Case Report - (2025) Volume 16, Issue 6
Unusually long survival (over 33 years) observed in 61 high-grade glioma patients treated in phase II studies of Antineoplastons A10 and AS2-1
Stanislaw R. Burzynski1*,
Tomasz J. Janicki1,
Gregory S. Burzynski1,
Radoslaw J. Siwiec2 and
Samuel W. Beenken3
1The Burzynski Clinic, Houston, Texas, USA
2Lubgen Farma, Poland
3Oncology Writings, Calera, Alabama, USA
*Correspondence:
Stanislaw R. Burzynski, The Burzynski Clinic,
USA,
Tel: 713-335-5697,
Email:
Received: 11-Nov-2025, Manuscript No. ipjnn-25-15774;
Editor assigned: 13-Nov-2025, Pre QC No. P-15774;
Reviewed: 27-Nov-2025, QC No. Q-15774;
Revised: 04-Dec-2025, Manuscript No. R-15774;
Published:
11-Dec-2025
Abstract
Background: High-Grade Gliomas (HGGs) are among the most aggressive and deadly primary brain tumors. Standard treatment includes surgery, external beam radiation and temozolomide, which can cause marked toxicity. Despite intensive therapy, the prognosis remains poor.
Objective: To assess long-term outcomes and safety in non-Glioblastoma (non-GBM) HGG patients treated with Antineoplastons A10 and AS2-1 (ANP) at the Burzynski Clinic (BC) under phase II protocols.
Methods: Sixty-one non-GBM HGG patients received intravenous ANP. Eligibility required Karnofsky/Lansky Performance Scores (KPS/LPS) of at least 60 and a life expectancy of at least 2 months. ANP was administered via a subclavian catheter and automated pump. Maximum tolerated doses of A10 and AS2-1 were achieved. Outcomes included objective response, survival and toxicity
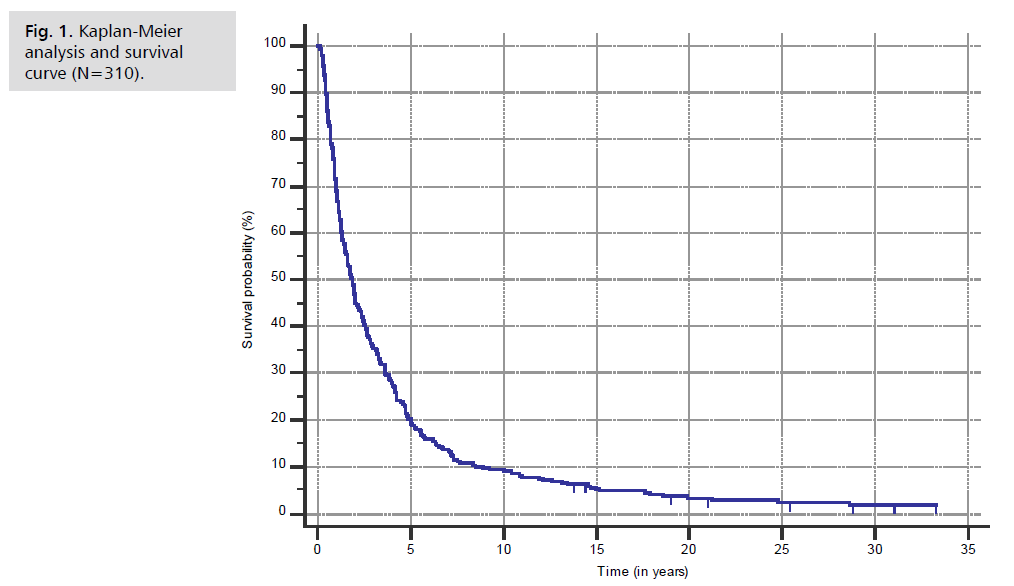
Results: As of October 2025, all 61 patients had survived for at least 5 years, with one patient surpassing 33 years. The criteria of a cure was met when 23 patients survived over 12 years. Ages ranged from 1.08 to 62.66 years (median 35.9). KPS/LPS scores ranged from 40 to 90 (median 60). Four patients experienced six Serious Adverse Events (SAE’s) possibly related to ANP (fever without infection, nausea, dizziness and three cases of somnolence); all recovered fully. In a Kaplan-Meier analysis of 310 non-GBM HGG patients treated at BC, median survival was 1.867 years.
Conclusions: ANP therapy shows significant potential for non-GBM HGG, with many long-term survivors and no observed long-term toxicity. This cohort represents the most extensive documented series of HGG survivors with unusually long-term survivals. Findings support ANP as a viable treatment option.
Keywords
Antineoplastons; Glioma; High-grade glioma; Long-term survival; Phase II studies
Introduction
Arising from glial progenitor cells, gliomas are a diverse group of primary tumors located within the Central Nervous System (CNS) [1]. Mainly affecting older adults, gliomas accounted for 22.9% of all newly diagnosed primary brain and other CNS tumors between 2017 and 2021 in the USA. Among these brain tumors, 14.0% of all tumors and 51.5% of all malignant tumors were Glioblastoma (GBM), a High-Grade Glioma (HGG). In recent years, GBM has caused the deaths of over 7,550 Americans annually [2].
The diagnosis of HGG involves clinical signs and symptoms, imaging studies, histological examination and molecular analysis of tumor tissue. The imaging study of choice is Magnetic Resonance Imaging (MRI) of the brain, which can be performed with or without gadolinium contrast. A brain MRI shows the anatomical relationship between the tumor and surrounding tissue, as well as tumor location, size and the extent of edema and necrosis.
Historically, brain tumor classification has relied on histological features. As a result, classification has been limited by diagnostic discrepancies, variable outcomes and different responses to therapy. However, recent advances in molecular profiling have enabled the integration of both morphological and molecular characteristics of brain tumors [3]. The 2021 World Health Organization (WHO) classification of CNS tumors introduced new types and subtypes often defined by their key molecular features [4]. Additionally, gliomas were categorized by age, with distinctions made between adult and pediatric types [5-7]. Adult-type diffuse HGG was subdivided based on two molecular markers: Isocitrate Dehydrogenase (IDH) and 1p/19q codeletion. Pediatric-type diffuse HGG was subdivided based on tumor location, histone mutations (including H3 K27-altered and H3 G34-mutant) and DNA methylation profiles [5].
Adult-type diffuse gliomas include 1) astrocytoma, IDH-mutant (WHO grades 2-4); 2) oligodendroglioma, IDH-mutant and 1p/19q-codeleted (WHO grades 2-3); and 3) GBM, IDH-wildtype (WHO grade 4). Pediatric-type diffuse high-grade gliomas included 1) diffuse midline glioma, H3 K27-altered (WHO grade 4); 2) diffuse hemispheric glioma, H3 G34-mutant (WHO grade 4); 3) diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype (WHO grade 4) and 4) infant-type hemispheric glioma [5].
HGGs are among the most aggressive and deadly types of brain tumors. They present with a wide range of clinical signs and symptoms that significantly reduce patients' Health-Related Quality of Life (HRQoL). These signs and symptoms can vary depending on tumor location and progression. However, common signs and symptoms include severe or chronic headaches, epileptic seizures, muscle weakness, numbness, nausea, vomiting, drowsiness, visual disturbances, concentration problems, memory loss and personality changes [8,9].
HGG is characterized by rapid growth and resistance to conventional therapies, which makes this tumor type a significant challenge in neuro-oncology. The treatment of HGG conventionally consists of a multimodal approach, which includes maximal safe Surgical Resection (SU), adjuvant Radiation Therapy (RT) and concurrent Chemotherapy (CH) with Temozolomide (TMZ) for six weeks, followed by TMZ for an additional six months [10, 11]. However, this approach is not curative. An available clinical trial is the preferred option [12]. The median Overall Survival (OS) for GBM patients receiving conventional multimodal treatment is approximately 13 months, with survival rates of 82% at six months, 55% at 12 months and 19% at 24 months [13]. Patients experience several side effects related to surgery and chemoradiotherapy, including ataxia, motor or language deficits, fatigue, insomnia and malaise. Long-term adverse effects include atrophy of brain tissue and cognitive deficits [14]. These acute and chronic Adverse Effects (AE) significantly decrease the HRQoL of HGG patients.
Recurrence of HGG, defined as tumor regrowth despite treatment, is a very negative prognostic factor [15]. Nearly all patients with HGG experience recurrence within several months and the prognosis is dismal [16]. In cases of GBM, recurrence in all patients is inevitable [17]. There is no standard therapy for recurrent HGG. Generally, the therapeutic strategy is tailored to each patient's specific requirements and guided by recommendations from a multidisciplinary tumor board that considers prior treatment, time to recurrence, performance status, corticosteroid dependence and molecular markers [12,15]. Again, enrollment in a clinical trial is preferred. Conventional therapy options for recurrent HGG include a second surgery, additional RT, additional TMZ or lomustine and bevacizumab [12]. This extra treatment will lead to additional AEs and further reduce patients' HRQoL.
Methods
Antineoplaston research began in 1967, when significant deficiencies were noticed in the peptide content of the serum of patients with cancer compared with healthy people. Initially, Antineoplastons were isolated from the blood and later from urine [18]. Subsequent studies of the isolated Antineoplastons demonstrated that Antineoplaston A10 (Atengenal) and Antineoplaston AS2-1 (Astugenal) were the most promising formulations. The chemical name of Antineoplaston A10 is 3-phenylacetylamino-2,6-piperidinedione. It consists of the cyclic form of L-glutamine connected by a peptide bond to a phenylacetyl residue. The mixture of synthetic Phenylacetyl Glutaminate (PG) and phenylacetyl isogluatminate (isoPG) in a 4:1 ratio, when dissolved in sterile water, constitutes an Antineoplaston A10 intravenous (IV) injection. Further metabolism of Antineoplaston A10 results in Phenylacetate (PN). Both metabolites, PG and PN, have anticancer activity. The mixture of PN and PG in a 4:1 ratio, dissolved in sterile water, constitutes Antineoplaston AS2-1 IV injection [19].
Antineoplastons A10 and AS2-1 (ANP) has been used in several phase II clinical studies [20-68]. Here we present 1) unusually long survival (from more than five years to over 33 years) of 61 HGG patients treated at the Burzynski Clinic (BC), between August 1992 and September 2004, according to phase II protocols of ANP and 2) the lack of long-term adverse sequelae. The eligibility criteria for protocol therapy included a Karnofsky/Lansky Performance Score (KPS/LPS) of 60-100% and a life expectancy of at least 2 months. All patients were treated according to single-arm, phase II studies, which administered ANP by IV injection. Some patients also received oral ANP for maintenance. Gradually increasing doses of IV ANP were administered via a subclavian catheter and infusion pump until the maximum tolerated doses of A10 and AS2-1 were achieved. The outcome criteria were 1) objective response (OR), 2) survival and 3) toxicity. All study patients and/or their legal guardians read, understood and signed an Informed Consent Document before treatment. Disease progression, unacceptable toxicity, physician decision, or patient request resulted in the termination of ANP.
