Keywords
TNBC; HER2neu; TIL; TLS; Prognostic; Predictive; Molecular; DFS; OS
Introduction
Invasive breast cancers (IBC) are heterogeneous with variable tumor biology and outcome [1]. The tumor grade, staging and receptor status are among the many prognostic factors that affect breast cancer outcome, but they are insufficient to give precise prognostication [2]. To improve outcome searching for new prognostic factors are required. Tumor microenvironment has been found to promote cancer growth. The importance of tumor microenvironment in promoting cancer growth has been increasingly recognized [3]. Immune system was found to play a role on oncogensis and tumor progression [4]. The prognostic and predictive role of tumor infiltrating lymphocytes (TILs) have been demonstrated in many solid tumors with its anti tumor activity [5,6]. Among breast cancer subtypes, triple negative breast cancer had the worst prognosis, but high TIL is associated with favorable prognosis and pathologic complete response in neoadjuvant setting [7,8]. The presence of ectopic lymphoid tissue known as tertiary lymphoid structures (TLS) resembling secondary lymphoid organs (SLO) have been described in many cancers including breast cancer [9-11]. TLS is formed of T and B cells, the former composed of germinal centre and small number of follicular dendritic cells while the latter is formed from mature dendritic cells and endothelial venules respectively. So, it is the site of immune response responsible for triggering, recruiting and activating TIL and thus improves antitumor response and prolongs survival [12-16].
The TLS and its correlation with different prognostic factors in invasive breast cancer with different molecular subtypes have been studied in very little trials [12-16]. But its correlation with type of chemotherapy was not discussed.
Patient and Methods
In this study, Eighty patients with stage II and III breast cancer according to AJCC TNM 8TH edition [17] in Tanta oncology and pathology department with luminal A, B and her 2 enriched and TNBC diagnosed with core needle biopsy and properly staged in the period from June 2012 to June 2018 received standard neoadjuvant protocol formed of AC (doxorubicin 60 mgm/m2 plus cyclophosphamide 600 mgm/m2 on day 1 every 3 weeks for 4 cycles followed by paclitaxel in dose of 80 mgm/m2 for 12 weeks). Patients with HER2 positive tumor received trastuzumab 6 mgm/kg (loading dose 8 mgm/kg) once every 3 weeks from the start of taxol start.
Prechemotherapy formalin fixed paraffin embedded core biopsies were collected. Hormone receptor positivity was defined as 1% or more for estrogen and progesterone receptors. The intensity of HER2 membrane staining was scored as 0, 1+, 2+ or 3+. Tumors with 3+ scores were classified as positive for HER2 over expression, whereas tumors with 0 or 1+ scores were considered as negative. FISH was done for her 2 neu +2 to determine whether they are negative or positive. Tumors were classified as ‘not amplified’ (FISH negative) if the calculated ratio was less than 2 and ‘amplified’ (FISH positive) if the ratio was 2 or greater. Ki 67 labeling index was recorded as high if more than 14%.
Assessment of tumor immune cell infiltrate
Immunohistochemistry: For Immunohistochemical staining, prechemotherapy 4μm sections of formalin fixed paraffin embedded core biopsies blocks were collected. The avidin-biotinperoxidase complex method was used for the immunostaining of monoclonal antibodies to CD3, CD4 (pan T cell marker), and CD20 (pan B cell marker) to label T and B cells [Dako, Glostrup, Denmark]. In brief, after dewaxing, inactivating endogenous peroxidase activity and blocking cross-reactivity with normal serum (Vectastain Elite Kit; Vector, Burlingame, CA, USA), the sections were incubated overnight at 4°C with a diluted solution of the primary antibodies. Location of the primary antibodies was achieved by subsequent application of a biotinylated antiprimary antibody, an avidin-biotin complex conjugated to horseradish peroxidase, and diaminobenzidine (Vectastain Elite Kit, Vector, Burlingame, CA). The slides were counter-stained by hematoxylin. Human lymph node was used as positive control. Negative controls were performed by omitting the primary antibody.
Immunohistochemical analysis of tumor infiltrating lymphocytes in tumor stroma was performed by two pathologists independently performed the categorizing and had no knowledge of the patients’ background history. The number of TILs in stroma surrounding the stained cancer cells was quantitatively measured in each field under 400x magnification. Areas of in situ carcinoma and crush artifacts were not included. Score 0 and 1 for <20% and >20% respectively and score one is considered positive (Figure 1) A case of Infiltrating duct carcinoma showed severe form of TLIs >20% (score 1) in direct contact to tumor cells (H&E 400x). The immune cell aggregates which were TLS positive showed the presence of CD20+ B lymphocytes within the follicles, with areas of CD3+, CD4+ T lymphocytes mainly in the periphery [T cell zone] resembling the highly organized structures of secondary lymphoid organs (Figure 2). Tertiary lymphoid structures with germinal center formation (2A and 2C) were detected by CD20+ B lymphocytes and CD3+ (2B) and CD4+ (2D) T lymphocytes in the T cell zone (streptavidin biotin 400x). Only TLS located in the adjacent stroma (in direct contact or within 0.5 mm of the tumor) were scored, with TLS positive 1% and TLS-negative tumors by their absence.
