats’ tail veins before and after cell transplantation, and serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin were measured with an automatic biochemistry analyzer (Toshiba-40FR).
Immunohistochemistry
The rats were sacrificed after transplantation and perfused with 4% formaldehyde (Ferak, Berlin, Germany). The livers were removed and cut into 0.5-1.0 cm3 pieces. The pieces were dehydrated and embedded in OCT (Sakura Finetek USA Inc, Torrance, CA) in liquid nitrogen. Cryosections (5 μm thickness) were cut and washed twice with PBS, then incubated overnight at 4°C with mouse anti-human nuclei monoclonal (Sigma, 1:100) and rabbit anti-human albumin antibodies (Chemicon, 1:100). After being washed with PBS, slides were incubated for 1 h at room temperature with anti-mouse Cy3 (1:200, Jackson Immunoresearch Laboratories, West Grove, PA) and antirabbit FITC (1:200, Jackson Immunoresearch Laboratories) antibodies for 1 h at room temperature. After being washed in PBS, slides were incubated for 20 min at room temperature with TOTO-3 iodide (Molecule Probe, 1:10000). After being washed with PBS, the sections were mounted with mounting medium (Vector Laboratories, Burlingame, CA) and viewed with a fluorescence microscope equipped with appropriate filters.
Statistical analysis
Group comparisons were made by one-way ANOVA followed by Dunnett’s test. Survival was assessed by use of Kaplan-Meier survival curves. A P-value < .05 was considered statistically significant.
Results
Morphologic features of WJ-MSCs after differentiation
Without culture in hepatogenic differentiation medium, the WJ-MSCs grew in typical spindle and fibrocyte-like adherent monolayers. After culture in hepatogenic differentiation medium, morphologic changes were observed in differentiated cells: Initially, the cells decreased in mass and later developed the polygonal morphology of hepatocytes (Figure 1).
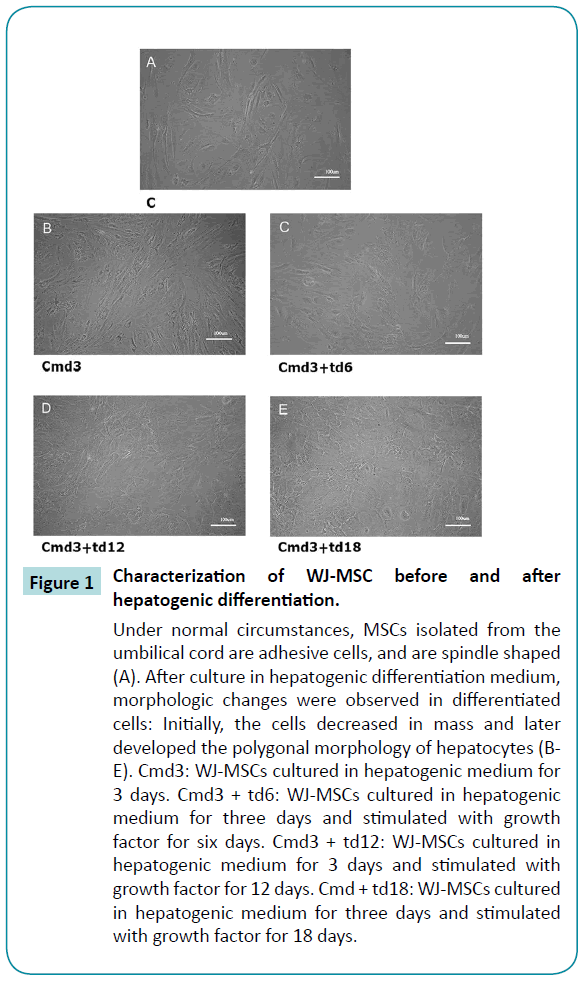
Figure 1: Characterization of WJ-MSC before and after hepatogenic differentiation.
Under normal circumstances, MSCs isolated from the umbilical cord are adhesive cells, and are spindle shaped (A). After culture in hepatogenic differentiation medium, morphologic changes were observed in differentiated cells: Initially, the cells decreased in mass and later developed the polygonal morphology of hepatocytes (BE). Cmd3: WJ-MSCs cultured in hepatogenic medium for 3 days. Cmd3 + td6: WJ-MSCs cultured in hepatogenic medium for three days and stimulated with growth factor for six days. Cmd3 + td12: WJ-MSCs cultured in hepatogenic medium for 3 days and stimulated with growth factor for 12 days. Cmd + td18: WJ-MSCs cultured in hepatogenic medium for three days and stimulated with growth factor for 18 days.
