Background: In most hospitals the preoperative decision to determine blood type and screen (TS) is based on historical rules. With the use of hospital information systems, the incidence of perioperative blood transfusions could be used to guide the decision to TS or not. Recently, systematic criteria for type and screen, based on the procedure’s probability of transfusion were introduced by Dexter et al. We used this algorithm for retrospective analyses on our perioperative data in our Anesthesia Information Management System (AIMS) system and evaluated the effects on frequency and costs in the TS policy.
Methods: Data of 20132 patients who underwent a surgical procedure, recorded in our AIMS were compiled. Preoperative data were added to the data set. For each procedure the median estimated blood loss (mEBL), the minimal blood loss threshold and the incidence of blood transfusion were analyzed. These data were used to guide future decisions for type and screen. A confidence interval of >95% with a transfusion incidence of < 5.0% was taken as safety limit to avoid unnecessary type and screen.
Results: A mEBL of 400 ml showed a transfusion incidence of >5% with a confidence interval of >95%. A costs analysis estimated a potential cost reduction of at least 97.3 % or € 150.000 a year, when looking at TS unnecessary performed over the past 2 years.
Conclusions: We determined in this study the minimal EBL to be more than 400 ml to advocate TS in our population. The regime is easy to implement with the use of an AIMS system and will most likely lead to a reduction in costs.
Keywords
Preoperative, Transfusion, Cost reduction, Blood transfusion
Introduction
Present type and screen (TS) policy in many hospitals is based on fixed protocols in the pre-operative assessment clinic. Patients determined to be at risk for blood transfusion, will have their status of TS assessed by the transfusion laboratory. The TS process consists of a blood type for ABO group and Rhesus (Rh) type and a screening for significant red blood cell directed antibodies [1,2]. The subsequent TS procedure searches for irregular antibodies, which can be found in approximately 0.8% of the blood donor population [3]. Ethical and economic considerations warrant a closer look at our clinical pathways and expenses [4,5]. Based on previous data, TS is not necessary in patients with a low transfusion risk. Studies have shown a cost reduction of $10 to $78 per TS procedure [2,4,6-8] when using a stricter TS policy. Unfortunately, most studies addressing a change in TS policies look only at one type of surgery [7-9], or propose regimes that are complex and still include the acquisition of blood samples from patients [4]. Recently, Dexter et al. proposed a simple regime to guide pre-operative TS testing [1]. Those authors used a calculated median estimated blood (medEBL) loss, which was derived from procedures performed in the past. The medEBL together with a selected threshold for minimal estimated blood loss (minEBL) was used to propose a regime for TS [1]. The minEBL was selected by taking the smallest medEBL for which the chance of erythrocyte transfusion was less than 5%. If the surgical procedure had a chance of more than 5 % that erythrocyte transfusion is needed, TS was advised.
The aim of our study was to assess whether the proposed regime for TS from Dexter et al. [1] could be implemented in a Dutch general teaching hospital where the majority (60%) of surgery consists of low complexity, high volume, ambulatory surgery and to calculate its theoretical impact on costs.
Our hypothesis was that the regime proposed by Dexter et al. [1] will be applicable in our hospital. In addition, we expect to find that a future implementation of this regime will be accompanied by a reduction in costs.
Methods
After our institutional review board gave approval (no.13.034, September 26th 2013) and waived us of the obligation to acquire informed consent the study was performed at the Diakonessenhuis Utrecht, a general teaching hospital in the Netherlands with approximately 600 beds. Except for transplantation, intracranial and cardiac surgery, all surgical procedures are performed. A major part of the surgical procedures (60%) is low complex, high volume, day case surgery.
The study has been checked and written, were possible, in accordance with the Strobe-statement guidelines on cohort studies. From March 2011 to March 2013 peri-operative data were compiled from all elective surgical procedures, using the Anesthesia Information Management System (AIMS) (Innovian VF6.2, Draeger, Lubeck). Patients under the age of 18 years were excluded from this study, in accordance with the Dexter et al. protocol [1]. The collected information included the type of procedure, as well as the registered blood loss and the peri-operative transfusions given up to the time of leaving the recovery room. Information from the pre-operative information system (Synopsis 1.2.18a, Clinical Information Systems, Glasgow) was used to add laboratory data and ASA physical status to this data set.
After extracting the data from the AIMS and the preoperative assessment system using Crystal Reports version XI, the datasets were linked using Microsoft ACCESS 2003 and the totals for blood loss and RBC transfusions were aggregated using SQL queries. The set was then statistically analyzed in Microsoft EXCEL (14.3), including the use of the function BETAINV. In order to exclude administrative errors and blood transfusions not related to surgery, cases with abnormal high blood transfusion were analyzed separately. If the transfusion was a pre-operative decision due to comorbidities, the case was excluded. If the decision for blood transfusion was made during surgery the case was included but was analyzed to verify that the amount of transfused RBC units in AIMS matched with the data provided by our transfusion laboratory.