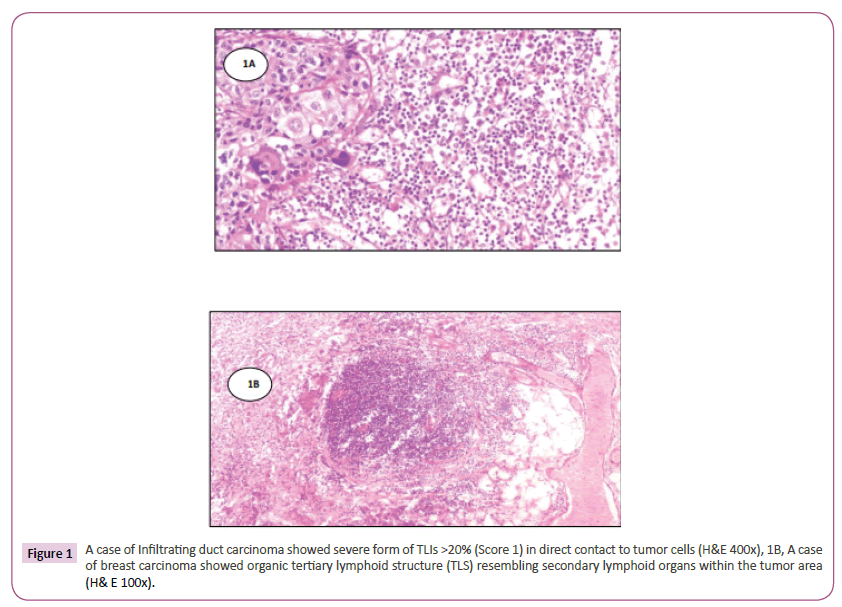
Figure 1: A case of Infiltrating duct carcinoma showed severe form of TLIs >20% (Score 1) in direct contact to tumor cells (H&E 400x), 1B, A case of breast carcinoma showed organic tertiary lymphoid structure (TLS) resembling secondary lymphoid organs within the tumor area (H& E 100x).
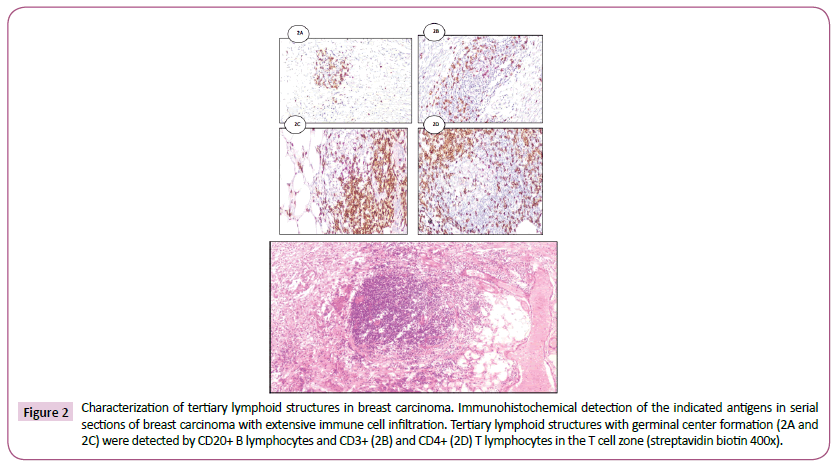
Figure 2: Characterization of tertiary lymphoid structures in breast carcinoma. Immunohistochemical detection of the indicated antigens in serial sections of breast carcinoma with extensive immune cell infiltration. Tertiary lymphoid structures with germinal center formation (2A and 2C) were detected by CD20+ B lymphocytes and CD3+ (2B) and CD4+ (2D) T lymphocytes in the T cell zone (streptavidin biotin 400x).
Response: Response evaluation was done according to RECIST 1.1 [18]. The surgery was done after 4 weeks from last cycle. Pathological complete response was defined as no residual disease in the breast on axillary lymph nodes or residual carcinoma in situ. After neoadjuvant chemotherapy breast conserving or modified radical mastectomy. Radiotherapy was given to all patients with breast conserving surgery and in patients for which modified radical mastectomy radiotherapy with 4 or more positive lymph nodes or tumor large than 5 cm with negative lymph nodes or close surgical margin. Overall survival (OS) time was defiened as the period from the start of neoadjuvant to the time of death from any cause or last follows up. Disease free survival (DFS) was defined as freedom from locoregional, and distant recurrence. Follow up by physical examination every 4- 6 months during the first 5 years. Mammogram is done annually US every 6 months, and CT and bone scintigraphy annually. The study protocol was approved by the Ethics Committee. Informed written consent was obtained from patients enrolled in the study.
