| HPV, ASC-US, Genotype, Gynaecological monitoring, Cervical cancer |
Abbreviations
|
| HPV: Human Papillomavirus; ASC-US: Atypical Squamous Cells of Undetermined Significance; CIN: Cervical Intraepithelial Neoplasia; DNA: Desoxyribonucleic Acid |
Background
|
| Persistent infection by some types of human papillomavirus (HPV) is recognized as a causal and necessary factor for the development of cervical cancer and a body of epidemiological data shows that these viruses are responsible for 99.7% of this type of neoplasia [1-5]. To date, more than 200 genotypes have been identified of which approximately 40 can infect the anogenital tract. HPV genotypes are classified into “high-risk” HPV (HR HPV), “probable high-risk” HPV and “low-risk” HPV (LR HPV) [2,6,7]. The HR group includes 15 HPV genotypes (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, 82) that are proven to be associated with cervical cancer, while the LR group includes 12 HPV genotypes (types 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, CP6108) lack malignant potential and are responsible for the development of benign HPV-associated lesions such as condylomata [8,9]. The HPV genotypes 26, 53, 66, 83 have been classified as probable HR as their causative role in the development of cervical cancer remains controversial [10-12]. |
| It is well recognized that the carcinogenic potential of HR HPV genotypes is highly heterogeneous within the group [13]. Hence, HPV16 is considered the most aggressive HR HPV, as it shows an increased probability of persistence as compared to other HR genotypes and this persistence is associated with the development and progression of precancerous Cervical Intraepithelial Neoplasia (CIN). HPV16 is the most prevalent genotype detected both in cervical cancer (at least 50% of cases) and in CIN. |
| US guidelines recommend the systematic screening for HPV16 and HPV18 genotypes in HR HPV positive women over 30 years of age displaying a cytologically normal Pap smear to determine whether immediate colposcopy is needed [14]. In France, HPV detection is recommended only for patients with Atypical Squamous Cells of Undetermined Significance (ASC-US) on cytology and the HPV genotype is not considered as a parameter for monitoring during patient follow-up. It has been demonstrated that HPV16/18 genotyping can eliminate the need for colposcopy in 40% to 60% of cases [15] and genotype identification could further decrease the number of unnecessary colposcopies performed, principally by demonstrating the absence of viral persistence. Moreover, new screening tests are emerging to provide some degree of genotyping with the aim of improving risk stratification among HPV-positive subjects and to allow the individualisation of followup protocols. However, the limited data on the cervical cancer risk associated with HR HPV genotypes other than HPV16 and HPV18 limits the usefulness of HPV genotyping as risk assessment for patients infected with these genotypes. Hence, large studies with follow-up are required to assess the carcinogenic potential of individual HPV genotypes and to justify systematic HPV genotyping in clinical management. |
| The primary aim of our retrospective study was to analyse the prevalence of HPV genotypes among women diagnosed with ASCUS Pap smears who were followed up in the University Hospital of Nice. The secondary aims were to determine, based on follow-up data: firstly, whether infection with a specific HPV genotype was predictive of the presence or absence of a precancerous lesion, and secondly whether incorporation of HPV genotyping tests into screening programs can improve the management of women with ASC-US cytology. |
Methods
|
|
Patients
|
| The study population included 613 cervical smears with ASC-US from women who attended at the University Hospital of Nice between 2008 and 2012. The cervical samples were collected using a cytobrush and were placed in the PreservCyt transport medium (Cytyc Corp, Marlborough, MA). Liquid-based cytology (ThinPrep) was performed and material in PreservCyt medium was then transferred from the Pathology Laboratory to the Laboratory of Virology for HPV genotyping. |
|
HPV genotyping on Papillo Check DNA microarray
|
| HPV genotyping was performed using the PapilloCheck® HPV DNA Chip (Greiner Bio-one GmbH) according to the manufacturer’s instructions. After extraction, a 350-bp fragment of the E1 open reading frame, an external PCR control, and a DNA fragment of the human ADAT1 gene were amplified with specific primers in the presence of 5 μL of purified DNA sample. Slides were scanned and analysed with CheckReport software (GBO). PapilloCheck microarray contains 28 probes, including 24 HPV probes and four control probes (orientation, hybridization, external PCR, and ADAT1), each in five replicate spots. This DNA-array system detects and genotypes 24 different HPV genotypes: 18 HR HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73, 82) and 6 LR HPV (6, 11, 40, 42, 43, 44). |
|
Patient follow-up
|