MRI scans of the brain with gadolinium enhancement were used for diagnosis, response evaluation and follow-up of these patients. Brain MRIs were performed serially every 8 weeks during the first 2 years of protocol ANP. Afterward, they were conducted as needed during follow-up. T2-weighted, T2-FLAIR, T1-weighted and contrast-enhanced T1-weighted images were obtained. HGG shows gadolinium enhancement so contrast-enhanced T1-weighted images were used to assess treatment effects [69,70]. Based on the brain MRI, the product of the two largest perpendicular diameters of each measurable (>5mm) and enhancing lesion was calculated. Tumor size was defined as the SUM of these products. The response criteria were as follows: a Complete Response (CR) was indicated by the total disappearance of all enhancing tumors. In contrast, a Partial Response (PR) was indicated by a 50% or greater reduction in the SUM. CR and PR required a confirmatory brain MRI performed at least four weeks after the initial finding. Progressive Disease (PD) was indicated by a 25% or greater increase in the SUM, or the presence of new measurable and enhancing disease. Stable Disease (SD) was defined as the absence of CR, PR, or PD [69,70].
The Phase II studies were conducted in accordance with the U.S. Code of Federal Regulations, Title 21, Parts 11, 50, 56 and 312; the Declaration of Helsinki (1964), including all amendments and revisions; the Good Clinical Practices: Consolidated Guideline (E6), International Conference on Harmonization (ICH) and Guidance for Industry (FDA). By participating in this study protocol, the investigators agreed to provide access to all appropriate documents for monitoring, auditing, Institutional Review Board (IRB) review and review by any authorized regulatory agency.
Results
A total of 310 HGG patients received treatment at the BC between May 1988 and March 2014 as part of Phase II ANP studies. GBM patients were excluded as they were the subjects of another article [66]. Of these patients, 170 were designated as "Special Exceptions" (54.8%) because they did not meet all eligibility criteria, often due to a KPS/LPS of less than 60. These 170 patients were individually approved by the Food and Drug Administration (FDA). Out of 310 HGG patients, 61 survived over 5 years from HGG diagnosis.
Fifty-nine of 310 non-GBM HGG patients (19.0%) were initially diagnosed with low-grade tumors that subsequently transformed into high-grade tumors. In such cases, only the dates for high-grade diagnoses were used to calculate survival. For these 310 patients, Kaplan-Meier survival analysis showed a median OSD of 1.867 years (95% CI 1.555 to 2.133). See Fig. 1., where the “Time” axis is presented in increments of five years and survival extended to over 30 years in our observation.
Before being seen at the BC, 18 of 61 long-term survivors underwent SU, followed by RT, and CH; 10 patients had SU and RT; 8 patients had SU only, 5 patients had RT only after initial biopsy; 7 patients had biopsy only; 16 patients had some combination of single SU +/- RT +/- CH. Several patients also had other treatments. Demographic details, prior treatment and overall survival are shown in Tab. 1.
| |
N=310 (All Patients) |
N=61 (OSD >5 years) |
| Sex |
|
|
| Male |
200 |
39 |
| Female |
110 |
22 |
| Age (at admission at BC) |
|
|
| Range |
0.41-76.66 |
1.08-62.66 |
| Median |
37.8 |
35.9 |
| Age groups (at admission at BC) |
|
|
| Below 21 |
64 |
10 |
| 21+ |
246 |
51 |
| KPS/LPS (at admission at BC) |
|
|
| Range |
30-100 |
40-90 |
| Median |
50 |
60 |
| Prior Treatment (single treatments/multiple treatments) |
|
|
| None |
3* |
- |
| Bx only |
46/1 |
7 |
| Bx, RT |
21/1 |
5/- |
| Bx, RT, CH |
23/12 |
1/2 |
| Bx, RT, CH, Other |
2/4 |
-/1 |
| Bx, RT, Other |
1/- |
1/- |
| Bx, CH |
3/1 |
-/1 |
| Bx, CH, Other |
1/1 |
1/- |
| SU |
34/4 |
7/1 |
| SU, RT |
28/11 |
9/1 |
| SU, CH |
5/2 |
3/- |
| SU, CH, Other |
1/ - |
3/- |
| SU, RT, CH, |
18/47 |
4/6 |
| SU, RT, CH, Other |
5/23 |
-/8 |
| SU, RT, Other |
6/1 |
-/1 |
| SU, Other |
3/- |
2/- |
| RT |
2**/ - |
- |
| Pathology |
|
|
| Anaplastic Astrocytoma |
189 |
40 |
| Anaplastic Astrocytoma/BSG |
8 |
- |
| Anaplastic Astrocytoma/DIPG |
22 |
5 |
| Anaplastic Astrocytoma/Mixed |
33 |
8 |
| Anaplastic Astrocytoma/Mixed/DIPG |
1 |
- |
| Anaplastic Astrocytoma/Visual Pathway |
2 |
- |
| Anaplastic Astrocytoma/Spine |
2 |
- |
| Anaplastic Glioma |
1 |
- |
| Anaplastic Oligoastrocytoma |
2 |
1 |
| Anaplastic Oligodendroglioma |
13 |
3 |
| Anaplastic Oligodendroglioma/DIPG |
1 |
- |
| Astrocytoma Fibrillary High Grade |
2 |
- |
| Astrocytoma Fibrillary High Grade/Spine |
1 |
- |
| Astrocytoma High Grade/Spine |
1 |
- |
| Astrocytoma Infiltrating/DIPG |
1 |
1 |
| Glioma High Grade |
15 |
1 |
| Glioma High Grade/Visual Pathway |
1 |
1 |
| Gliomatosis Cerebri |
2 |
- |
| Oligoastrocytoma High Grade |
11 |
1 |
| Xantoastrocytoma, grade 4 |
2 |
- |
| Overall Survival from diagnosis |
|
|
| Over 6 months |
86.77% |
NA |
| Over 5 years |
19.68% |
100% |
| Over 12 years |
7.41% |
37.70% |
Note: BC-Burzynski Clinic, BSG-Brain Stem Glioma, Bx-Biopsy, CH-Chemotherapy, DIPG-Diffuse Intrinsic Pontine Glioma, KPS-Karnofsky Performance Score, LPS-Lansky Performance Score, OSD-Overall Survival from Diagnosis, RT-Radiation Therapy, SU-Surgery Other treatment: Accutane, artemisinin, Avastin, BCG (Bacillus Calmette-Guérin) immunotherapy, cis-retinoic acid, clinical trial, Dimethyl Sulfoxide (DMSO), electromagnetic therapy, erlotinib, Gleevec, high-dose vitamin C and vitamin E, hyperthermia, Insulin Potentiation Therapy (IPT), interferon, Lymphokine-Activated Killer (LAK) cells, monoclonal therapy, poly ICLC - dsRNA molecule used as an immune stimulant in cancer immunotherapy, shark cartilage, sodium phenylbutyrate, tamoxifen, temozolomide, thalidomide, and unknown alternative therapy *Three patients had no prior treatment: one patient was diagnosed with a butterfly malignant glioma and the biopsy was not necessary; the second patient had a tumor consistent with malignant glioma and was not a candidate for surgery; the third patient was diagnosed with a high-grade glioma, and the biopsy was not performed because of the possibility of permanent damage to the visual pathway. This patient has also had complications with previous anesthesia. **Two patients were treated with RT without a biopsy due to the dangerous location of the tumor. One had a brain CT and angiogram, the other had a brain MRI for diagnosis.
Tab. 1. Demographics, prior treatment, pathology and OSD.
Fifty-three of the 61 long-term survivors (86.9%) had anaplastic astrocytoma, of which eight were described as "mixed" and five had histological features of diffuse intrinsic pontine glioma. Eight patients had one of five other HGG diagnoses. Pathologists with academic affiliations, including prominent neuropathologists, made the diagnoses. Details of the diagnosis, prior treatment and tumor status at the start of ANP are presented in Tab. 2.