Statistical Methods
SPSS version 19.0 was used for statistical analysis. The associations between TILs, tertiary lymphocyte structure and different clinicpathologic factors were done. Analysis of pCR was carried out using a binary logistic regression model. The Kaplan–Meier method was used to estimate DFS, and OS, with p-value<0.05 was considered statistically significant [19]. Cutoff values for different biomarkers included in this study were chosen before statistical analysis. Use Cox regression hazard model for multivariate analysis [20].
Results
Intrinsic subtypes among eighty patients studied from June 2012 to June 2018 with breast cancer histopathologically examined in pathology department, Tanta university hospital who received neoadjuvant chemotherapy in oncology department, Tanta university hospital were distributed as following hormone positive breast cancer (HRBC) in 32 (52.5% of whom 24(30%) and 18 (22.5%) were luminal A and B respectively), HER2 enriched subtype in 14 (17.5%), and TNBC in 23 (30%) patients.
According to tumor infiltrating lymphocyte, TNBC (83.3% p-value<0.0001) and HER2 enriched (78.6%, p-value<0.0001) had high tumor infiltrating lymphocyte as seen in Table 1. Also, the factors with statistical significance were age, menopausal status, histology, type of surgery, pathological CR. Thirty-three patients (41.2%) and 47(58.8%) achieved pCR and non-pCR in respectively. According to intrinsic subtype, pCR was achieved in 4 (16.7%), 8(44.4%), 8 (57.1%), 13(54.2%) patients with luminal A, B, HER2 enriched and triple negative breast cancer respectively which was statistically significant (p-value 0.027). Tertiary lymphoid structure is present in 43/80 (53.7%) for which pCR was reported in 62.8% versus 16.2% in patients with present and absent tertiary lymphocyte structure respectively which was statistically significant (p-value=0.001) (Table 2). In patients with her 2 enriched subtype overall survival was higher in TIL more than>20% versus low (p-value=0.032) and in present TLS than absent (p-value=0.117). DFS were significantly longer in high TIL than in low one and in present TLS versus absent respectively and this was statistically significant (p-value=0.027, 0.013 respectively) (Figure 3) survival according to tumor infiltrating lymphocyte and tertiary lymphocyte structure for HER2 enriched subtype). Also, in patients with triple negative overall survival was higher in TIL more than>20% versus low (p-value=0.001) and in present TLS than absent (p-value=0.001). DFS was significantly longer in high TIL than in low one and in present TLS versus absent respectively (p-value=0.003, 0.057 respectively) (Figure 4) (Survival according to tumor infiltrating lymphocyte and tertiary lymphocyte aggregate for triple negative breast cancer subtype). As regards, hormone positive subtypes and different breast subtypes no statistically, significant difference between TIL and TLS and OS and disease-free survival except for So with TIL in different breast subtypes (p=0.008) (Figures 5 and 6) Survival according to tumor infiltrating lymphocyte and tertiary lymphocyte structure for hormone enriched subtype and all breast subtypes respectively. The multivariate analysis maintains statistical difference between DFS with TIL and TLS with both TNBC and HER2 enriched breast subtype but not with hormone positive and all breast subtypes (Table 3).
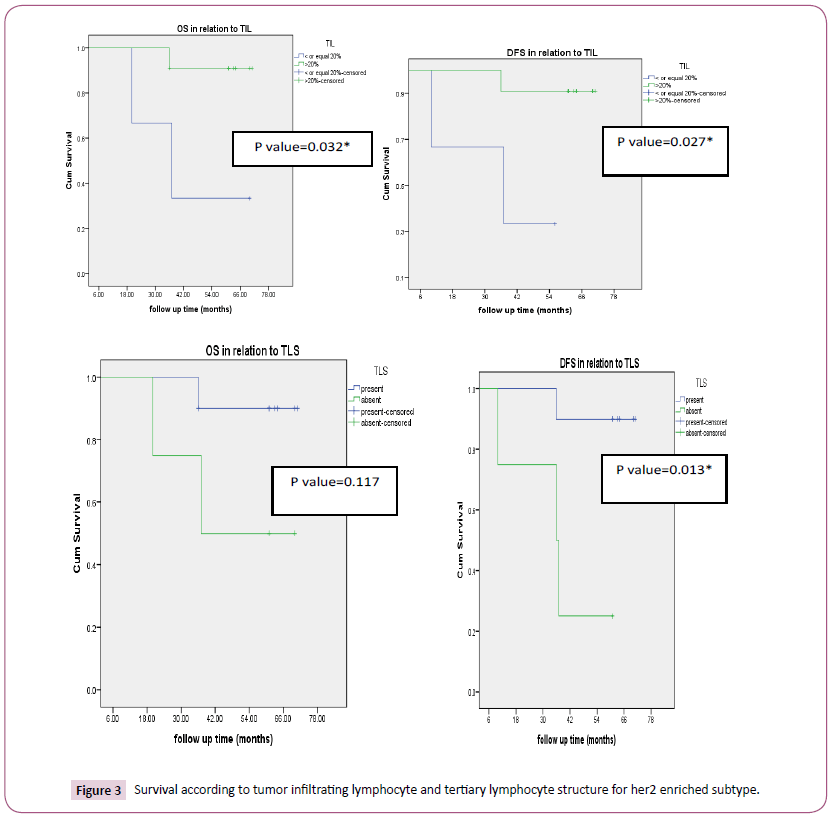
Figure 3: Survival according to tumor infiltrating lymphocyte and tertiary lymphocyte structure for her2 enriched subtype.