Expression of hepatocyte-related specific genes after differentiation
To determine whether the WJ-MSCs had differentiated into hepatocyte-like cells, we tested for the presence of hepatocyterelated specific genes by use of reverse transcriptase-PCR and real-time PCR. WJ-MSCs before differentiation had no evidence of hepatocyte-specific gene primer and expressed very low levels of albumin,glucose-6-phosphatase,alpha 1-antitrypsin tryptophan 2,3-dioxygenase and Cytochrome P450 3A4 genes. However, after three days of differentiation, real-time PCR revealed evidence of the hepatocyte-specific marker genes, and the gene expressions gradually increased with longer periods of differentiation. Meanwhile, the expression levels of alpha-fetoprotein gradually decreased (Figure 2). The expression levels of hepatocyte-specific genes were mostly similar on days 12 and 18 after differentiation, but in some cases, the expression levels were greater on day 12 than on day 18. Thus, we chose to use 12-day differentiated cells in the experiments described below.
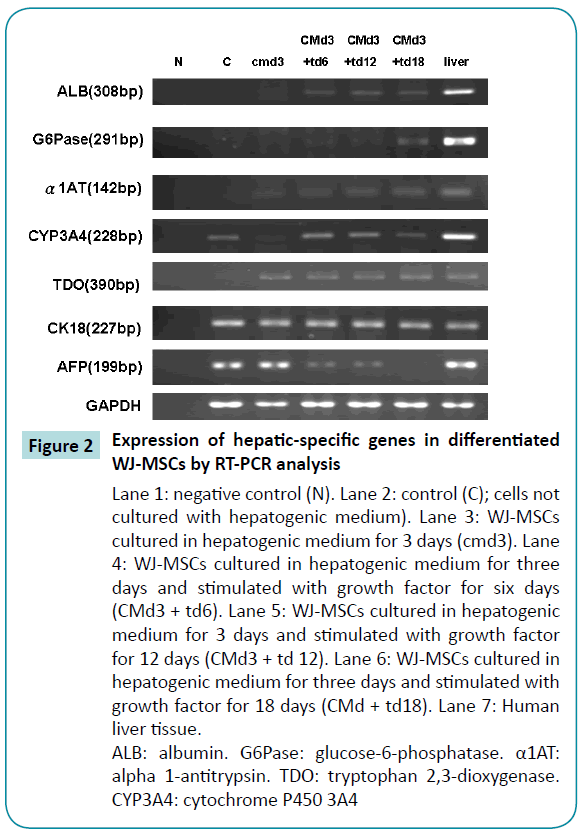
Figure 2: Expression of hepatic-specific genes in differentiated WJ-MSCs by RT-PCR analysis
Lane 1: negative control (N). Lane 2: control (C); cells not cultured with hepatogenic medium). Lane 3: WJ-MSCs cultured in hepatogenic medium for 3 days (cmd3). Lane 4: WJ-MSCs cultured in hepatogenic medium for three days and stimulated with growth factor for six days (CMd3 + td6). Lane 5: WJ-MSCs cultured in hepatogenic medium for 3 days and stimulated with growth factor for 12 days (CMd3 + td 12). Lane 6: WJ-MSCs cultured in hepatogenic medium for three days and stimulated with growth factor for 18 days (CMd + td18). Lane 7: Human liver tissue.
ALB: albumin. G6Pase: glucose-6-phosphatase. α1AT: alpha 1-antitrypsin. TDO: tryptophan 2,3-dioxygenase. CYP3A4: cytochrome P450 3A4
Immunohistochemical evidence of albumin expression in undifferentiated and differentiated WJ-MSCs
The results of immunofluorescence analysis of albumin expression in undifferentiated and differentiated MSCs are shown in Figure 3. The undifferentiated cells did not express green fluorescence (cy2) (Figure 3A and B), which means that they did not produce albumin. After three days culture in the hepatogenic differentiation medium, expression of albumin was present in a number of cells, although the cells still exhibited primitive spindle shape morphology (Figure 3C and D). After six days incubation with growth factor, almost all of the WJ-MSCs were producing albumin (Figure 3E and F). The cells also gradually developed the polygonal morphology of hepatocytes, and by 12 days, hepatocyte morphology had further matured, with abundant granules present (Figure 3G and H).