| Case |
Date |
Pathology |
Hospital name |
Prior treatment |
Tumor characteristic |
| 1 |
02/15/88 |
Anaplastic Astrocytoma |
Cooper Hospital/University Medical Center, Camden, NJ |
SU |
Supratentorial; Single lesion; Primary |
| 2 |
07/29/87 |
Moderately anaplastic astrocytoma |
UCSF Medical Center, San Francisco, CA |
Bx, RT |
DIPG/Solitary/NE only |
| 3 |
10/19/87 |
Anaplastic Astrocytoma |
National Cancer Institute report |
SU, RT |
Supratentorial; Single lesion; Primary |
| 4 |
04/28/87 |
Moderately anaplastic astrocytoma |
UCSF Medical Center, San Francisco, CA |
SU, RT, CH |
Supratentorial; Single lesion; Primary |
| 5 |
04/22/88 |
Anaplastic Astrocytoma |
University of Alberta Hospitals, Alberta, Canada |
SU, RT, CH |
DIPG/Solitary E+NE |
| 6 |
09/14/90 |
Giganto-Cellular Anaplastic Astrocytoma |
Children's Hospital of Philadelphia, Philadelphia, PA |
SU, RT |
Supratentorial; Single lesion; Primary |
| 7 |
08/28/92 |
Anaplastic Astrocytoma, Osseous Metaplasia. |
New York University Medical Center, New York, NY |
SU, RT, tamoxifen |
DIPG/Solitary E+NE |
| 8 |
12/03/1993 |
Anaplastic Astrocytoma |
Princess Margaret Hospital for Children, Subiaco, Australia |
Bx |
Supratentorial; Single lesion; Primary |
| 01/03/1994 |
Astrocytoma grade 1-2 |
Children's Hospital of Philadelphia, Philadelphia, PA |
| 9 |
1/y/90 |
Anaplastic Astrocytoma |
Not available |
2SU, 4Bx, RT, 2CH, CT – no data, tamoxifen |
Supratentorial; Single lesion; Primary |
| 10/25/93 |
Oligodendroglioma |
National Institute of Health Medical Center, Bethesda, MD |
| 12/06/1993 |
Anaplastic Oligodendroglioma |
National Institute of Health Medical Center, Bethesda, MD |
| 01/25/94 |
Anaplastic Oligodendroglioma |
National Institute of Health Medical Center, Bethesda, MD |
| 11/14/94 |
Oligodendroglioma |
National Institute of Health Medical Center, Bethesda, MD |
| 03/21/95 |
Anaplastic Oligodendroglioma |
National Institute of Health Medical Center, Bethesda, MD |
| 10 |
12/08/1994 |
Mixed Glioma grade 3-4 |
St. Vincent Medical Center, Toledo, OH |
SU, RT |
Supratentorial; Single lesion; Primary |
| 11 |
09/29/94 |
Anaplastic Oligoastrocytoma |
The New York Hospital, New York, NY |
SU, RT |
Supratentorial; Single lesion; Primary |
| 12 |
08/30/95 |
Anaplastic Astrocytoma |
The Methodist Hospital, Houston, TX |
Bx, RT |
Supratentorial; Single lesion; Primary |
| 13 |
02/26/96 |
High Grade Glioma |
The Moses H. Cone Memorial Hospital, Greensboro, NC |
SU |
Supratentorial; Single lesion; Primary |
| 03/29/96 |
Mixed Astrocytoma-Oligodendroglioma |
Children's Hospital of Philadelphia, Philadelphia, PA |
| 14 |
02/23/96 |
Anaplastic Astrocytoma |
Oregon Health Sciences University, Portland, OR |
SU |
Supratentorial; Single lesion; Primary |
| 04/03/1996 |
Anaplastic Astrocytoma |
Children's Hospital of Philadelphia, Philadelphia, PA |
| 15 |
9/y/90 |
Astrocytoma Infiltrating |
Medical College of Ohio, Toledo, OH |
SU, 2Bx, RT, CH |
Supratentorial; Single lesion; Primary |
| 10/18/95 |
Astrocytoma Infiltrating |
Cleveland Clinic, Cleveland, OH |
| 02/06/1996 |
Astrocytoma infiltrating |
Cleveland Clinic, Cleveland, OH |
| 16 |
02/16/96 |
Astrocytoma, possible anaplastic |
Air Force USAC Medical Center, Wright Patterson, OH |
Bx, RT |
Supratentorial; Multifocal; Primary |
| 03/08/1996 |
Astrocytoma Infiltrating |
Air Force USAC Medical Center, Wright Patterson, OH |
| 09/12/1996 |
Astrocytoma |
Children's Hospital of Philadelphia, Philadelphia, PA |
| 17 |
10/06/1989 |
Moderate anaplastic astrocytoma |
Huntington Memorial Hospital, Pasadena, CA |
Bx, RT |
Supratentorial; Single lesion; Primary |
| 18 |
09/03/1991 |
Small cell astrocytoma grade 4 |
Swedish Medical Center, Englewood, Co |
2SU, RT, CH |
Supratentorial; Multicentric; Primary |
| 09/05/1991 |
Glioblastoma Multiforme, small cell variant |
University of Colorado Health Science Center, Aurora, CO |
| 09/09/1991 |
Small cell malignant astrocytoma |
Mayo Clinic, Rochester, MN |
| 02/03/1997 |
High Grade Glioma |
California Pacific Medical Center, San Francisco, CA |
| 19 |
12/23/91 |
Mixed Malignant Glioma. |
UCSF Medical Center, San Francisco, CA |
2SU, RT, CH |
Supratentorial; Multifocal; Primary |
| 12/15/95 |
Mixed Malignant Glioma |
UCSF Medical Center, San Francisco, CA |
| 20 |
02/24/97 |
Anaplastic astrocytoma |
University of Pennsylvania Medical Center, Philadelphia, PA |
SU |
Supratentorial; Single lesion; Primary |
| 21 |
07/18/85 |
Astrocytoma |
The Children's Hospital of Philadelphia, Philadelphia, PA |
SU, Bx, RT, 2CH |
Supratentorial; Single lesion; Primary |
| 08/10/1992 |
Anaplastic mixed glioma |
Zale Lipshy University Hospital, Dallas, TX |
| 03/28/97 |
Anaplastic astrocytoma |
The Children's Hospital of Philadelphia, Philadelphia, PA |
| 22 |
04/23/93 |
Astrocytoma |
St. Joseph's Hospital, Houston, TX |
2Bx, RT |
Supratentorial; Single lesion; Primary |
| 05/11/1993 |
Glioma Infiltrating |
St. Joseph's Hospital, Houston, TX |
| 07/23/96 |
Anaplastic Astrocytoma |
Clinic at the University of Florida, Gainesville, FL |
| 23 |
12/10/1996 |
Anaplastic mixed glioma |
North Shore University Hospital, Manhasset, NY |
SU, CH |
Supratentorial; Single lesion; Secondary |
| 04/30/97 |
High grade glioma/Anaplastic Oligodendroglioma |
Memorial Hospital for Cancer and Allied Diseases, New York, NY |
| 07/25/97 |
Anaplastic Astrocytoma |
North Shore University Hospital, Manhasset, NY |
| 24 |
4/y/91 |
Astrocytoma |
University of Pennsylvania Medical Center, Philadelphia, PA |
Bx, SU, RT |
Supratentorial; Multifocal; Secondary |
| 06/02/1997 |
Anaplastic Astrocytoma |
University of Pennsylvania Medical Center, Philadelphia, PA |
| 25 |
04/24/92 |
Oligoastrocytoma type III |
Centre Hospitalier Sainte Anne, Paris, France |
SU, RT |
Supratentorial; Single lesion; Primary |
| 26 |
9/y/91 |
Mixed glioma grade 2/3 |
New York Medical Center, New York, NY |
2SU, RT, CH |
Supratentorial; Single lesion; Primary |
| 04/27/98 |
High grade mixed glial-neuronal tumor |
University Medical Center, New York, NY |
| 27 |
08/21/98 |
Astrocytoma Fibrillary, grade 3 |
Foothills Hospital, Department of Histopathology, Calgary, Alberta, Canada |
SU |
Supratentorial; Single lesion; Primary |
| 28 |
01/06/1989 |
Anaplastic Astrocytoma |
UCSF Medical Center, San Francisco, CA |
Bx, RT, 2CH, Accutane, tamoxifen |
DIPG/Solitary E+NE |
| 29 |
4/y/92 |
Anaplastic Astrocytoma |
Brookhaven Memorial Hospital Medical Center, Patchogue, NY |
3SU, RT, 2CH |
Supratentorial; Multicentric; Secondary |
| 08/05/1993 |
Astrocytoma |
Brookhaven Memorial Hospital Medical Center, Patchogue, NY |
| 08/20/93 |
Anaplastic Astrocytoma |
Stoney Brook University Hospital, Stoney Brook, NY |
| 04/14/94 |
Anaplastic Astrocytoma |
Brookhaven Memorial Hospital Medical Center, Patchogue, NY |
| 11/09/1998 |
Anaplastic Astrocytoma |
Memorial Hospital for Cancer and Allied Diseases, New York, NY |
| 30 |
12/24/97 |
Anaplastic Astrocytoma |
Not available |
2SU, RT, 3CH, tamoxifen |
Supra & Infratentorial; Multicentric; Primary |
| 10/14/98 |
Anaplastic Astrocytoma |
UCSF Medical Center, San Francisco, CA |
| 31 |
05/10/1996 |
Astrocytoma fibrillary, grade 2 |
Community Hospitals, Indianapolis, IN |
Bx |
Supratentorial; Single lesion; Primary |
| 05/15/96 |
Astrocytoma |
Indiana University Medical Center, Indianapolis, IN |
| 07/26/96 |
Astrocytoma Fibrillary, grade 2 |
The Johns Hopkins Hospital, Baltimore, MD |
| 32 |
07/27/98 |
Anaplastic Astrocytoma |
Sutter General Hospital, Sacramento, CA |
Bx, RT, CH |
Supratentorial; Single lesion; Primary |
| 08/25/98 |
Anaplastic Astrocytoma |
Roth Medical Group, Sacramento, CA |
| 33 |
05/15/00 |
Anaplastic Astrocytoma |
Saint John's Health Center, Santa Monica, CA |
Bx |
Supratentorial; Multifocal; Primary |
| 34 |
05/06/1996 |
Astrocytoma Malignant |
Not available |
2SU |
Supratentorial; Single lesion; Primary |
| 07/31/00 |
Anaplastic Astrocytoma |
University Jagiellonum Collegium Medicum, Krakow, Poland |
| 35 |
11/07/2000 |
Astrocytoma Fibrillary /Anaplastic Astrocytoma |
United Hospital Allina Health System, St. Paul, MN |
Bx |
Supratentorial; Multifocal; Primary |
| 36 |
04/26/00 |
Glial neoplasia, high grade |
Beth Israel Medical Center-North Division, New York, NY |
2SU, RT |
Supratentorial; Single lesion; Primary |
| 12/20/00 |
Anaplastic Astrocytoma |
Beth Israel Medical Center-North Division, New York, NY |