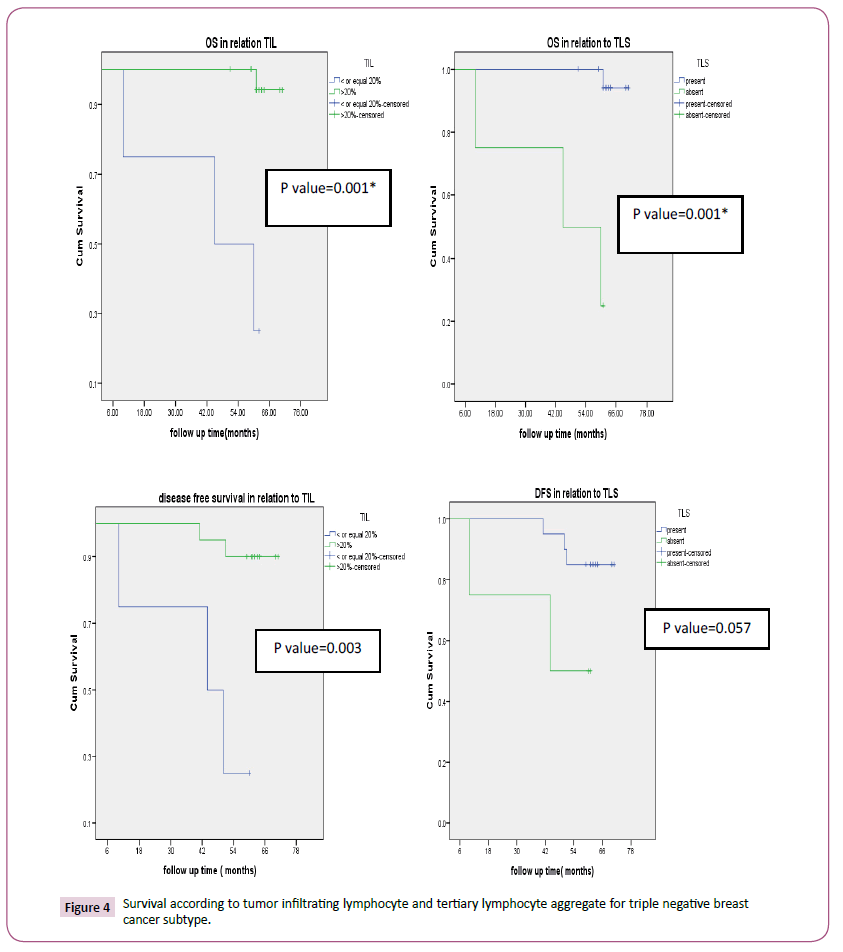
Figure 4:Survival according to tumor infiltrating lymphocyte and tertiary lymphocyte aggregate for triple negative breast cancer subtype.
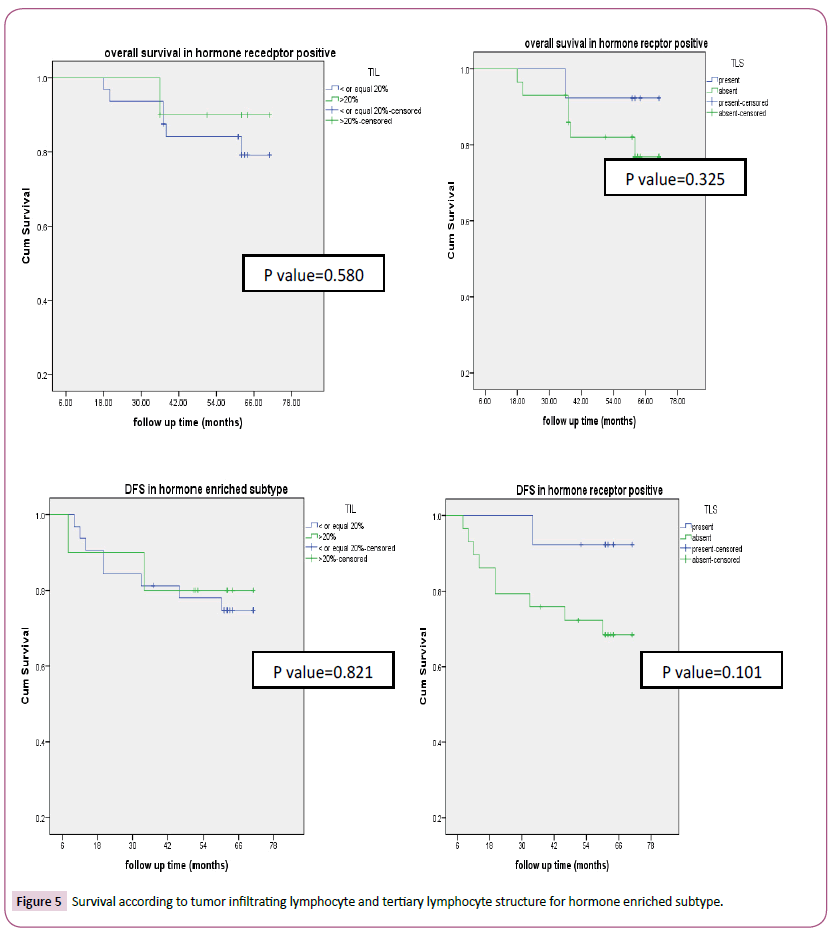
Figure 5: Survival according to tumor infiltrating lymphocyte and tertiary lymphocyte structure for hormone enriched subtype.