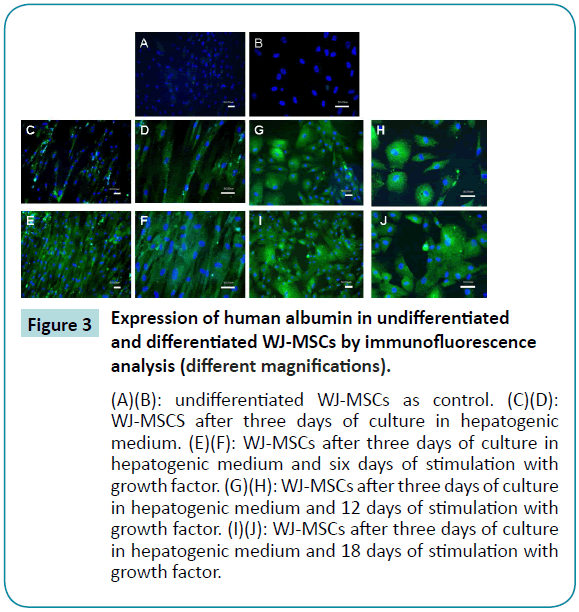
Figure 3: Expression of human albumin in undifferentiated and differentiated WJ-MSCs by immunofluorescence analysis (different magnifications).
(A)(B): undifferentiated WJ-MSCs as control. (C)(D): WJ-MSCS after three days of culture in hepatogenic medium. (E)(F): WJ-MSCs after three days of culture in hepatogenic medium and six days of stimulation with growth factor. (G)(H): WJ-MSCs after three days of culture in hepatogenic medium and 12 days of stimulation with growth factor. (I)(J): WJ-MSCs after three days of culture in hepatogenic medium and 18 days of stimulation with growth factor.
Connexin32 expression in undifferentiated and differentiated WJ-MSCs
The results of immunofluorescence staining for connexin32 revealed that neither undifferentiated WJ-MSCs nor WJ-MSCs cultured in differentiation medium for three days expressed this hepatocyte gap-junction protein (Figure 4A-D). After six days differentiation with growth factor, the cells had begun to express connexin32 (Figure 4E and F), but the connexin32 was spread out in the cytoplasm, suggesting that it did not yet possess gap junction capability. At 12 to 18 days of differentiation, brighter green fluorescence was present between cells (Figure 4G-J), and we postulate that connexin32 then was functional.
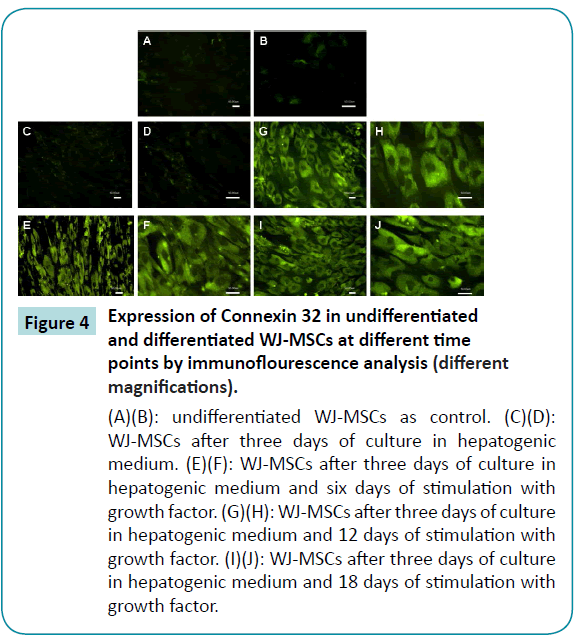
Figure 4: Expression of Connexin 32 in undifferentiated and differentiated WJ-MSCs at different time points by immunoflourescence analysis (different magnifications).
(A)(B): undifferentiated WJ-MSCs as control. (C)(D): WJ-MSCs after three days of culture in hepatogenic medium. (E)(F): WJ-MSCs after three days of culture in hepatogenic medium and six days of stimulation with growth factor. (G)(H): WJ-MSCs after three days of culture in hepatogenic medium and 12 days of stimulation with growth factor. (I)(J): WJ-MSCs after three days of culture in hepatogenic medium and 18 days of stimulation with growth factor.