| 37 |
05-12-1995 |
Anaplastic Astrocytoma |
New York University Medical Center, New York, NY |
SU, Bx, CH |
Supratentorial; Single lesion; Primary |
| 01/16/98 |
Oligoastrocytoma, grade 3 |
UCSF Medical Center, San Francisco, CA |
| 38 |
04/30/02 |
Anaplastic Astrocytoma |
UCSF Medical Center, San Francisco, CA |
Bx |
Supratentorial; Single lesion; Primary |
| 39 |
11/06/1995 |
Anaplastic Astrocytoma |
Armed Forces Institute Of Pathology, Washington, DC |
SU, RT, CH |
Supratentorial; Single lesion; Primary |
| 40 |
07/10/1992 |
Astrocytoma Infiltrating with pilocytic features grade 2/3 |
Westchester County Medical Center, Grasslands Reservation Valhalla, NY |
SU, CH |
Supratentorial; Single lesion; Primary; Visual Pathway |
| 07/23/92 |
Juvenile pilocytic astrocytoma |
Memorial Sloan-Kettering Cancer Center, New York, NY |
| 10/13/92 |
Anaplastic Astrocytoma |
Westchester County Medical Center, Grasslands Reservation Valhalla, NY |
| 11/05/1992 |
Juvenile pilocytic astrocytoma |
Memorial Sloan-Kettering Cancer Center, New York, NY |
| 07/12/1994 |
Optic nerve involved by high grade glioma |
Westchester County Medical Center, Grasslands Reservation Valhalla, NY |
| 41 |
03/09/1998 |
Anaplastic Astrocytoma |
Akron General Medical Center, Akron, OH |
Bx |
Supratentorial; Single lesion; Primary |
| 42 |
03/11/1992 |
Astrocytoma |
Hackensack Medical Center, Department of Pathology, Hackensack, NJ |
SU, alternative therapy |
Supratentorial; Single lesion; Primary /NE only |
| 02/09/2000 |
Anaplastic Oligodendroglioma |
New York Presbyterian Hospital/Columbia Presbyterian Center, New York, NY |
| 03/15/00 |
Anaplastic Oligodendroglioma |
Memorial Hospital for Cancer and Allied Diseases, New York, NY |
| 43 |
08/05/2003 |
Oligoastrocytoma mixed, grade 3 |
UCLA Medical Center, Department of Pathology in Los Angeles, CA |
Bx,2CH |
Supratentorial; Single lesion; Primary /NE only |
| 44 |
02/26/03 |
Anaplastic Astrocytoma |
Hartford Hospital, Harford, CT |
Bx, CH, Gleevec |
Supratentorial; Single lesion; Primary /NE only |
| 45 |
06/01/1999 |
Oligoastrocytoma Mixed |
Robert Wood Johnson University Hospital, New Brunswick, NJ |
SU, high dose vitamins C and E |
Supratentorial; Single lesion; Primary |
| 07/28/99 |
Oligoastrocytoma Mixed, grade 3 |
Department of Defense, Armed Forces Institute Of Pathology, Washington, DC |
| 09/01/1999 |
Oligodendroglioma |
Mayo Clinic, Rochester, MN |
| 09/07/1999 |
Oligodendroglioma |
University of Kansas Medical Center, Kansas City |
| 09/21/99 |
Astrocytoma |
The Johns Hopkins Hospital, Baltimore, MD |
| 10/07/1999 |
Glioneuronal tumor with neuropil-like islands |
Robert Wood Johnson University Hospital, New Brunswick, NJ |
| 46 |
07/15/83 |
Astrocytoma Malignant |
Medical Center Del Oro Hospital, Houston, TX |
5SU, Bx, 2RT, 5CH, interferon, Accutane |
Supratentorial; Multicentric; Primary |
| 03/07/2003 |
Glioma favor Oligoastrocytoma |
St. Luke's Baylor Hospital, Houston, TX |
| 11/21/03 |
Anaplastic mixed glioma |
St. Luke's Baylor Hospital, Houston, TX |
| 06/28/04 |
High Grade Glioma |
M.D. Anderson Cancer Center, Houston, TX |
| 07/05/2004 |
Anaplastic Astrocytoma |
M.D. Anderson Cancer Center, Houston, TX |
| 47 |
07/07/2003 |
Astrocytoma Fibrillary, grade 3 |
Mayo Clinic, Rochester, MN |
4SU, 2RT, 2CH, LAK cells |
Supratentorial; Single lesion; Primary |
| 07/08/2003 |
Anaplastic Astrocytoma |
Hoag Memorial Hospital Presbyterian, Newport Beach, CA |
| 07/09/2003 |
Astrocytoma Fibrillary, grade 3 |
Hoag Memorial Hospital Presbyterian, Newport Beach, CA |
| 03/22/04 |
Anaplastic Astrocytoma |
Hoag Memorial Hospital Presbyterian, Newport Beach, CA |
| 48 |
05/28/96 |
Astrocytoma, grade 2 with focal grade 3 |
Santa Barbara Cottage Hospital, Santa Barbara, CA |
3SU, Bx, 4RT, 5CH, PB, Accutane, thalidomide |
Infratentorial; Single lesion; Primary |
| 06/04/1996 |
Oligodendroglioma |
Massachusetts General Hospital, Boston, MA |
| 04/02/1998 |
Oligoastrocytoma, grade 3 |
UCSF Medical Center, San Francisco, CA |
| 05/02/2001 |
Glioma infiltrating |
UCSF Medical Center, San Francisco, CA |
| 02/06/2002 |
Anaplastic Oligoastrocytoma |
UCSF Medical Center, San Francisco, CA |
| 03/02/2004 |
Anaplastic Astrocytoma |
UCSF Medical Center, San Francisco, CA |
| 49 |
09/28/04 |
Anaplastic Astrocytoma |
Pathology at Massachusetts General Hospital, Boston, MA |
3SU, RT, 2CH, Clinical trial CC-8490 |
Supratentorial; Single lesion; Primary |
| 10/01/2004 |
Anaplastic Astrocytoma |
Pathology at Massachusetts General Hospital, Boston, MA |
| 04/25/05 |
Astrocytoma |
Not readable |
| 50 |
7/y/1999 |
Gemistocytic Astrocytoma |
Arhus University Hospital, Arhus, Denmark |
2SU, RT, CH |
Supratentorial; Single lesion; Secondary |
| 11/13/01 |
Anaplastic Astrocytoma |
| 51 |
08/24/05 |
Anaplastic Astrocytoma |
UT Southwestern Medical Center, University Hospitals & Clinics, Zale Lipshy Laboratory, Dallas, TX |
SU |
Supratentorial; Single lesion; L-M, Primary |
| 52 |
10/07/2003 |
Anaplastic Astrocytoma |
Cedars-Sinai Medical Center, Los Angeles, CA |
SU, RT |
Supratentorial; Single lesion; Primary |
| 53 |
06/12/2007 |
Anaplastic Astrocytoma |
St. Anthony Health Services, Denver, CO |
SU |
Supratentorial; Single lesion; Primary |
| 54 |
10/20/06 |
Oligoastrocytoma, astrocytic predominant, grade 2 |
Mayo Clinic, Rochester, MN |
Bx, RT, 2CH |
Supratentorial; Single lesion; Primary |
| 12/09/2006 |
Anaplastic Astrocytoma |
University of Michigan, Ann Arbor, MI |
| 55 |
04/06/2010 |
Astrocytoma Infiltrating Diffuse |
M.D. Anderson Cancer Center, Houston, TX |
Bx, RT, anticancer treatment unknown |
DIPG/Solitary/NE only |
| 56 |
06/22/07 |
Anaplastic Astrocytoma |
Michael Reese Hospital, Chicago, IL |
SU, RT |
Supratentorial; Single lesion; Primary |
| 57 |
02/11/2010 |
Oligoastrocytoma, grade 2 |
St. Joseph Hospital and Medical Center, Phoenix, AZ |
2SU, Bx, 3RT, CH, bevacizumab |
Supratentorial; Multicentric; Primary |
| 05/12/2010 |
Astrocytoma with Gemistocytic features, grade 2 |
St. Joseph Hospital and Medical Center, Phoenix, AZ |
| 08/24/10 |
Anaplastic Astrocytoma, gemistocytic |
St. Joseph Hospital and Medical Center, Phoenix, AZ |
| 58 |
04/01/2011 |
Anaplastic Glioma |
Royal Free Hampstead NHS Trust, London, U.K. |
SU, RT |
Supratentorial; Single lesion; Primary |
| 59 |
04/13/11 |
Astrocytoma Infiltrating |
Fleni Hospital Montañeses 2325, C1428, Buenos Aires, Argentina |
Bx, RT, 2CH |
DIPG/Single lesion; Primary/NE only |
| 11/08/2011 |
Astrocytoma Infiltrating |
M.D. Anderson Cancer Center, Houston, TX |
| 60 |
01/24/12 |
Anaplastic Astrocytoma/thalamic |
UPMC- Presbyterian, Pittsburg, PA |
Bx |
Supratentorial; Single lesion; Primary |
| 61 |
01/05/2006 |
Anaplastic Oligodendroglioma |
Barnes-Jewish Hospital Washington University Medical Center, St. Louis, MO |
2SU, RT, 2CH, PB, Tarceva, pazopanib, bevacizumab |
Supratentorial; Single lesion; Primary |
| 08/05/2011 |
Anaplastic Oligodendroglioma |
Barnes-Jewish Hospital Washington University Medical Center, St. Louis, MO |
Note: ANP-Antineoplastons, Bx-Biopsy, CH-Chemotherapy, DIPG-Diffuse Intrinsic Pontine Glioma, E-Enhanced signal, LAK- Lymphokine-Activated Killer cells, LM – Leptomeningeal involvement, NE-Non Enhanced signal, PB-Phenylbutyrate, RT-Radiation Therapy, SU-Surgery
Tab. 2. Diagnosis, prior treatment and tumor status at the start of ANP.
At admission to BC, the age of these patients ranged from 1.08 to 62.66 years, with a median age of 35.9 years. There were 22 females and 39 males. KPS/LPS scores ranged from 40 to 90, with a median score of 60. Twenty-five patients (41.0%) were not eligible for protocol ANP but, after FDA approval, were treated as Special Exceptions (SEs) according to protocol.
All ORs were confirmed by prominent neuroradiologists who were not affiliated with BC. ORs consisted of CR in 13 cases, PR in 4 cases, SD in 20 cases and PD in 14 cases. Ten cases were not evaluable. Overall Survival from Diagnosis (OSD) was more than 12 years in 23 patients, more than 20 years in 8 patients and more than 30 years in 2 patients. As of October 2025, 1 patient had an OSD of more than 33 years. Eight patients (13.1%) were alive and doing well at the last follow-up. The best response to ANP, patient status at last follow-up and OSD following ANP for all 61 long-term survivors are described in Tab. 3.