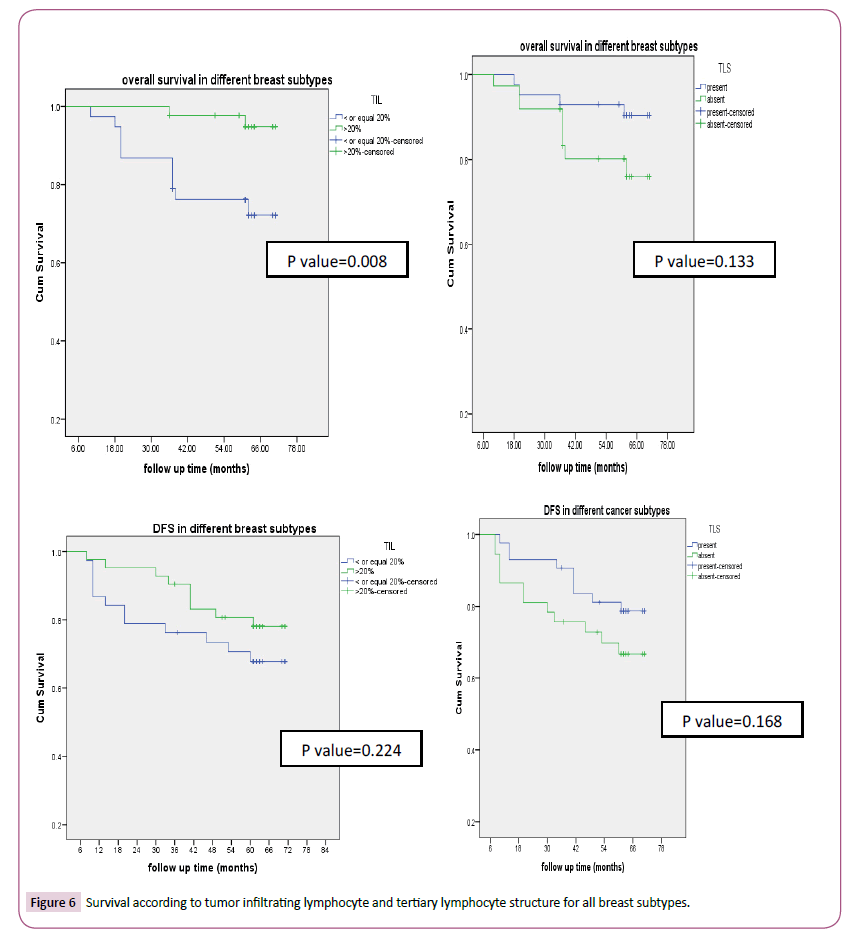
Figure 6:Survival according to tumor infiltrating lymphocyte and tertiary lymphocyte structure for all breast subtypes.