Ammonia metabolism capability in undifferentiated and differentiated WJ-MSCs
Normal liver cells are capable of metabolizing ammonia to urea, which is less toxic than ammonia. In order to show that differentiated WJ-MSCs were capable of performing this physiological function, we placed the cells in a medium containing ammonia for 36 h and assayed the medium to determine whether ammonia concentrations had decreased. The results of the assay indicated that undifferentiated WJ-MSCs did not possess the ability to metabolize ammonia, whereas differentiated WJ-MSCs, possessed the ability, which was most pronounced after 12 days of differentiation (Figure 5).
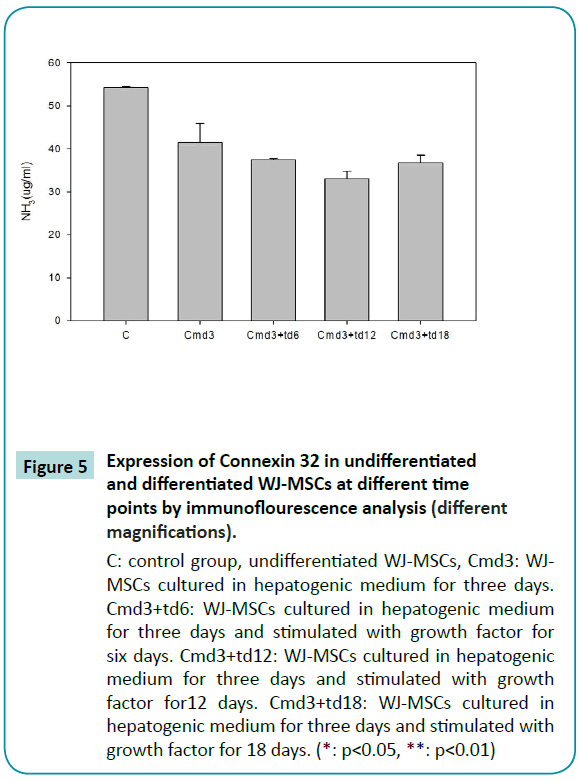
Figure 5: Expression of Connexin 32 in undifferentiated and differentiated WJ-MSCs at different time points by immunoflourescence analysis (different magnifications).
C: control group, undifferentiated WJ-MSCs, Cmd3: WJMSCs cultured in hepatogenic medium for three days. Cmd3+td6: WJ-MSCs cultured in hepatogenic medium for three days and stimulated with growth factor for six days. Cmd3+td12: WJ-MSCs cultured in hepatogenic medium for three days and stimulated with growth factor for12 days. Cmd3+td18: WJ-MSCs cultured in hepatogenic medium for three days and stimulated with growth factor for 18 days. (*: p<0.05, **: p<0.01)
Glycogen accumulation in undifferentiated and differentiated WJ-MSCs demonstrated with periodic acid-Schiff staining
A necessary function of human hepatocytes is the storage of glycogen. Glycogen in tissues can be detected by staining with periodic acid-Schiff (PAS) reagent, with glycogen oxidized to aldehyde, producing a purple color. Results of our experiments with PAS staining of WJ-MSCs revealed that WJ-MSCs six days after differentiation were much darker than undifferentiated WJ-MSCs (Figure 6A-F) WJ-MSCs 12 days and 18 days after differentiation had even much darker purple stain (Figure 6G-J). The results indicated that the differentiated WJ-MSCs had increased glycogen storage.
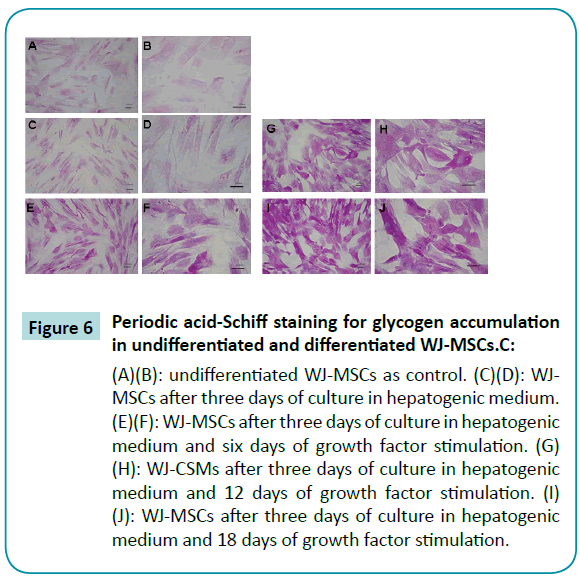
Figure 6: Periodic acid-Schiff staining for glycogen accumulation in undifferentiated and differentiated WJ-MSCs.C:
(A)(B): undifferentiated WJ-MSCs as control. (C)(D): WJMSCs after three days of culture in hepatogenic medium. (E)(F): WJ-MSCs after three days of culture in hepatogenic medium and six days of growth factor stimulation. (G) (H): WJ-CSMs after three days of culture in hepatogenic medium and 12 days of growth factor stimulation. (I) (J): WJ-MSCs after three days of culture in hepatogenic medium and 18 days of growth factor stimulation.