| Case |
Sex |
Age at admission (years) |
Protocol |
Diagnosis at admission |
KPS/LPS at admission |
Start Date |
Days on protocol |
Best response on treatment |
Post ANP treatment |
Cause of death or last contact date |
OSD years |
| 1 |
F |
13.67 |
BT-03 |
Anaplastic Astrocytoma |
70 |
01/19/1989 |
97 |
PD |
None |
unknown |
11.88 |
| 2 |
F |
36.41 |
BT-03 |
Anaplastic Astrocytoma/DIPG |
60 |
07/12/1988 |
394 |
CR |
None |
pneumonia |
28.60 |
| 3 |
M |
30.42 |
BT-03 |
Anaplastic Astrocytoma |
70 |
07/08/1988 |
262 |
SD |
None |
unknown |
6.18 |
| 4 |
M |
48.42 |
BT-04 |
Anaplastic Astrocytoma |
70 |
04/12/1990 |
654 |
PR |
None |
HGG |
9.25 |
| 5 |
M |
26.58 |
BT-03 |
Anaplastic Astrocytoma/DIPG |
40 |
10/30/1989 |
113 |
CR |
None |
chronic toxicity from RT |
24.76 |
| 6 |
M |
9.33 |
BT-04 |
Anaplastic Astrocytoma |
80 |
10/08/1990 |
90 |
SD |
SU 5/91 |
HGG |
5.02 |
| 7 |
M |
8.25 |
CAN-01 |
Anaplastic Astrocytoma/DIPG |
60 |
10/26/1992 |
2533 (3641 po) |
CR |
None |
unknown |
19.92 |
| 8 |
F |
12.50 |
CAN-01 |
Anaplastic Astrocytoma |
60 |
01/07/1994 |
1235 (458 po) |
CR |
None |
Alive 12/5/24 |
31.03 (+) |
| 9 |
M |
27.25 |
CAN-01 |
Anaplastic Oligodendroglioma |
90 |
05/30/1995 |
|
NE |
None |
malignancy |
7.55 |
| 10 |
M |
32.58 |
CAN-01 |
Anaplastic Astrocytoma/Mixed |
80 |
07/26/1995 |
1639 (876 po) |
CR |
None |
unknown |
6.41 |
| 11 |
M |
55.25 |
CAN-01 |
Anaplastic Astrocytoma/Mixed |
70 |
08/22/1995 |
433 |
SD |
None |
different malignancy - sinus cancer |
17.59 |
| 12 |
M |
62.67 |
CAN-01 |
Anaplastic Astrocytoma |
60 |
01/31/1996 |
104 |
SD |
None |
unknown |
5.58 |
| 13 |
M |
54.33 |
BT-18 |
Anaplastic Astrocytoma/Mixed |
80 |
03/27/1996 |
519 |
CR |
RT |
unknown |
14.52 |
| 14 |
F |
50.42 |
BT-08 |
Anaplastic Astrocytoma |
90 |
04/08/1996 |
554 (218 po) |
SD |
None |
unknown |
7.30 |
| 15 |
M |
41.92 |
BT-15SE |
Anaplastic Astrocytoma Infiltrating |
70 |
05/02/1996 |
130 |
SD |
None |
HGG |
7.30 |
| 16 |
M |
26.58 |
BT-09 |
Anaplastic Astrocytoma |
80 |
09/17/1996 |
1103 |
PR |
SU 10/99 |
HGG |
5.21 |
| 17 |
M |
44.50 |
BT-15 |
Anaplastic Astrocytoma |
60 |
11/15/1996 |
377 |
SD |
None |
unknown |
12.57 |
| 18 |
F |
30.67 |
BT-09 |
Glioma High Grade |
60 |
02/12/1997 |
53 |
PD |
None |
HGG |
5.59 |
| 19 |
M |
38.42 |
BT-18 |
Anaplastic Astrocytoma/Mixed |
60 |
02/17/1997 |
35 |
PD |
None |
HGG |
5.68 |
| 20 |
F |
29.75 |
BT-08SE |
Anaplastic Astrocytoma |
90 |
03/27/1997 |
134 |
SD |
None |
unknown |
5.62 |
| 21 |
M |
44.00 |
BT-15 |
Anaplastic Astrocytoma |
90 |
04/12/1997 |
74 |
PD |
None |
HGG |
5.10 |
| 22 |
F |
45.42 |
BT-15 |
Anaplastic Astrocytoma |
80 |
10/14/1998 |
224 (773 po) |
CR |
None |
unknown |
14.96 |
| 23 |
M |
26.67 |
BT-18 |
Anaplastic Oligodendroglioma |
80 |
06/06/1997 |
31 |
PD |
RT, SU 07/97 |
Alive 10/10/25 |
28.83 (+) |
| 24 |
F |
48.83 |
BT-15SE |
Anaplastic Astrocytoma |
50 |
03/11/1998 |
133 |
NE |
None |
HGG |
5.49 |
| 25 |
F |
40.83 |
BT-18 |
Anaplastic Astrocytoma/Mixed |
60 |
06/17/1998 |
138 |
PD |
None |
unknown |
6.66 |
| 26 |
M |
35.50 |
BT-18 |
Anaplastic Astrocytoma/Mixed |
60 |
06/24/1998 |
145 |
PD |
None |
HGG |
7.18 |
| 27 |
F |
17.83 |
BT-10 |
Anaplastic Astrocytoma |
80 |
10/20/1998 |
268 (680 po) |
SD |
SU 01/04, RT |
HGG |
10.46 |
| 28 |
M |
42.50 |
BT-11SE |
Anaplastic Astrocytoma/DIPG |
40 |
03/19/1999 |
179 |
PD |
None |
HGG |
10.88 |
| 29 |
M |
41.25 |
BT-15SE |
Anaplastic Astrocytoma |
40 |
03/29/1999 |
85 |
PD |
None (shunt) |
septicemia |
7.26 |
| 30 |
F |
13.75 |
BT-22SE |
Anaplastic Astrocytoma |
40 |
10/01/1999 |
399 |
SD |
None |
unknown |
13.16 |
| 31 |
F |
36.92 |
BT-09SE |
Anaplastic Astrocytoma |
50 |
10/15/1999 |
78 (556 po) |
SD |
SU 03/02, RT |
unknown |
7.12 |
| 32 |
F |
37.25 |
BT-15 |
Anaplastic Astrocytoma |
60 |
01/12/2000 |
184 (763 po) |
CR |
None |
unknown |
5.28 |
| 33 |
F |
31.92 |
BT-08 |
Anaplastic Astrocytoma |
60 |
06/06/2000 |
56 (454 po) |
CR |
None |
Alive 10/13/25 |
25.40 (+) |
| 34 |
M |
33.67 |
BT-09SE |
Anaplastic Astrocytoma |
50 |
11/09/2000 |
197 |
PD |
RT |
unknown |
5.80 |
| 35 |
M |
52.50 |
BT-08 |
Anaplastic Astrocytoma |
60 |
02/07/2001 |
57 |
NE |
SU 8/12, RT, 3xCH, OT |
unknown |
17.87 |
| 36 |
M |
41.50 |
BT-15 |
Anaplastic Astrocytoma |
60 |
02/22/2001 |
162 |
PR |
None |
HGG |
5.04 |
| 37 |
M |
35.92 |
BT-18SE |
Oligoastrocytoma High Grade |
50 |
02/22/2002 |
90 |
PD |
None |
HGG |
7.05 |
| 38 |
F |
3.58 |
BT-22SE |
Anaplastic Astrocytoma |
50 |
05/23/2002 |
621 |
SD |
SU 07/04, 2005, RT, CH, OT |
unknown |
18.55 |
| 39 |
M |
40.33 |
BT-15 |
Anaplastic Astrocytoma |
60 |
01/23/2003 |
257 (305 po) |
SD |
None |
HGG |
10.05 |
| 40 |
M |
12.25 |
BT-23SE |
Glioma High Grade/Visual Pathway |
50 |
04/09/2003 |
479 (1983 po) |
PR |
None |
Alive 10/10/25 |
33.25 (+) |
| 41 |
F |
41.58 |
BT-09 |
Anaplastic Astrocytoma |
80 |
05/27/2003 |
170 |
PD |
SU 11/03 |
unknown |
7.33 |
| 42 |
M |
43.08 |
BT-09 |
Anaplastic Astrocytoma |
90 |
12/03/2003 |
664 |
SD |
SU 10/05 |
unknown |
8.47 |
| 43 |
M |
49.25 |
BT-18 |
Anaplastic Astrocytoma/Mixed |
70 |
02/10/2004 |
42 |
NE |
2xRT |
HGG |
8.34 |
| 44 |
F |
1.08 |
BT-06SE |
Anaplastic Astrocytoma |
50 |
02/26/2004 |
841 |
SD |
SU 03/08, 3xCH, TT |
unknown |
10.98 |
| 45 |
M |
33.25 |
BT-09 |
Anaplastic Astrocytoma/Mixed |
70 |
04/13/2004 |
255 |
SD |
SU 02/05, RT,CH |
unknown |
15.17 |
| 46 |
M |
43.25 |
BT-15SE |
Anaplastic Astrocytoma |
40 |
09/22/2004 |
3 |
NE |
None |
HGG |
21.25 |
| 47 |
M |
37.00 |
BT-09SE |
Anaplastic Astrocytoma |
50 |
02/09/2005 |
531 (300 po) |
CR |
None |
unknown |
14.72 |
| 48 |
M |
30.58 |
BT-15SE |
Anaplastic Astrocytoma |
50 |
06/15/2005 |
37 |
NE |
TT |
unknown |
10.42 |
| 49 |
M |
47.00 |
BT-15SE |
Anaplastic Astrocytoma |
50 |
07/01/2005 |
298 |
SD |
None |
Alive 10/09/25 |
21.03 (+) |
| 50 |
M |
28.33 |
BT-15SE |
Anaplastic Astrocytoma |
50 |
07/07/2005 |
9 |
NE |
None |
HGG |
6.00 |
| 51 |
F |
24.42 |
BT-08SE |
Anaplastic Astrocytoma |
40 |
10/17/2005 |
2193 |
CR |
None |
HGG |
6.16 |
| 52 |
M |
30.58 |
BT-18 |
Anaplastic Oligoastrocytoma |
70 |
01/18/2007 |
84 |
PD |
SU 5/07, CH |
unknown |
6.37 |
| 53 |
M |
36.25 |
BT-08 |
Anaplastic Astrocytoma |
80 |
08/17/2007 |
427 |
CR |
SU 12/08, RT, CH, TT |
unknown |
13.41 |
| 54 |
F |
23.25 |
BT-15 |
Anaplastic Astrocytoma |
80 |
09/13/2007 |
474 |
SD |
None |
Alive 10/09/25 |
18.97 (+) |
| 55 |
M |
27.50 |
BT-09SE |
Anaplastic Astrocytoma/DIPG |
50 |
01/06/2011 |
213 |
SD |
None |
unknown |
8.90 |
| 56 |
M |
56.00 |
BT-21SE |
Anaplastic Astrocytoma |
90 |
06/30/2011 |
39 |
NE |
SU 12/11, 2xOT |
unknown |
5.24 |
| 57 |
M |
50.33 |
BT-15SE |
Anaplastic Astrocytoma |
50 |
08/31/2011 |
95 |
NE |
TT, CH |
unknown |
6.74 |
| 58 |
F |
27.33 |
BT-09SE |
Anaplastic Astrocytoma |
50 |
12/14/2011 |
535 |
CR |
None |
post viral treatment |
12.13 |
| 59 |
F |
25.67 |
BT-09SE |
Astrocytoma Infiltrating/DIPG |
90 |
02/02/2012 |
506 |
SD |
None |
Alive 09/09/25 |
14.41 (+) |
| 60 |
F |
20.00 |
BT-09SE |
Anaplastic Astrocytoma |
90 |
03/09/2012 |
52 |
PD |
RT, CH, TT |
Alive 10/23/25 |
13.75 (+) |
| 61 |
M |
43.50 |
BT-09SE |
Anaplastic Oligodendroglioma |
90 |
07/18/2012 |
9 |
NE |
None |
HGG |
6.57 |
Note: ANP-Antineoplastons, CH-Chemotherapy, CR-Complete Response, DIPG-Diffuse Intrinsic Pontine Glioma, HGG-High Grade Glioma, KPS-Karnofsky Performance Score, LPS-Lansky Performance Score, NE-Non Evaluable, OSD-Overall Survival Since Diagnosis, OT-Other Therapy, PD-Progressive Disease, po-per ora, PR-Partial Response, RT-Radiation Therapy, SD-Stable Disease, SE-Special Exemption, SU-Surgery, TT-Targeted.