To evaluate the economic implications of the new TS policy, we compared data from our laboratory on TS performed versus the need for TS when this regime would have been implemented in the period from March 2011 to March 2013.
Table 1 describes the method by Dexter et al. used to determine for which procedures TS is not indicated [1]. The medEBL was calculated for all scheduled procedures. In addition, the minEBL threshold was determined as the lowest medEBL with a 95% confidence interval for which the chance on erythrocyte transfusion was more than 5%. For all procedures with at least 19 cases and with a medEBL below the minEBL threshold, the 95% lower confidence limit was calculated. If the need for blood transfusion is less than 5%: no TS is indicated. For instance, the transfusion incidence (Ti=9) for hemi-colectomies (n=103) was 8.7% with a median EBL of <500. The lower 95% confidence limit is 0.043, which is below 0.05. This procedure is thus just below the threshold of requiring TS.
| Procedures performed in the past with N ≥ 19 |
| Step 1 |
Calculate median estimated blood loss (EBL)
(Absent data regarded as not being larger than median) |
| Step 2 |
Select minimal EBL threshold
Smallest median EBL where the lower 95% confidence limit for erythrocyte transfusion was >5%
Formula: 1-BETAINV(1-0,05,n-m+1,m) |
| Step 3 |
If median EBL→threshold à calculate 95% confidence limit for m. |
| Conclusion |
If value step 3→5% à no TS indicated |
| EBL= estimated blood loss, N= sample size, m= transfusion incidence |
Table 1: Regime proposed by Dexter et al. [1].
Results
See Figure 1 for complete inclusion.
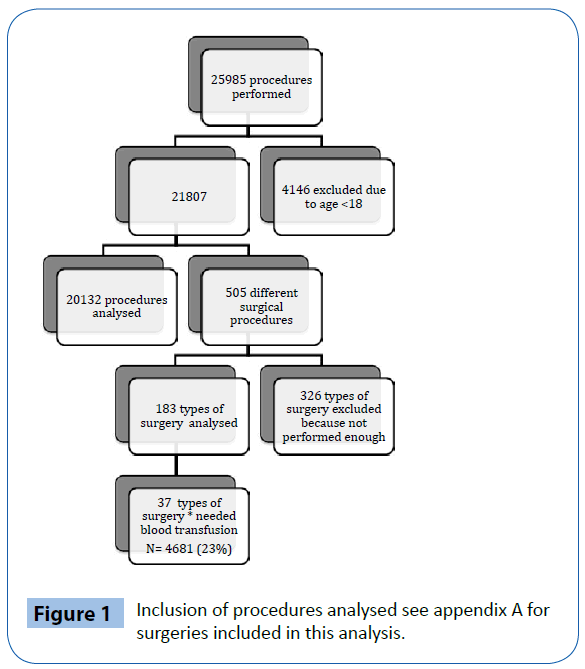
Figure 1: Inclusion of procedures analysed see appendix A for surgeries included in this analysis.
EBL recorded in our AIMS was 10% (Table 2). The overall incidence of blood transfusion (at least one unit of RBC) was 0.66% (144 out of 21807). Twenty-one patients received autologous blood but did not need donor blood. One operation was logged with an abnormally high need for RBC transfusion. This specific case has been excluded due to the fact that there was no intra-operative blood loss or transfusion recorded. The smallest medEBL for which the lower confidence limit is more than 5% is 500 ml. At 400 ml the limit can be confirmed to be below 5%. The threshold for minEBL was defined to be ≤ 400 ml. Blood loss is measured in quantities of 100ml (Table 3).
| |
(N) |
(%) |
| Total |
21.807 |
100.0% |
| Male |
9.957 |
45.6% |
| Female |
11.850 |
54.3% |
| ASA 3 en 4 |
1.865 |
8.6% |
| Blood loss recorded |
2.181 |
10.0% |
| Transfusion |
144 |
0.66% |
Table 2: Demographics.
| Median EBL |
Amount of cases with blood loss
(N) |
Transfusion incidence
(%) |
Lower 95% CL |
| 900 |
46 |
43.5 |
0.310 |
| 700 |
76 |
35.5 |
0.264 |
| 500 |
69 |
15.9 |
0.092 |
| 400 |
62 |
10.3 |
0.036 |
| 300 |
510 |
0.6 |
0.002 |
| 200 |
634 |
3.6 |
0.018 |
| 100 |
150 |
3.0 |
0.008 |
Table 3: Table used for determining the ‘minimal EBL’ threshold.
Cost analyses
The process of TS requires two samples for correct identification. In both samples blood type will be matched (€ 3.52) and then an antigen screening (€ 42.27) will be done as well. Total costs for one TS in an uneventful procedure will be (2 × € 3,52) + € 42,27= € 49,31. We have recorded 6461 TS in 21807 procedures. 6005 applied to procedures that we evaluated according to Dexter’s regime. Of these, 165 TS would have been performed based on Dexter’s algorithm for necessity. 2351 TS were assessed for procedures that never needed blood transfusions and 3489 TS were assessed for procedures that did not have a >5% chance for blood transfusion within a 95% confidence interval. According to the algorithm proposed by Dexter, the reduction on TS would have been approximately 11 TS/ day or € 150.000 euro per year. Which accounts to a cost reduction of 97.3% of our expenses on TS.