Weight changes in rats with acute liver failure after transplantation of 12-day different????iated WJ-MSCs
By injecting rats with two doses of CCl4 weekly for two weeks, we established the rat model of acute liver failure. We found that untreated rats died one day after receiving the fourth injection of CCl4. However, when 1*106 12-day differentiated WJ-MSCs had been injected into the portal vein after the third dose of CCl4, the rats survived the fourth injection of CCl4. We also found that the rats’ weight decreased for four days after the fourth injection then began increasing on the seventh day, at a rate similar to that of sham animals (Figure 7A). These results indicated that the 12-day differentiated WJ-MSCs were capable of protecting rats from fatal acute liver failure.
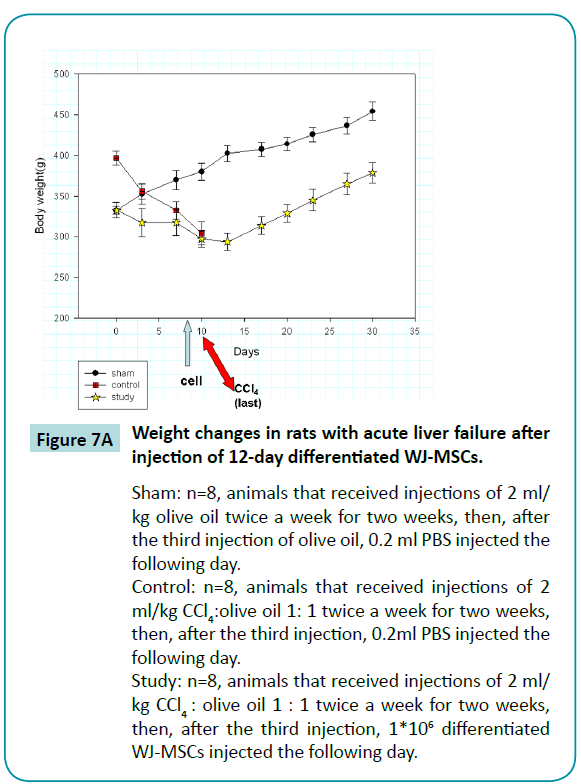
Figure 7a: Weight changes in rats with acute liver failure after injection of 12-day differentiated WJ-MSCs.
Sham: n=8, animals that received injections of 2 ml/kg olive oil twice a week for two weeks, then, after the third injection of olive oil, 0.2 ml PBS injected the following day.
Control: n=8, animals that received injections of 2 ml/kg CCl4:olive oil 1: 1 twice a week for two weeks, then, after the third injection, 0.2ml PBS injected the following day.
Study: n=8, animals that received injections of 2 ml/kg CCl4 : olive oil 1 : 1 twice a week for two weeks, then, after the third injection, 1*106 differentiated WJ-MSCs injected the following day.
Serum liver function tests after transplantation of 12-day differentiated WJ-MSCs into acute liver-failure rats
Serum AST, ALT, and total bilirubin values increased abruptly after the injection of CCl4 (Figure 7B-D) in the rats of control and study groups, reaching peak or near-peak values within eight hours after the first injection. In control animals, values for these liver tests remained high and abruptly increased (especially AST and bilirubin) after the last injection of CCl4 on day 10. All control animals died within 1 day after the last injection. In contrast, the liver function test values in rats of study group, who was injected with 12-day differentiated WJ-MSCs on day 9, did not rise after the last injection but declined and returned to baseline values at about day 12. Thus, we postulate that the differentiated WJ-MSCs were capable of repairing CCl4-induced damage to the livers.