Tab. 3. Best response to ANP, overall survival from diagnosis and status at last follow-up.
Two representative cases are described:
Case# 1 (Case 8 in Tab. 2. and Tab. 3.)
In November 1993, a 12-year-old Caucasian female, with no prior health issues, was found to have a contrast-enhancing tumor in the left temporal lobe, which crossed the midline and compressed the superior pons. Biopsy revealed pleomorphic tumor cells with mitotic figures. Review of the microscopic sections of tumor tissue by experienced pathologists provided a diagnosis of Anaplastic Astrocytoma (AA). No treatment was started initially, but a brain MRI performed nine weeks later showed tumor progression.
In January 1994, the patient was seen at the BC. Symptoms included headaches, nausea, memory loss, slurred speech and fatigue. She usually experienced four focal seizures per day, some days more, some days less, with occasional grand mal seizures. Physical exam revealed hesitant and slurred speech. LPS was 60. MRI of the brain confirmed a 4.8 cm × 2.1 cm infiltrating, enhancing mass in the left temporal lobe that crossed the midline and compressed the brainstem.
Treatment with IV ANP began on January 7, 1994, per the phase II protocol. Dosages of A10 and AS2-1 were gradually increased until maximum tolerated doses were achieved. Follow-up brain MRIs at 3 and 14 months showed no significant change in tumor size, but by 13 months, all symptoms had been resolved while seizures persisted. Brain MRIs at 23 and 29 months of treatment showed tumor shrinkage by 96% and 98%, respectively, indicating a PR. At 32 months, brain MRI showed no enhancement, indicating achievement of a CR. Persistent seizures were felt to de due to scarring. IV ANP was discontinued at 40 months and oral maintenance ANP was initiated. Brain MRIs at 45, 51 and 55 months revealed a persistent CR. After 56 months of treatment, oral ANP was also discontinued. Three months later, scar tissue in the tumor bed was removed and the seizures ended. Follow-up brain MRIs at 6 and 15 years post-ANP showed no evidence of tumor; the CR was persistent. At the last follow-up in December 2024, the patient was doing well and living a normal life. Her OSD at that time was more than 31 years. There have been no long-term adverse sequelae.
Case #2 (Case 33 in Tab. 2. and Tab. 3.)
In May 2000, a 31-year-old female developed right upper extremity clumsiness and weakness of the right leg. She sought medical attention and was referred to a neurologist. MRI of the brain, performed on May 10, 2000, revealed a 2.0 cm2 mass in the left parietal lobe. Computerized Tomography (CT) scans of the chest, abdomen and pelvis were performed on May 11, 2000 and found to be normal. On May 15, 2000, the patient underwent a stereotactic biopsy of the parietal tumor. Histological examination of tumor tissue slides revealed an AA.
When the patient presented at the BC, she had not had SU, RT, or CH. A baseline MRI of the brain, with gadolinium contrast, performed on June 1, 2000, showed a 2.0 cm2 non-enhancing mass and two smaller enhancing masses (0.02 cm2 and 0.15 cm2) in the left parietal lobe. On June 6, 2000, the patient began IV ANP therapy and the ANP dosages were gradually increased to the maximum tolerated dosages. Throughout her IV ANP therapy, the patient experienced elevations in transaminases, which, on occasion, interrupted her therapy. MRI of the brain on July 3, 2000, one month after initiation of ANP therapy, demonstrated complete resolution of the enhancing disease in the left parietal lobe, indicating the achievement of a CR. However, the 2.0 cm2 non-enhancing tumor persisted. The MRI of the brain, performed on July 31, 2000, demonstrated a persistent CR. On August 1, 2000, IV ANP therapy was terminated, while oral maintenance ANP began on August 4, 2000.
The patient continued taking oral ANP until October 31, 2001, when she elected to stop all ANP therapy. Long-term follow-up brain MRI on February 6, 2017, showed a persistent CR and complete disappearance of the 2.0 cm2 non-enhancing nodule, indicating an enduring CR and complete tumor regression. The patient experienced two serious adverse events possibly related to ANP, both of which were resolved completely.
At the last follow-up in October 2025, she was doing well, had no evidence of tumor recurrence and had an OSD of more than 25 years and five months.
Discussion
An emerging therapeutic approach is Tumor-Treating Fields (TTFields) [71]. These fields are a non-invasive cancer therapy that uses low-intensity, intermediate-frequency, alternating electric fields to disrupt cancer cell division by interfering with mitotic spindle formation and chromosome segregation, resulting in mitotic arrest and apoptosis. Therapy is delivered via transducer arrays placed on the scalp and connected to a portable device [71].
The EF-14 phase III trial evaluated TTFields in combination with maintenance temozolomide and following standard chemoradiotherapy in patients with newly diagnosed GBM. Patients receiving TTFields plus TMZ demonstrated significantly improved progression-free survival (6.7 months vs. 4.0 months) and overall survival (20.9 months vs. 16.0 months) compared to TMZ alone. There was no significant increase in toxicity. Two-year survival rates were also higher in the TTFields arm (43% vs. 30%) [71]. Subsequent analyses have confirmed the findings of the EF-14 trial. TTFields therapy has been correlated with survival benefits and with outcomes similar to those observed in EF-14 [72].
The EF-14 results were published in 2014 and the study design did not fully account for the molecular markers that have become increasingly important for cohort stratification in clinical studies. Because of the diagnostic criteria presented in the 2021 WHO classification of CNS tumors [4,5], challenges to the design of clinical trials for HGG are significant. For example, IDH1/2 mutations define a distinct subset of gliomas with improved survival [73]. Genetic analyses also distinguish primary GBM, characterized by EGFR amplification and PTEN loss, from secondary GBM, arising from lower-grade gliomas with TP53 and IDH mutations [74].
In 2024, Barrera and colleagues reported a retrospective survival analysis of 80 HGG patients who underwent surgical resection and were treated from 2012 to 2015 at the Cancer Institute of the Americas Clinic in Medellin, Colombia [75]. The histological diagnosis was based on the 2007 WHO classification [76], not the diagnostic criteria presented above. Clinical, demographic and lifestyle characteristics were analyzed, along with genetic instability in white blood cells. The Kaplan-Meier analysis indicated an average survival of two years and two months. A Cox proportional hazards model showed that patient age, exposure to polycyclic hydrocarbons at work and the number of sister-chromatid exchanges in lymphocytes at the first sampling were significantly associated with survival in the multivariate analysis.
We report here on a survival analysis of 310 non-GBM HGG patients treated in phase II studies of ANP at BC between May 1988 and March 2014. Survival of more than 5 years up to more than 33 years was observed in 61 patients (19.6%). All these patients were seen after their diagnoses were established at outside academic institutions with prominent neuroradiologists confirming the ORs. The case of one of these patients was reviewed and confirmed by experts from the National Cancer Institute [77].
The mechanism of action of ANP differs from that of RT or CH. The growth of normal cells is controlled by cell cycle progression genes (oncogenes) and by cell cycle arrest genes (tumor suppressor genes). In cancer, alterations in these control genes in malignant cells favor aggressive cell proliferation. Evidence shows that ANP affects more than 535 gene aberrations in the malignant genome and functions as a "molecular switch" that "turns on" tumor-suppressor genes and "turns off" oncogenes [78]. Hence, the antineoplastic action of ANP involves restoration of cell cycle control, induction of programmed cell death and interference with cancer cell metabolism and nuclear transport.
In 1986, we published an article proposing that the neoplastic process is a disease of information processing [79]. The neoplastic process develops according to the "program" encoded in a network of mutated genes [32,79-87]. The technology now utilized can detect the DNA of these mutated genes in blood at concentrations as low as one billionth of a gram/mL [88]. Laboratories, such as Foundation Medicine, Guardant360 and Tempus AI, can provide results within two weeks and many insurance policies in the USA cover the tests.
At the BC, DNA analyses are compared with a list of 535 genomic aberrations compiled from early laboratory data on the effects of ANP on the entire genome of GBM [78] and from clinical data derived from blood and tissue testing of patients treated at the BC [88]. For each patient whose blood was tested, the number of genomic aberrations found to be affected by ANP determined the patient's candidacy for ANP.
We have found the genomic aberrations affected by ANP through our testing of blood samples from patients with over 70 different cancer diagnoses, including brain tumors [89-91]. Our goal is to correlate the ANP-related removal of genomic aberrations from patients’ blood with radiological response and survival. Based on our genomic testing, 114 aberrations significant in driving HGG progression [92] were affected and removed from the patients’ blood by ANP, including 51 aberrations of TP53, 19 of PIK3CA, 11 of NF1, 9 of PTEN, 5 of EGFR, 3 each of CCND1, IDH, and PDGFRA, and 1 each of CCND2, CDK6, CDKN2A, and CDKN2B. Some of these changes may have been influenced by other prescription drugs given to patients with advanced disease. New data will supplement these results once the number of tested genes increases beyond the 600-800 genes currently being tested. Based on this new information, we will include additional targeting agents along with ANP in treatment regimes, likely improving the rate of ORs and length of survival [87].
The survival of 61 evaluable, non-GBM HGG patients patients from 5 years to 33 years and the survival of 23 evaluable patients in this group from 12 years to 33 years, is very unusual. Surviving patients have no adverse long-term sequelae related to ANP and live normal lives, with healthy children. Thirty patients experienced Serious Adverse Events (SAEs) that were unrelated to ANP and all fully recovered. Four patients experienced six SAEs that were possibly related to ANP (fever without infection, nausea, dizziness and somnolence) and all fully recovered, as well. The authors are not aware of similar findings from other clinical studies of HGG.
Based on the OSD data shown above and the lack of consistently corresponding ORs, it is very likely that another ANP mechanism of action contributes to the unusually long survival observed in the 61 HGG patients. Additional publications describing the unusually long survival of patients receiving ANP in phase II studies of GBM, Recurrent Medulloblastoma (RMB) and Diffuse Intrinsic Pontine Glioma (DIPG) have already been published [66-68].
Conclusion
We present here the unusually long OSD seen in 61 non-GBM HGG patients treated in Phase II clinical studies with ANP, a therapy that avoids the long-term sequelae of RT and CH. The survival of 23 HGG patients for more than 12 years implies a cure. Because these studies were performed before current genomic testing techniques were available, these new technologies will permit better clinical trial design, improved cohort stratification and more accurate results.