| Comprehensive data regarding age, HPV genotyping result and clinical monitoring were collected for HPV-positive women. For each patient followed, Pap smear cytology results and the result of any biopsies were documented. The age of the HPV-negative women was recorded as part of an investigation into the variation of HPV prevalence with age. |
|
Statistical analysis
|
| All statistical analyses were performed with SPSS version 11.0 and the significance level software was set at 0.05. Data are presented as percentages and comparisons of qualitative variables performed by Chi-square or Pearson correlation coefficient when appropriate or a Fisher’s exact test. |
| The Odds Ratios with 95% confidence intervals were calculated using binomial logistic regression. |
Results
|
| From 2008-2012, 613 women with ASC-US attended at the University Hospital of Nice. The age of the participants ranged from 16 to 82 years (median 34.7 years). The overall prevalence of HPV infection in this study was 51% of the 613 smears, with 313 positive for at least one HPV detected by PapilloCheck® DNA microarray (Table 1). We observed that the number of cases of multi-infection decreased with age, reflecting viral clearance (data not showed). |
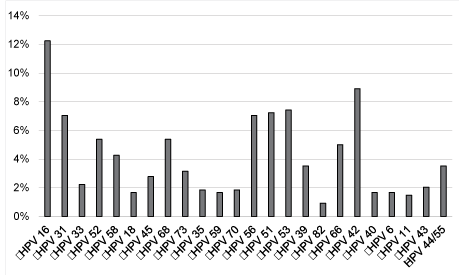
| The most frequent HR HPV were, in descending order, HPV 16 (12%), 31 (7%), 51 (7%), 53 (7%), 56 (7%), 52 (5%), 66 (5%), 68 (5%), 58 (4%) and HPV 42 was the most prevalent LR HPV in our cohort (Figure 2). |
| We next analysed patients follow-up to assess the correlation between infection with specific HR HPV genotypes and the presence of pre-cancerous lesions in patients with an initial ASCUS cytology (Table 1). For each patient followed, we documented Pap smear cytology results and the histological diagnosis resulting from any biopsies performed. The high-grade lesions (CIN2 or 3) found at biopsy when monitoring was more frequent between 20-40 years, compare to 41-60 years. |
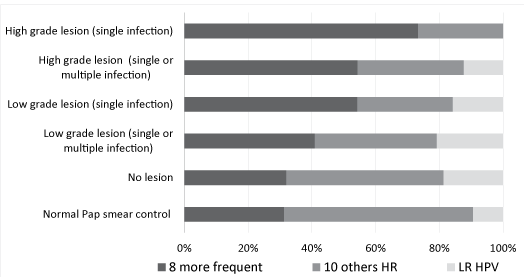
| Because the number of positives cases for each HR HPV genotype was relatively small, we regrouped the 8 most frequent genotypes (HPV 16, 18, 31, 33, 45, 52, 58, 68) found in invasive cervical cancer according to deSanjosé [16] and the French study EDITH [17] and the 10 other genotypes detected by the PapilloCheck DNA microarray (HPV 35, 39, 51, 53, 56, 59, 66, 70, 73, 82). We presented also infections by LR HPV (HPV 6, 11, 40, 42, 43, 44) detected with the PapilloCheck® HPV DNA Chip. Distribution of the 3 categories of HPV found in the initial Pap smear based on results obtained during the monitoring was compared. We calculated the relative frequencies of each HPV genotype category (category 1; 8 most frequent HR HPV found in invasive cervical cancer, category 2; 10 least frequent HR HPV found in invasive cervical cancer and category 3; LR HPV), according to lesion grade (Figure 3). The analysis of the results using logistic regression allowed us to conclude that the presence of one of the eight HPV genotypes of category 1 was associated with a 3.4-fold higher risk (95% CI 1.20-9.94) of discovering a CIN2 or 3 lesions at biopsy and that HPV16 mono- and poly-infections increased this risk by 7.1-fold (95% CI 2.10-24.52). Furthermore, we observed that HPV genotypes of category 2 were present in 59.4% of ASCUS smears of women who, during follow-up, had cytologically normal smears without treatment. Finally, we assessed lesion grade in relation to age of patients and observed no correlation between these two parameters (data not showed). |
Discussion
|
| Our study provides a comprehensive description of the profile of HR HPV infection in a cohort of women with ASC-US and evaluates the risk of discovering a malignant lesion based on the genotype present in the ASC-US Pap smear. This is the first investigation of the distribution of HPV genotypes in women with ASC-US cytology in the south of France. |
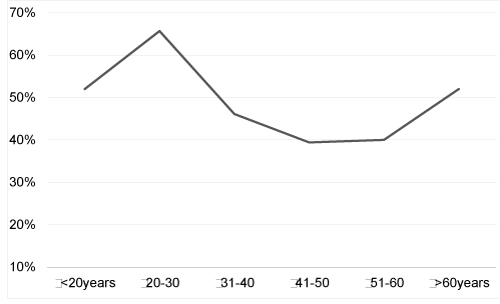
| Firstly, over one third (37%) of the cohort was less than 30 years old, probably because of selection bias reflecting that relatively young women came to see for the first time a gynecologist in our service. |
| We found that 51% of women having ASC-US cytology were HPV positive, in accordance with the prevalence of HPV infection found in the American ALTS study which was 50.7% (ASC-US LSIL Triage Study [18]). |
|
Age related prevalence
|
| Of interest, we observed a U-shaped curve of HPV prevalence by age group in our ASC-US cohort consistent with the prevalence curve observed in other countries including Italy [19], Hong Kong [20] and China [21] and in deSanjose’s worldwide metaanalysis [22]. However, our results are in contrast with some other international reviews [23] and with the HPV prevalence observed in several European countries like Denmark [24] or the Netherlands [25]. They are also inconsistent with the HPV prevalence seen in a recent French study [26]. The discrepancy observed between these various studies is well known [27] and is probably linked to variability in recruitment criteria. Indeed HPV prevalence analysis gives disparate results when conducted on patients diagnosed with low grade cytology or on the global population regardless of the cytological grade of Pap smears. |