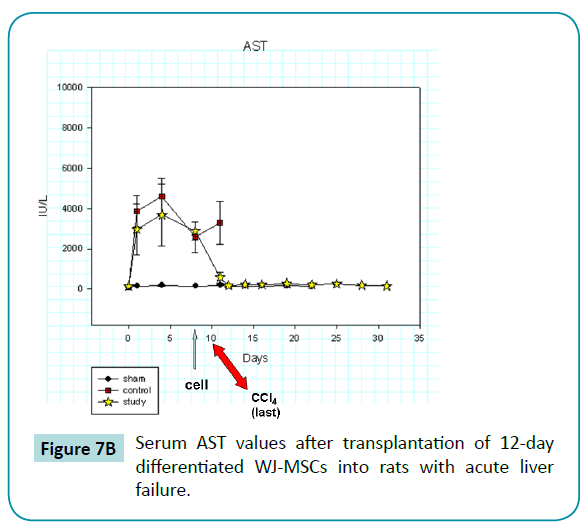
Figure 7b: Serum AST values after transplantation of 12-day differentiated WJ-MSCs into rats with acute liver failure.
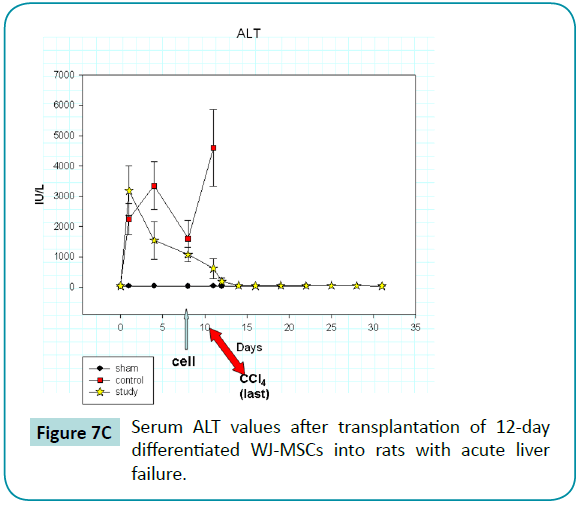
Figure 7c: Serum ALT values after transplantation of 12-day differentiated WJ-MSCs into rats with acute liver failure.
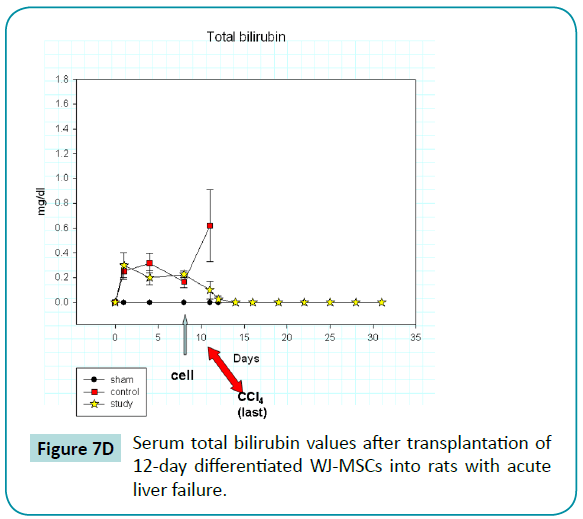
Figure 7d: Serum total bilirubin values after transplantation of 12-day differentiated WJ-MSCs into rats with acute liver failure.
Immunohistochemical staining of frozen tissue sections of rat livers 30 days after transplantation with 12-day differentiated WJMSCs
Immunohistochemical staining of frozen tissue sections of rat livers 30 days after the transplantation of 12-day differentiated WJ-MSCs revealed co-localization of human nuclei expression and human albumin expression (Figure 8). These findings are evidence that the differentiated WJ-MSCs survived and maintained a function of normal liver cells for more than 30 days after transplantation.
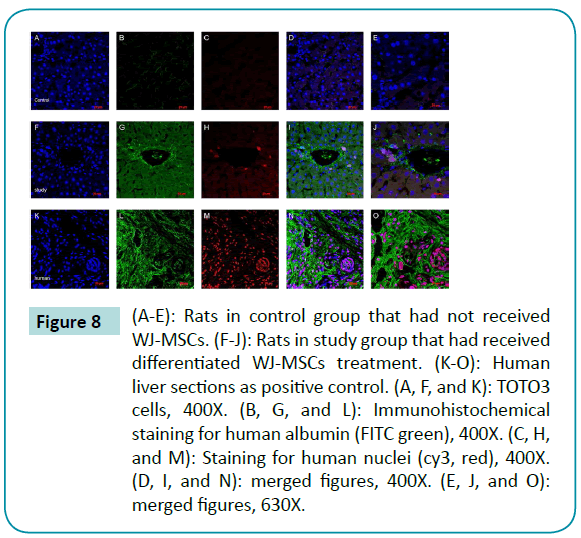
Figure 8: (A-E): Rats in control group that had not received WJ-MSCs. (F-J): Rats in study group that had received differentiated WJ-MSCs treatment. (K-O): Human liver sections as positive control. (A, F, and K): TOTO3 cells, 400X. (B, G, and L): Immunohistochemical staining for human albumin (FITC green), 400X. (C, H, and M): Staining for human nuclei (cy3, red), 400X. (D, I, and N): merged figures, 400X. (E, J, and O): merged figures, 630X.