ANP has proven to be an attractive option for a wide range of patients with persistent, recurrent, disseminated and/or metastatic brain tumors. Multiple Phase II clinical studies of Antineoplaston therapy in various advanced primary brain tumors, conducted under the Burzynski Research Institute's IND # 43,742, have now been completed and numerous articles have been published. The results presented here show that ANP is an effective option for treating HGG without causing long-term toxicity. Our group of HGG patients with exceptionally long-term survival is the largest series of HGG survivors documented in the world literature. Our findings support ANP as a viable treatment choice for these aggressive brain tumors.
Acknowledgement
The authors would like to express their appreciation to Carolyn Powers and Tanya Miller for their involvement.
Conflict of Interest
All the Authors of this paper have declared that there is no conflict of interest.
References
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353(8):811-822.
Google Scholar, Cross Ref, Indexed at
- Price M, Ballard C, Benedetti J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2017–2021. Neuro-Oncol. 2024;26(6):vi1.
Google Scholar, Cross Ref, Indexed at
- Depond CC, Bauchet L, Elhairech D, et al. Survival after newly-diagnosed high-grade glioma surgery: What can we learn from the French National healthcare database?. Brain Tumor Pathol. 2024;12(3):162-171.
Google Scholar, Cross Ref, Indexed at
- Antonelli M, Poliani PL. Adult type diffuse gliomas in the new 2021 WHO Classification. Pathologica. 2022;114(6):397.
Google Scholar, Cross Ref, Indexed at
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-oncology. 2021;23(8):1231-1251.
Google Scholar, Cross Ref, Indexed at
- Mesfin FB, Karsonovich T, Al-Dhahir MA. Gliomas. StatPearls Publishing. 2025.
Google Scholar, Indexed at
- Frosina G. Recapitulating the key advances in the diagnosis and prognosis of high-grade gliomas: Second half of 2021 update. Int J Mol Sci. 2023;24(7):6375.
Google Scholar, Cross Ref, Indexed at
- Ramos ME, Leon AR, Punzalan-Sotelo AM. Diffused Multifocal High Grade Glioma. J Health Med Sci. 2022;5(2).
Google Scholar
- Walbert T, Schultz L, Mikkelsen T, et al. Prospective assessment of end-of-life symptoms and quality of life in patients with high-grade glioma. Neuro-Oncol Prac. 2024;11(6):733-739.
Google Scholar, Cross Ref, Indexed at
- Fisher JP, Adamson DC. Current FDA-approved therapies for high-grade malignant gliomas. Biomedicines. 2021;9(3):324.
Google Scholar, Cross Ref, Indexed at
- von Bueren AO, Kwiecien R, Gielen GH, et al. Final analysis of the HIT-HGG-2007 trial (ISRCTN19852453): Significant survival benefit for pontine and non-pontine pediatric high-grade gliomas in comparison to previous HIT-GBM-C/-D trials. Neuro-Oncol. 2022;24(1):i63-i64. .
Google Scholar, Cross Ref, Indexed at
- Segura PP, Quintela NV, García MM, et al. SEOM-GEINO clinical guidelines for high-grade gliomas of adulthood (2022). Clin Transl Oncol. 2023;25(9):2634-2646.
Google Scholar, Cross Ref, Indexed at
- Fekete B, Werlenius K, Tisell M, et al. What predicts survival in glioblastoma? A population-based study of changes in clinical management and outcome. Front Surg. 2023;10:1249366.
Google Scholar, Cross Ref, Indexed at
- Dirven L, Aaronson NK, Heimans JJ, et al. Health-related quality of life in high-grade glioma patients. Chin J Cancer. 2014;33(1):40.
Google Scholar, Cross Ref, Indexed at
- Bendari A, Sham S, Hammoud H, et al. “Comprehensive Analysis of Factors Influencing Recurrence and Survival in Glioblastoma: Implications for Treatment Strategies”. J Mol Pathol. 2024;5(4):520-532.
Google Scholar, Cross Ref, Indexed at
- Huang M, Li S, Li P, et al. Drug clinical trials on high-grade gliomas: Challenges and hopes. Cancer Biol Med. 2024;20(12):947.
Google Scholar, Cross Ref, Indexed at
- Ciammella P, Cozzi S, Botti A, et al. Safety of inhomogeneous dose distribution IMRT for high-grade glioma reirradiation: A prospective phase I/II trial (GLIORAD TRIAL). Cancers. 2022;14(19):4604.
Google Scholar, Cross Ref, Indexed at
- Burzynski S. Antineoplastons: biochemical defense against cancer. Phy Chem Phys. 1976;8(3):275-279.
Google Scholar, Indexed at
- Reyes-Botero G, Laigle-Donadey F, Mokhtari K, et al. Temozolomide after radiotherapy in recurrent “low grade” diffuse brainstem glioma in adults. J Neuro-Oncol. 2014;120(3):581-6.
Google Scholar, Cross Ref, Indexed at
- Burzynski S, Burzynski G, Janicki T, et al. Recurrent/disseminated choroid plexus carcinoma: overall Survival of> 23.6 years in a one-year and nine-month-old female treated with Antineoplastons. J Med Res Case. 2024;6(1).
Google Scholar
- Burzynski SR, Burzynski GS, Janicki T, et al. Recurrent desmoplastic infantile ganglioglioma treated with antineoplastons: partial response and overall survival of> 11.8 Years in a ten-month-old female. Int J Brain Disord Treat. 2024;10:50.
Google Scholar
- Burzynski S, Burzynski G, Janicki T, et al. Recurrent pilocytic astrocytoma: treatment with antineoplastons, complete response, and> 27 years overall survival. Recent Adv Clin Trials. 2024;4(1):1-7.
Google Scholar
- Burzynski S, Burzynski G, Janicki T, et al. Progressive, multicentric, midline, low-grade glioma: survival greater than 24.8 years in three patients treated with antineoplastons A10 and AS2-1 in phase II studies. Annal Cas Rep Rev. 2024;423:2.
Google Scholar
- Burzynski SR, Burzynski GS, Janicki TJ, et al. Persistent pineoblastoma: complete response and> 26 years overall survival in a ten-month-old female treated with antineoplastons. Biomed Res Clin Prac. 2023;7:1-5.
Google Scholar
- Burzynski S, Burzynski G, Janicki T, et al. Recurrent Medulloblastoma: Complete Response and > than 21 Years and Five Months Overall Survival in a One-Year and Seven-Month-Old Male Treated with Antineoplastons. J Oncol Res Rev Rep. 2023;4(4):3-6.
- Burzynski S, Burzynski G, Janicki T, et al. Newly diagnosed Anaplastic Astrocytoma:> 23 Year Survival in a 31-Year and 11-Month-Old Female Treated with Antineoplastons. Neurol Neurosci. 2023;4(2):1-6.
Google Scholar
- Burzynski SR, Burzynski GS, Janicki TJ, et al. Inoperable Optic Pathway Glioma: A Seven-Year-Old Male with> 35 Years Overall Survival Following Treatment with Antineoplastons. Eur J Clin Med. 2023;4(5):9-14.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Burzynski G, Janicki T, et al. A 25-year-female with Diffuse Intrinsic Pontine Glioma Surviving for More than Nine Years Following Treatment with Antineoplastons. Cancer Res. 2022;7(1):1-7.
Google Scholar, Cross Ref, Indexed at
- Burzynski S, Burzynski G, Janicki T, et al. Newly diagnosed Glioblastoma: Partial Response and> 27 Years Overall Survival in a 37-Year-Old Male Treated with Antineoplastons. Recent Adv Clin Trials. 2022;1(2):1-7.
Google Scholar, Cross Ref, Indexed at
- Burzynski S, Burzynski G, Janicki T, et al. Newly diagnosed Multicentric Pilocytic Astrocytoma: Complete Response and> 22 Years Survival in a Six Year and Nine-month-old Female Treated with Antineoplastons. Internat J Clin Oncol and Cancer Res. 2022;7(3):76-82.
Google Scholar
- Burzynski S, Burzynski G, Janicki T, et al. Recurrent and progressive ganglioglioma in an 11-year-old male treated with antineoplastons: Partial response with more than nine years and nine months survival and complete resolution of clinical symptoms/signs. Biomed Res J. 2022;37:1-3.
Google Scholar
- Burzynski SR, Janicki T, Burzynski G. Comprehensive genomic profiling of recurrent classic glioblastoma in a patient surviving eleven years following antineoplaston therapy. Cancer Clin Oncol. 2015;4(2):41-52.
Google Scholar, Cross Ref, Indexed at
- Burzynski S, Burzynski G, Janicki T, et al. Outcomes in Four Children with Persistent, Recurrent, and Progressive Gangliogliomas Treated in Phase II Studies with Antineoplastons A10 and AS2-1. Neurol Neurosci. 2022;3(3):1-9.
Google Scholar
- Burzynski S, Burzynski G, Janicki T, et al. Recurrent/Persistent Glioblastoma: Complete Response and 24 Years Disease-Free-Survival in a 45-Year-Old Female Treated with Antineoplastons. Cancer Ther. 2022;7(3):1-6.
Google Scholar
- Burzynski SR, Janicki T, Burzynski GS, et al. Long-term survival (27.7 years) following IV Antineoplaston Therapy (ANP) in a 36-year-old-female with a progressive diffuse intrinsic pontine glioma (DIPG). Int J Radiol Imaging Technol. 2021;7:073-078.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Burzynski GS, Janicki T, et al. Long-term survival (23 years) in a 26-year-old male after Antineoplaston therapy for a progressive, diffuse intrinsic pontine glioma: A case report. Int J Brain Disorder Treat. 2021;6:038-044.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki T, Burzynski GS, et al. Resolution of clinical signs, a complete response, and long-term survival (> 23 Years) in a 3 and ½ month female with a newly diagnosed diffuse intrinsic pontine glioma treated with antineoplastons. Biomed Res Clin Prac. 2021;6:1-6.
Google Scholar
- Burzynski SR, Janicki T, Burzynski GS, et al. Diffuse intrinsic pontine glioma in an 11-year-old female treated with antineoplastons: Complete response and> 25-year survival. Pediatr Neonatal Med. 2021;1(2):1-5.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. A phase II study of Antineoplastons A10 and AS2-1 in children with brain tumors. Final Report (Protocol BT-10). J Cancer Ther. 2017;8(02):173.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS. A Phase II Study of Antineoplastons A10 and AS2-1 in Children with Low-Grade Astrocytomas—Final Report (Protocol BT-13). J Cancer Ther. 2016;7(12):837-850.