| The second peak of increased HPV prevalence observed in women aged over 60 years can be explained in two ways. Firstly, impaired immune responses, resulting from hormonal changes at menopause, may alter HPV clearance or induce reactivation of clinically silent pre-existing HPV infections [22]. Furthermore, reduction of the size of the squamocolumnar junction, and replacement of the cervical mucosa by atrophic stratified squamous epithelium may promote HPV infections in older women [28]. However, if hormonal changes and immune senescence are the main mechanisms leading to this kind of agerelated HPV infection pattern, increased HPV prevalence should be consistently observed in post-menopausal women across the world. |
| A second interpretation is that the second peak of increased HPV prevalence may be related to changes in the sexual behaviour of women and their partners and variation in this second peak depends upon sexual customs around the world. Indeed an increased number of lifetime sexual partners and changes in sexual attitude are closely related to social changes and are likely to influence the transmission of HPV [22,29]. In this way worldwide variations in HPV prevalence as a function of age appear to reflect differences in sexual behaviour and sexual customs across geographical regions. |
|
HPV genotypes in our ASC-US cohort
|
| In our study, we observed the distribution of 18 HR HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73, 82) and 6 LR HPV (6, 11, 40, 42, 43, 44/55) in 313 patients with ASC-US cytology in Nice. The most prevalent HR HPV detected in our population was HPV 16 (12%) either as a single infection or as part of multiple infections, followed by HPV 53, HPV 51, HPV 56 and HPV 31 (7% each) according to a recent French study [30]. The large American ALTS study showed contrasting results with an HPV genotype prevalence of 14% for HPV 16, followed by HPV 52 (8%), HPV 51 (7%), HPV 18 (6%), HPV 31 (6%), HPV 59 (5%), HPV 56 (5%), HPV 58 (5%), HPV 53 (5%), and HPV 39 (5%). |
| We observed a low prevalence of HPV 18 (2%) probably due to a sampling bias. Indeed, the use of some kinds of cytobrushes might limit the recovery of endo-cervical cells, which are the preferential location for HPV 18 infections. |
| Comparison of HPV prevalence between studies is problematic owing to variability in recruitment criteria (global population or selection of patients with specific cytological grades) and technical discrepancies (use of dissimilar HPV genotyping kits exhibiting different thresholds of sensitivity for the detection of some HPV genotypes) [19]. |
|
Viral epidemiology based on patient monitoring result
|
| First of all, consistent with other studies, no correlation was seen between age of patients and lesion severity. These data highlight the need to reconsider patient screening and monitoring strategies currently based on age, as the assessment of sexual behaviour appears to be more relevant [31- 33]. |
| Next, we analysed the predictive value of HPV genotyping results for the presence of a malignant lesion by documenting the relative frequencies of HPV genotypes according to grade of lesion based on patient data, including biopsy results, collected during one year’s follow-up. |
| These preliminary data show that probably HPV genotyping may help clinicians to take the decision whether immediate colposcopy is needed or not in women with ASC-US cytology according to HPV genotype. Indeed, it appears that a woman without infection by HPV 16, 18, 31, 33, 45, 52, 58, 68 should be reconvened 6 months or 12 months later for a Pap smear control while a woman infected by one of these HPV should be referred to immediate colposcopy. |
Conclusions
|
| In summary our data define HPV 31, 33, 45, 52, 58 and 68 as highly oncogenic HPV genotypes, besides the prototypic oncogenic HPV genotypes 16 and 18 and also suggest that these HR HPV genotypes are associated with particular risks of malignant progression supporting the usefulness of HPV genotyping for the triage of HPV positive patients. Indeed HPV genotyping may probably assist the clinician in the management of patients with ASC-US Pap smear because the absence of one of the 8 should reassure the clinician and not lead the women to colposcopy. Conversely the presence of one of the 8 justifies closer followup and should lead the clinician to prescribe a colposcopy. |
| In order to prove that the management of patients with ASC-US cytology could be based on HPV genotype, a large international prospective study must be conducted. |
Acknowledgment
|
| This work was supported by institutional grants from Conseil Général 06 and CHU de Nice. |
| The English grammar of the manuscript was edited by Dr Michael Coutts, consultant gynaecological pathologist, Maidstone Hospital, Kent, UK. |
| The statistical analyses were performed by Dr Sebastien Gonfrier |
Tables at a glance
|
 |
| Table 1 |
|
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |
| |