Discussion
We found that the TS regime proposed by Dexter et al. [1] applied in a hospital with low complexity, high volume, day case surgery could lead to a significant cost reduction in comparison with our conventional TS regime. This is remarkable especially in view of our low transfusion incidence of 8.5% in comparison with 41.8% by Dexter et al. Frank et al. [1,10] showed a similar cost reduction, but employing a different regime to optimize TS orders, looking not only at TS but also at cross matching. Cheng et al. [11] looked more closely at transfusion history to determine the need for TS, but these authors did not include blood loss from prior surgeries as a factor to guide TS indication.
There are some limitations to our study. Due to the great variability for procedures performed at our hospital, some procedures were not frequently enough performed to be included. Reich et al. stated that cases with extremely high or unexpectedly low blood loss where clinically implausible and therefore would bias the clinical use of the regime proposed [12]. In our case there has been only one case with an exceptionally high blood loss, and transfusion ratio, which we were able to track down and excluded. All other cases had a plausible transfusion incidence and seemed in accordance with expectations. We showed a higher incidence of no blood loss reports in our study (90%) in comparison to other studies (53-62%) [1,10]. The high volume of low complexity procedures performed at our hospital can explain this. Most of these surgeries have less than 100 ml blood loss. Since we measure blood loss in 100 ml units, blood loss less than 100 ml was not recorded. In addition Reich et al. stated that it might be wise to exclude patients that are anemic prior to surgery. In accordance with Dexter we did not exclude these patients, because these patients are part of the population that needs to be screened for an operation. In our study population 33% of blood transfusions where given to patients with blood loss of less than 300 ml. Therefore, it is highly likely that these patients were anemic prior to surgery. Dexter et al. did not mention exclusion of anemic patients. To us, by considering all anemic patients for TS, our proposed regimen would be on the safe side, since the threshold for TS would be lower when anemic patients would have been excluded. In the future, a prospective study might show an even more profound reduction in costs when excluding anemic patients. The cost reduction estimate we have shown is probably still on the conservative side. We did not calculate additional tests that need to be done in case of irregular antibodies. The costs of testing these antibodies range from € 105.68 to € 211.35.
Based on our results, we estimate that implementing the Dexter regime in hospitals primarily performing low complex, high volume surgery may lead to a significant cost reduction.
7724
References
- Dexter F, Ledolter J, Davis E, Witkowski TA, Herman JH, et al. (2012) Systematic criteria for type and screen based on procedure's probability of erythrocyte transfusion. Anesthesiology 116: 768-778.
- Friedberg RC, Jones BA, Walsh MK (2003) Type and screen completion for scheduled surgical procedures. A College of American Pathologists Q-Probes study of 894type and screen tests in 108 institutions. Arch Pathol Lab Med 127: 533-540.
- Tormey CA, Fisk J, Stack G (2008) Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion 48: 2069-2076.
- Van Klei WA, Moons KG, Leyssius AT, Knape JT, Rutten CL, et al. (2001) A reduction in type and screen: preoperative prediction of RBC transfusions in surgery procedures with intermediate transfusion risks. Br J Anaesth 87: 250-257.
- Martin J, Cheng D (2013) Role of the anesthesiologist in the wider governance of healthcare and health economics. Can J Anaesth 60: 918-928.
- Frank SR, Masear C (2013) Optimizing preoperative blood ordering with data acquired from an anesthesia information management system. Anesthesiology 118: 1286-1297.
- Prichard RS, O'Keefe M, McLaughlin R, Malone C, Sweeney KJ, et al. (2011) A study of pre-operative type and screen in breast surgery: improved efficiency and cost saving. Ir J Med Sci 180: 513-516.
- Ransom SB, McNeeleySG, Malone JM (1996) A cost-effectiveness evaluation of preoperative type-and-screen testing for vaginal hysterectomy. Am J ObstetGynecol 175: 1201-1203.
- Fenner M, Kessler P, Holst S, Nkenke E, Neukam FW, et al. (2009) Blood transfusion in bimaxillaryorthognathic operations: need for testing of type and screen. Br J Oral MaxillofacSurg 47: 612-615.
- Frank SM, Rothschild JA, Masear CG, Rivers RJ, Merritt WT, et al. (2013) Optimizing preoperative blood ordering with data acquired from an anesthesia information management system. Anesthesiology 118: 1286-1297.
- Cheng CK, Trethewey D, Brousseau P, Sadek I (2008) Creation of a maximum surgical blood ordering schedule via novel low-overhead database method. Transfusion 48: 2268-2269.
- Reich DL, Pessin MS (2012) Rational preoperative blood type and screen testing criteria. Anesthesiology 116: 749-750.