Discussion
The goals of this study were to produce functioning hepatocytelike cells from human WJ-MSCs and transplant the cells into CCl4-treated rats to prevent or ameliorate liver failure. The major findings were these: in vitro studies showed that WJMSCs, stimulated to differentiate into hepatocyte-like cells, had morphologic and functional characteristics of hepatocytes (polygonal shape and intracellular granules; expression of albumin, glucose-6-phosphatase, alpha 1-antitrypsin, tryptophan 2,3-dioxygenase,Cytochrome P450 3A4 genes; immunohistochemical evidence of albumin production and connexin32 expression; metabolism of ammonia into urea; and glycogen accumulation). RT-PCR experiments revealed that the differentiated cells had time-dependent decrease in alphafetoprotein levels, which is additional evidence of maturation of WJ-MSCs differentiated into hepatocyte-like cells since alpha-fetoprotein is a marker of premature hepatocytes and is not found in mature hepatocytes. In experiments with CCl4- treated rats, the differentiated WJ-MSCs protected the rats from increases in serum AST, ALT, and bilirubin concentrations and death, as occurred after the last CCl4exposure in the control rats. Moreover, immunohistochemical study of rat liver tissues 30 days after transplantation revealed that the differentiated WJ-MSCs had survived and were producing albumin. Thus, the cumulative evidence indicates that hepatocyte-like cells differentiated from WJ-MSCs can protect against liver failure in this animal model.
In recent years, research on MSCs has found that the cells are capable not only of differentiation into various cell types, but also of differentiation into cells from different germ layers [16]. Moreover, MSCs are involved in immune regulation through suppression of T lymphocytes [17]. In organ transplantation, a common complication is graft-versus-host disease. This disease, which is very difficult to treat, occurs when host T lymphocytes attack the graft tissue. Thus, the immunosuppressive capability of MSCs may be an additional advantage if they are used in regeneration cell therapy in comparison with whole human organ transplantation.
In agreement with our data, others [11] have found by RT-PCR analysis that undifferentiated WJ-MSCs have low levels of hepatic marker genes. Thus, we speculate that WJ-MSCs are more suitable for hepatogenic differentiation than MSCs from other sources. Also, compared with other sources of MSCs, which achieve the same degree of differentiation through 20 days induction [13], WJ-MSCs appear to possess superior hepatogenic differentiation capability.
Xenotransplantation of MSCs into non-immune suppressed animals reportedly does not cause acute rejection [18], a finding which suggests that MSCs are not recognized by the immune system. Our results agree with this possibility since no immune rejection of transplanted WJ-MSCs into rats with acute liver failure was observed after 30 days. These observations suggest another advantage of using MSCs in the treatment of liver failure.
In this study, we used differentiated cells rather than undifferentiated cells for transplantation. The bases for this strategy are the results of studies in which direct transplantation of undifferentiated cells into animals with liver damage caused liver fibrosis [12]. In those studies, few cells underwent hepatic differentiation, whereas most exhibited a fibroblastic morphology and gathered in fibrotic septa. Our results suggest that this kind of problem can be avoided by using differentiated MSCs. Also, when MSCs are induced to differentiate into hepatocytes outside the body, the chances of the cells differentiating into other types of cells when introduced into the body probably decreases greatly.
WJ-MSCs have been injected directly into the liver parenchyma of rats with CCL4-induced acute liver [19]. In that work, the authors slowly injected WJ-MSCs (about 5×105 cells) into the right lobe of the liver parenchyma via laparotomy. In response to this treatment, values of serum liver enzymes declined and liver failure was reversed. The clinical applicability of this cell transplantation approach, however, seems limited. Our approach, with infusion of differentiated hepatocyte-like cells via the portal vein seems more feasible for clinical application.