| Clinicopathological characteristics |
TIL<20% |
TIL>20% |
p-value |
| N |
% |
N |
% |
|
| Age |
| < 55 |
9 |
23.70% |
32 |
76.20% |
0.000* |
| >55 |
29 |
67.30% |
10 |
23.80% |
-- |
| Menopausal |
3 |
-- |
-- |
-- |
-- |
| premenopausal |
6 |
15.80% |
26 |
61.90% |
0.000* |
| postmenopausal |
30 |
78.90% |
8 |
19% |
-- |
| NOS |
2 |
5.30% |
8 |
19% |
-- |
| Sex |
| Female |
36 |
94.70% |
34 |
81% |
0.062 |
| Male |
2 |
5.30% |
8 |
19 |
|
| Performance status |
|    0-1 |
28 |
73.70% |
24 |
57.10% |
0.121 |
| 2 |
10 |
26.30% |
1 |
42.90% |
|
| Histology |
| IDC |
36 |
94.70% |
30 |
71.40% |
0.006* |
| ILC |
2 |
5.30% |
12 |
28.60% |
|
| Stage |
|
|
|
|
|
| IIb |
2 |
5.30% |
4 |
9.50% |
|
| IIIa |
24 |
63.20% |
14 |
33.30% |
|
| IIIb |
10 |
26.30% |
22 |
52.40% |
0.055 |
| IIIc |
2 |
5.30% |
2 |
4.80% |
|
| Type of surgery |
| MRM |
28 |
73.70% |
6 |
14.30% |
0.000* |
| BCS |
10 |
26.30% |
36 |
85.70% |
|
| Ki 67 |
| <  14% |
17 |
44.70% |
27 |
64.30% |
0.079 |
| >14% |
21 |
55.30% |
15 |
35.70% |
|
| LVSI |
| present |
22 |
57.90% |
28 |
66.70% |
0.418 |
| absent |
16 |
42.10% |
14 |
33.30% |
|
| Intrinsic subtype |
| Her 2 enriched |
3 |
7.90% |
11 |
26.20% |
0.032* |
| Non her2 enriched |
35 |
92.10% |
31 |
73.80% |
|
| Intrinsic subtype |
| TNBC |
4 |
10.50% |
20 |
47.60% |
0.000* |
| Non TNBC |
34 |
89.50% |
22 |
52.40% |
|
| Intrinsic subtype |
| HRBC |
32 |
84.20% |
10 |
23.80% |
0.000* |
| Non HRBC |
6 |
15.80% |
32 |
76.20% |
|
| Response |
| Pathological CR |
11 |
28.90% |
22 |
52.40% |
0.033* |
| Non pCR |
27 |
71.10% |
20 |
47.60% |
|
Table 1: Correlation between different clinicopathological and tumor infiltrating lymphocytes in different breast cancer intrinsic subtypes.
| Response |
Subtypes |
p-value |
| luminal A |
luminal B |
her2enriched |
TNBC |
| N |
% |
N |
% |
N |
% |
N |
% |
| pCR |
4 |
16.7% |
8 |
44.4% |
8 |
57.1% |
13 |
54.2% |
0.027 |
| Non pCR |
20 |
83.3% |
10 |
55.6% |
6 |
42.9% |
11 |
45.8% |
|
Table 2a: Pathological complete response.
| Tertiary lymphocyte structure |
Response |
Total |
p-value |
| pCR |
Non pCR |
| Present |
Count |
27 |
16 |
43 |
0.001* |
| % within TLS |
62.8% |
37.2% |
100.0% |
| % within response |
81.8% |
34.0% |
53.8% |
| % of Total |
33.8% |
20.0% |
53.8% |
| Absent |
Count |
6 |
31 |
37 |
| % within TLS |
16.2% |
83.8% |
100.0% |
| % within response |
18.2% |
66.0% |
46.3% |
| % of Total |
7.5% |
38.8% |
46.3% |
| Total |
Count |
33 |
47 |
80 |
| % within TLS |
41.3% |
58.8% |
100.0% |
| % within response |
100.0% |
100.0% |
100.0% |
| % of Total |
41.3% |
58.8% |
100.0% |
Table 2b: Breast cancer intrinsic subtypes and TLS.
| Parameters |
Univariate analysis |
Multivariate analysis |
| Sig |
Sig |
| All breast subtypes |
| Menopause |
0.153 |
0.08 |
| Histology |
0.08 |
0.015* |
| PS |
0.151 |
0.012* |
| Stage |
0.191 |
0.16 |
| Grade |
0.834 |
0.402 |
| LVSI |
0.16 |
0.576 |
| ki67per |
0.121 |
0.355 |
| Surgery |
0.263 |
0.37 |
| TIL |
0.501 |
0.915 |
| TLS |
0.834 |
0.359 |
| Pathological response |
| TNBC |
0.589 |
0.116 |
| Menopause |
0.902 |
0.397 |
| Histology |
0.098 |
0.045 |
| PS |
0.359 |
0.712 |
| Stage |
0.635 |
0.723 |
| Grade |
0.596 |
0.799 |
| LVSI |
0.682 |
0.726 |
| ki67per |
0.49 |
0.89 |
| Surgery |
0.829 |
0.639 |
| TIL |
0.007* |
0.002* |
| TLS |
0.046 |
0.004* |
| Pathological CR |
0.22 |
0.397 |
| HRBC Menopause |
0.221 |
0.752 |
| Histology |
0.865 |
0.939 |
| PS |
0.423 |
0.232 |
| Stage |
0.71 |
0;606 |
| grade |
0.245 |
0.82 |
| LVSI |
0.306 |
0.019* |
| Ki67 |
0.906 |
0.176 |
| Surgery |
0.186 |
0.154 |
| TIL |
0.802 |
0.915 |
| TLS |
0.142 |
0.793 |
| Pathological compete response |
| Her2 enriched subtype |
0.202 |
0.904 |
| Menopause |
0.306 |
- |
| Histology |
0.617 |
0.829 |
| PS |
0.517 |
0.897 |
| Stage |
0.284 |
0.817 |
| grade |
0.699 |
0.983 |
| LVSI |
0.862 |
0.526 |
| Ki67 |
0.1 |
0.671 |
| Surgery |
0.063 |
- |
| TIL |
0.014* |
0.002* |
| TLS |
0.004* |
0.003* |
| Pathological CR |
0.354 |
0.524 |
Table 3: Univariate and multivariate analysis with disease free survival in relation to different breast subtypes.