Google Scholar
- Burzynski S, Janicki TJ, Burzynski GS. Primary CNS tumors and leptomeningeal, disseminated, and/or multicentric disease in children treated in phase II studies with antineoplastons A10 and AS2-1. Cancer Clin Oncol. 2016;5(2):38-48.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. A phase II study of Antineoplastons A10 and AS2-1 in patients with brainstem gliomas. The report on non-diffuse intrinsic pontine glioma (Protocol BT-11). J Cancer Ther. 2015;6(4):334-344.
Google Scholar
- Burzynski SR, Janicki TJ, Burzynski GS. A Phase II Study of Antineoplastons A10 and AS2-1 in Adult Patients with Primary Brain Tumors-Final Report (Protocol BT-09). J Cancer Ther. 2015;6(12):1063.
Google Scholar
- Burzynski SR, Burzynski GS, Janicki TJ, et al. Complete response and Long-term survival (> 20 years) of a child with tectal glioma: A case report. Pediatr Neurosurg. 2015;50(2):99-103.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. A phase II study of antineoplastons A10 and AS2-1 in adult patients with newly-diagnosed anaplastic astrocytoma. Final report (Protocol BT-08). Clin Oncol. 2015;4:28-38.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS. A Phase II Study of Antineoplastons A10 and AS2-1 Injections in Adult Patients with Recurrent Anaplastic Astrocytoma—Final Report (Protocol BT-15). Clin Oncol. 2015;4:13-23.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Burzynski GS, Marszalek A, et al. Long-term survival (over 20 years), complete response and normal childhood development in medulloblastoma treated with Antineoplastons A10 and AS2-1. J Neurol Stroke. 2015;2(3):00054.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Burzynski GS, Marszalek A, et al. Long-term survival over 21 years and pathologically confirmed complete response in pediatric anaplastic astrocytoma: A case report. J Neurol Stroke. 2015;2(6):00072.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Burzynski GS, Brookman S. A case of sustained objective response of recurrent/progressive diffuse intrinsic pontine glioma with phenylbutyrate and targeted agents. J Cancer Ther. 2014;6(01):40.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. A phase II study of antineoplastons A10 and AS2-1 in children with high-grade glioma. Final Report (Protocol BT-06), and review of recent trials. J Cancer Ther. 2014;5(6):565-77.
Google Scholar
- Burzynski SR, Janicki TJ, Burzynski GS. A phase II study of antineoplastons A10 and AS2-1 in adult patients with recurrent glioblastoma multiforme: Final report (Protocol BT-21). J Cancer Ther. 2014;5(10):946-56.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. A phase II study of antineoplastons A10 and AS2-1 in children with recurrent, refractory or progressive primary brain tumors—Final report (Protocol BT-22). J Cancer Ther. 2014;5(10):977-88.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. Preliminary findings on the use of targeted therapy with pazopanib and other agents in combination with sodium phenylbutyrate in the treatment of glioblastoma multiforme. J Cancer Ther. 2014;5(14):1423.
Google Scholar, Cross Ref, Indexed at
- Burzynski G, Janicki T, Marszalek A, et al. PT-02: Long-term survival (> 20 years) of a child with brainstem glioma treated with antineoplastons A10 and as2-1: A case report. Neuro-Oncol. 2014 Nov;16(5):v175.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. The response and survival of children with recurrent diffuse intrinsic pontine glioma based on phase II study of antineoplastons A10 and AS2-1 in patients with brainstem glioma. Child's Nerv Syst. 2014;30(12):2051-61.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. Long-term survival (> 13 years) in a child with recurrent diffuse pontine gliosarcoma: a case report. Pediatr Hematol Oncol. 2014;36(7):e433-9.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR. Recent clinical trials in diffuse intrinsic brainstem glioma. Cancer Ther. 2007;5:379-390.
Google Scholar
- Burzynski SR, Janicki TJ, Weaver RA, et al. Targeted therapy with antineoplastons A10 and AS2-1 of high-grade, recurrent and progressive brainstem glioma. Integr Cancer Ther. 2006;5(1):40-7.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR. Treatments for astrocytic tumors in children: current and emerging strategies. Paediatr Drugs. 2006;8(3):167-178.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Weaver RA, Janicki T, et al. Long-term survival of high-risk pediatric patients with primitive neuroectodermal tumors treated with antineoplastons A10 and AS2-1. Integr Cancer Ther. 2005;4(2):168-177.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Weaver RA, Lewy RI, et al. Phase II study of antineoplaston A10 and AS2-1 in children with recurrent and progressive multicentric glioma: A preliminary report. Drugs in R & D. 2004;5(6):315-26.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Lewy RI, Weaver R, et al. Long-term survival and complete response of a patient with recurrent diffuse intrinsic brain stem glioblastoma multiforme. Integr Cancer Ther. 2004;3(3):257-261.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Weaver RA, Bestak M, et al. Treatment of primitive neuroectodermal tumors (PNET) with antineoplastons A10 and AS2-1 (ANP). Peliminary results of phase II studies. In Neuro-Oncol. 2004;6(4):428-428.
Google Scholar
- Burzynski SR, Weaver RA, Bestak M,et al. Phase II studies of antineoplastons A10 and AS2-1 (ANP) in children with atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system. A preliminary report. In Neuro-Oncol. 2004;6(4):427-427).
Google Scholar
- Burzynski SR, Conde AB, Peters A, et al. A retrospective study of antineoplastons A10 and AS2-1 in primary brain tumours. Clin Drug Investig. 1999;18(1):1-0.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Burzynski GS, Janicki TJ, et al. Unusually long survival from diagnosis (more than three years to more than 29 years) in 27 glioblastoma patients treated with antineoplastons in phase II studies. Neurol Neurosci 2025;6(7): 036-045.
Cross Ref, Indexed at
- Burzynski SR, Burzynski GS, Janicki TJ, et al. Unusually Long Survival (More Than Five Years to More Than 31 Years) in Twelve Patients with Relapsed Medulloblastoma Treated with Antineoplastons in Phase II Studies. Eur J Clin Exp Med. 2025;6(4):1-8.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. Diffuse intrinsic pontine glioma: Unusually long survival of 28 patients (from 3 years to over 29 years) in phase 2 studies with antineoplastons A10 and AS2-1. J Neurol. 2025;16(03):001-11.
Google Scholar
- Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. Clin Oncol. 2010;28(11):1963-1972.
Google Scholar, Cross Ref, Indexed at
- Chinot OL, Macdonald DR, Abrey LE, et al. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013;13(5):347.
Google Scholar, Cross Ref, Indexed at
- Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. Jama. 2015;314(23):2535-2543.
Google Scholar, Cross Ref, Indexed at
- Ballo MT, Conlon P, Lavy-Shahaf G, et al. Real-world experience with tumor treating fields (TTFields) in newly diagnosed glioblastoma: A survival meta-analysis with systematic review. Clin Oncol. 2023;41(16):2059.
Google Scholar, Cross Ref, Indexed at
- Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765-773.
Google Scholar, Cross Ref, Indexed at
- Ohgaki H. Genetic pathways to glioblastomas. Neuropathol. 2005 Mar;25(1):1-7.
Google Scholar, Cross Ref, Indexed at
- Barrera LM, Ortiz LD, Grisales HD, et al. Survival analysis and associated factors of high-grade glioma patients. Biomedica. 2024;44(2):191-206.
Google Scholar, Cross Ref, Indexed at
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97-109.
Google Scholar, Cross Ref, Indexed at
- Hawkins MJ, Friedman MA. National Cancer Institute's evaluation of unconventional cancer treatments. J Natl Cancer Inst. 1992;84(22):1699-1702.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Patil SS. The effect of antineoplastons A10 and AS2-1 and metabolites of sodium phenylbutyrate on gene expression in glioblastoma multiforme. J Cancer Ther. 2014;2014.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR. Antineoplastons: history of the research (I). Drugs Exp Clin Res. 1986;12:1-9.
Google Scholar, Indexed at
- Burzynski SR. Targeted therapy for brain tumors. Brain Cancer: Therapy and Surgical Interventions (Horizons in Cancer Research). Nova Science Publishers Inc. 2006:77-111.
Google Scholar
- Burzynski SR, Janicki TJ, Burzynski GS, et al. Preliminary Findings on the Use of Targeted Therapy in Combination with Sodium Phenylbutyrate in Advanced Malignant Mesothelioma: A Strategy for Improved Survival. J Cancer Ther. 2014;5(12):1127-1144.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. Preliminary findings on the use of targeted therapy in combination with sodium phenylbutyrate in colorectal cancer after failure of second-line therapy-a potential strategy for improved survival. J Cancer Ther. 2014;5(13):1270.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Janicki TJ, Burzynski GS, et al. Preliminary Findings on the Use of Targeted Therapy in Combination with Sodium Phenylbutyrate in Recurrent Advanced Pancreatic Cancer-A Potential Strategy for Improved Survival. J Cancer Ther. 2014;5(12):1072-1091.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Burzynski GS, Janicki TJ. Recurrent glioblastoma multiforme-A strategy for long-term survival. J Cancer Ther. 2014;5(10):957-976.
Google Scholar
- Burzynski SR, Janicki T, Beenken S. Treatment of recurrent glioblastoma multiforme (rGBM) with Antineoplaston AS2-1 in combination with targeted therapy. Cancer Clin Oncol. 2019;8:1-5.
Google Scholar, Cross Ref, Indexed at
- Best MG, Sol N, Zijl S, et al. Liquid biopsies in patients with diffuse glioma. Acta Neuropathol. 2015;129(6):849-865.
Google Scholar, Cross Ref, Indexed at
- Burzynski SR, Inventor. Methods for the treatment of recurrent glioblastoma (RGBM). United States patent US. 2020.
Google Scholar
- Burzynski SR, Inventor. Methods for the treatment of glioblastoma multiforme. United States patent US. 2022.
Google Scholar
- Burzynski SR, Inventor. Methods for the treatment of leptomeningeal disease. United States patent US. 2023.
Google Scholar
- Bax DA, Mackay A, Little SE, et al. A distinct spectrum of copy number aberrations in pediatric high-grade gliomas. Clin Cancer Res. 2010;16(13):3368-3377.
Google Scholar, Cross Ref, Indexed at
- Giambra M, Messuti E, Di Cristofori A, et al. Characterizing the genomic profile in high-grade gliomas: from tumor core to peritumoral brain zone, passing through glioma-derived tumorspheres. Biology. 2021;10(11):1157.
Google Scholar, Cross Ref, Indexed at
- Senger D, Cairncross JG, Forsyth PA. Long-term survivors of glioblastoma: Statistical aberration or important unrecognized molecular subtype?. Cancer Res J. 2003;9(3):214-221.
Google Scholar, Cross Ref, Indexed at