In conclusion, WJ-MSCs isolated from the human umbilical cord could differentiated into hepatocyte-like cells in vitro. The hepatocyte-like cells had morphologic features and functional characteristics of mature hepatocytes: hepatocyte-related genes, conversion of ammonia to urea, albumin synthesis, glycogen storage, and declining values of alpha-fetoprotein. Transplantation of hepatocyte-like cells into the portal vein of rats with carbon tetrachloride-induced acute liver failure prevented irreversible liver damage and death of the animals. by ameliorating the rising values of serum ALT, AST, and bilirubin. Immunohistochemical study of rat liver tissues 30 days after transplantation revealed that the hepatocyte-like cells had survived and were producing albumin. These findings may serve as a reference for future research in cell therapy of liver failure, including experiments in larger-animal models.
6518
References
- O'Grady, J.G., Schalm, S.W., Williams, R.Acute liver failure: redefining the syndromes.Lancet 1993; 342: 273-275.
- Horwitz, E.M., Keating, A.Nonhematopoieticmesenchymal stem cells: what are they?Cytotherapy2000; 2: 387-388.
- Pittenger, M.F., Mackay, A.M., Beck, S.C., Jaiswal, R.K., Douglas, R., et al. Multilineage potential of adult human mesenchymal stem cells.Science 1999; 284: 143-147.
- Ye, X., Kuang, J. Li,X., Tang, G. Microstructure, properties and temperature evolution of electro-pulsing treated functionally graded Ti-6Al-4V alloy strip. J Alloy Compd 2014; 599:1-9.
- Ye, X., Tang, G., Song, G., Kuang, J. Effect of electropulsing treatment on the microstructure, texture, and mechanical properties of cold-rolled Ti-6Al-4V alloy. J Mater Res 2014; 29:1500-1512.
- Wang, H.S., Hung, S.C., Peng, S.T., Huang, C.C., Wei, HM., et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord.Stem Cells 2004; 22: 1330-1337.
- Lee, O.K., Kuo T.K., Chen W.M., Lee K.D., Hsieh S.L., et al. Isolation of multipotentmesenchymal stem cells from umbilical cord blood.Blood2004; 103: 1669-1675.
- Tsai. M.S., Lee. J.L., Chang. Y.J., Hwang. S.M. Isolation of human multipotentmesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol.Hum Reprod2004; 19: 1450-1456.
- Zuk. P.A., Zhu. M., Ashjian. P., De Ugarte. D.A., Huang JI., et al. Human adipose tissue is a source of multipotent stem cells.MolBiol Cell 2002; 13: 4279-4295.
- Cameron. G.R., Karijnartne W.A.E., Carbon tetrachloride cirrhosis in relation to liver regeneration. J. Pathol. Bacteriol 1936, 42:1-21.
- Campard. D., Lysy.P.A., Najimi. M., Sokal. E.M. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells.Gastroenterology 2008; 134: 833-848.
- diBonzo. L.V, Ferrero. I., Cravanzola. C., Mareschi. K., Rustichell. D., et al. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 2008, 57:223-231.
- Lee. K.D., Kuo. T.K., Whang-Peng. J., Chung. Y.F., Lin. C.T., et al. In vitro hepatic differentiation of human mesenchymal stem cells.Hepatology2004; 40: 1275-1284.
- Zhao. W., Li.JJ., Cao. DY., Li. X., Zhang. LY., et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis.World J Gastroenterol 2012; 18: 1048-1058.
- Zhao. L., Feng. Z., Hu. B., Chi. X., Jiao. S.,Ex vivo-expanded bone marrow mesenchymal stem cells facilitate recovery from chemically induced acute liver damage.Hepatogastroenterology2012; 59: 2389-2394.
- Jiang. Y., Vaessen. B., Lenvik. T., Blackstad. M., Reyes. M., et al. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain..ExpHematol2002; 30: 896-904.
- Uccelli. A., Moretta. L., Pistoia. V.,Immunoregulatory function of mesenchymal stem cells.Eur J Immunol2006; 36: 2566-2573.
- Medicetty. S., Bledsoe.A.R., Fahrenholtz. C.B., Troyer. D., Weiss. M.L. Transplantation of pig stem cells into rat brain: proliferation during the first 8 weeks..ExpNeurol2004; 190: 32-41.
- Tsai. P.C., Fu. T.W., Chen. Y.M., Ko. T.L., Chen. T.H., et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis.Liver Transpl2009; 15: 484-495.
