Discussion
The emerging role of new cancer immunotherapeutic drugs highlights the importance of tumor immune response. The detailed characteristics of tumor immune environment in breast cancer will help to identify patients who will benefit from these new drugs. Different immune response interacts to produce immunological parameters including tumor infiltrating lymphocytes which are considered predictors of response to neoadjuvant chemotherapy in breast cancer and tumor lymphocyte structure are considered the site of active immunological response [21]. TIL were found to be high in HER2 and TNBC and correlated with better response to neoadjuvant chemotherapy [6-8,20-24]. In the present study, we found 47.6%, 26.2% of our TNBC and HER2 enriched patients had TIL more than other breast subtypes, which is similar to that reported by Stanton et al. [25].
In this study we noted that high tumor infiltrate are associated with formation of tertiary lymphoid structure composed of B follicle with germinal center nearby or surrounded by T cell zone similar to secondary lymphoid follicles [26]. In this study, TLS were detected in 53.7% of the tumors in whole breast groups, 83.7% in triple negative subgroup which is lower than lee et al. who reported that TLS were 96.7% because they scored it depending on H&E which makes the differentiation between TLS and lymphoid aggregates very difficult. Also, in our study we use dual CD3/CD20 IHC to identify the B cell follicles and T cell zone [27].
Again, we reported a high TLS (71.4%) in HER2 enriched which is similar to that reported by Liu et al. 12]. One of the main problems of chemotherapeutic agents is immune escape of cancer cell [28]. The present study included patients treated with NAC (AC followed by paclitaxel with/without trastuzumab). Paclitaxel improve cytotoxic and regulatory T-lymphocytes sensitivity [29]. Immunogenic death is caused by cyclophosphamide and anthracycline [30].
Tipple negative and HER2 enriched breast subtypes are associated with high pathological CR than other breast subtypes (p=0.027) as reported by Prat et al. [31,32]. Higher pathological CR was reported with high tumor infiltrating lymphocytes (0.027) and positive lymphoid structure (p=0.001) as reported by other authors [20-23,33]. Increased number of tumor infiltrating lymphocytes and tertiary lymphoid structure are associated with longer OS and DFS for TNBC and HER2-positive breast cancer [34]. In univariate, longer DFS are associated with high tumor infiltrating lymphocytes and positive lymphoid structure in both TNBC and her 2 enriched and is maintained in multivariate analysis [27].
Conclusion
In conclusion, for predicting treatment response in patients with TNBC and HER2 enriched subtype, TILs and TLS may be prognostic and predictive marker but this is not true for hormone receptorpositive subtypes of breast cancer. Limitation of our study: More studies are important to evaluate the role of these immune markers in tumor relapse. But as shown in our study the number of recurrences is very low so larger trials with large number of patients are needed to confirm this conclusion.
Ethics Approval and Consent to Participate
• Written consent was taken from patients and hospital approval of university hospital.
• Consent for publication from all authors and university hospital.
• Data and material are available.
Conflicts of Interest
Authors have no conflicts of interest to disclose.
Funding
Funding by authors, no support
Author’s Contributions
Author 1: The idea of research, collection of the data and analysing.
Author 2: Pathological interpretation.
Author 3: Discussion and comparing with others.
Acknowledgements
Thanks to staff of Department of Oncology.
Author’s information refers to Lamiss Mohamed or Lamiss Sad at Pubmed.
• Prognostic and predictive role of ERCC1 protein expression in locally advanced stage III non-small cell lung cancer. Medical Oncology in 2014.
• Non-methylated MGMT as predictive factor in newly diagnosed glioblastoma multiforme treated with bevacizumab concurrent with radiotherapy followed by adjuvant bevacizumab plus irinotecan versus temozolomide concurrent with radiotherapy followed by adjuvant temozolomide in June 2018.
• Genetic variations of selected genes using target deep sequencing in colorectal cancer patients Oct 2017, Journal of Cancer Science and Therapy.
23657
References
- Akram M, Iqbal M, Daniyal M, Ullah Khan A (2017) Awareness and current knowledge of breast cancer. Biol Res 50: 33.
- Janet MG, Sharima R, Connie E, Jeanne R (2017) State of the evidence 2017: an update on the connection between breast cancer and the environment Environ Health 16: 94.
- Goetz JG (2012) Tumor microenvironment indoctrination: An emerging hallmark of cancer. Cell Adh Migr 6: 190-192.
- Aquino MT, Malhotra A, Mishra MK, Shanker A (2015) Challenges and future perspectives of T cell immunotherapy in cancer. Immunol Lett 166: 117-133.
- Yu X, Zhang Z, Wang Z, Wu P, Qiu F, et al. (2016) Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: A systematic review and meta-analysis. Clin Transl Oncol 18: 497-506.
- Wein L, Savas P, Luen SJ, Virassamy B, Salgado R, et al. (2017) Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: Current and future directions. Front Oncol 7: 156.
- Tian T, Ruan M, Yang W, Shui R (2016) Evaluation of the prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers. Oncotarget 7: 44395-44405.
- Pruneri G, Gray KP, Vingiani A, Viale G, Curigliano G, et al. (2016) Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat 158: 323-331.
- Hiraoka N, Ino Y, Yamazaki-Itoh R (2000) Tertiary lymphoid organs in cancer tissues. Front Immunol 7: 244.
- Liu S, Chen B, Burugu S, Leung S, Gao D, et al. (2017) Role of cytotoxic tumor-infiltrating lymphocytes in predicting outcomes in metastatic HER2-positive breast cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol 3: e172085.
- Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, et al. (2012) 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? 2: 765.
- Liu X, Tsang JYS, Hlaing T, Hu J, Ni YB, et al. (2017) Distinct tertiary lymphoid structure associations and their prognostic relevance in HER2 positive and negative breast cancers. Oncologist 22: 1316-1324.
- Lee HJ, Kim JY, Park IA, Song IH, Yu JH, et al. (2015) Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol 144: 278-288.
- Jang N, Kwon HJ, Park MH, Kang SH, Bae YK (2018) Prognostic value of tumor-infiltrating lymphocyte density assessed using a standardized method based on molecular subtypes and adjuvant chemotherapy in invasive breast cancer. Ann Surg Oncol p: 12.
- Song IH, Heo SH, Bang WS, Park HS, Park IA, et al. (2017) Predictive value of tertiary lymphoid structures assessed by high endothelial venule Counts in the neoadjuvant setting of triple-negative breast cancer. Cancer Res Treat 49: 399-407.
- Morita M, Yamaguchi R, Tanaka M, Tse GM, Yamaguchi M, et al. (2016) Two progressive pathways of microinvasive carcinoma: low-grade luminal pathway and high-grade HER2 pathway based on high tumour-infiltrating lymphocytes. J Clin Pathol 69: 890-898.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline. Eur J Cancer 45: 228-247.
- Kaplan EL, Meier P (1958) Non-parametric estimation from incomplete observations. J Ame Statist Assn 53: 457-481.
- Cox DR (1972) Regression models and life-tables. JR Stat Soc B 34: 187-220.
- Barnes TA, Amir E (2017) HYPE or HOPE: The prognostic value of infiltrating immune cells in cancer. Br J Cancer 117: 451-460.
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, et al. (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32: 2959-2966.
- Denkert C, Minckwitz G, Brase JC, Sinn BV, Gade S, et al. (2015) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 33: 983-991.
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, et al. (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26: 259-271.
- Stanton SE, Adams S, Disis ML (2016) Variation in the incidence and magnitude of tumor infiltrating lymphocytes in breast cancer subtypes: A systemic review. JAMA ONCOL 2: 1354-1360.
- Solinas C, Garaud S, De Silva P, Boisson A, Van den EG, et al. (2017) Immune checkpoint molecules on tumor-infiltrating lymphocytes and their association with tertiary lymphoid structures in human breast cancer. Front Immunol 8: 1412.
- Lee HJ, Park IA, Song IH, Shin SJ, Kim JY, et al. (2015) Tertiary lymphoid structures: prognostic significance and relationship with tumor-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol 16: 203089.
- Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, et al. (2018) Prediction of treatment response to neoadjuvant chemotherapy in breast cancer by subtype using tumor-infiltrating lymphocytes. Anticancer Res 38: 2311-2321.
- Pandya PH, Murray ME, Pollok KE, Renbarger JL (2016) The Immune System in Cancer Pathogenesis: Potential Therapeutic Approaches. J Immunol Res 16: 4273943.
- Liu Y, Cao X (2016) Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med 94: 509-522.
- Prat A, Fan C, FernÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂández A, Hoadley KA, Martinello R, et al. (2015) Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med 13: 303.
- Prat A, Pascual T, Adamo B (2017) Intrinsic molecular subtypes of HER2+ breast cancer. Oncotarget 43: 73362-3.
- Song IH, Heo SH, Bang WS, Park HS, Park IA, et al. (2017) Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast cancer. Cancer Res Treat 49: 399-407.
- Krishnamurti U, Wetherilt CS, Yang J, Peng L, Li X (2017) Tumor-infiltrating lymphocytes are significantly associated with better overall survival and disease-free survival in triple-negative but not estrogen receptor-positive breast cancers. Hum Pathol 64: 7-12.











