Review Article - (2023) Volume 15, Issue 1
Updated Review on Proniosomal Transdermal Drug Delivery System
Dr. Ram Babu Sharma*,
Deepak Kashyap and
Hitesh Thakur
Himalayan Institute of Pharmacy, Kala Amb Distt. Sirmaur (H.P.)-173030, India
*Correspondence:
Dr. Ram Babu Sharma, Himalayan Institute of Pharmacy, Kala Amb Distt. Sirmaur (H.P.)-173030,
India,
Tel: + 7018379926,
Email:
Received: 30-Dec-2022, Manuscript No. Ijddr-23-13351;
Editor assigned: 09-Jan-2023, Pre QC No. Ijddr-23-13351;
Reviewed: 23-Jan-2023, QC No. Ijddr-23-13351;
Revised: 27-Jan-2023, Manuscript No. Ijddr-23-13351;
Published:
31-Jan-2023, DOI: 10.36648-0975-9344-15.1-989
Abstract
Scientists worked to stabilise without having an impact on the noisome drug
delivery method its marital characteristics, which led to the creation of the
potential drug carrier proniosome. Drug delivery methods using niosomes and
liposomes have disadvantages that proniosomes do not. Dry formulations of a
non-ionic, water-soluble surfactant are called proniosomes and are applied to a
carrier system. Proniosome hydration results in the formation of niosomes.
They have the potential to increase the dissolution, accessibility, and uptake
of various medications by addressing the instability issues with niosomes and
liposomes. In addition, they provide a flexible method of drug delivery for a variety
of both hydrophilic and hydrophobic medicines. They can deliver medications
using a number of techniques to the intended site of action, offering a controlled
drug release of the medication and a reduction in any potentially harmful side
effects. It's critical to be knowledgeable of each study's limitations, which each
have their own benefits and drawbacks, in order to get the right study results.
Ecological, prospective, retrospective, case-control, case-crossover, or crosssectional
cohort designs are all possible for observational studies. A vital subclass
of observational experiments of the diagnosis research designs, which compare
the accuracy of different diagnostic approaches and tests to other diagnostic
measures, can be used to derive important findings. Only data collected utilising a
valid scientific methodology and the appropriate statistical methods can be used
in biomedical research to draw meaningful findings. As a result, it's critical to pick a
solid study strategy in order to offer a just and impartial evaluation of the research
concerns. This review focuses on a variety of proniosome-related topics including
- advantages, preparation, mechanism of action, materials and their specification,
study design, characterization &evaluation parameter.
Keywords
Proniosomes; Niosomal; Factorial designs; Relationship; TDS
INTRODUCTION
In a dry formulation, proniosomes are surfactant-coated carrier
with water solubility. When stirred in a hot water solution to form
a noisome dispersion, they quickly rehydrate prior to application.
Proniosomes maintain their physical stability while being stored
and transported. Drugs that are encased in the vesicular structure
of proniosomes have a longer shelf life in the bloodstream, have
better tissue penetration, and are less toxic. From a technical
perspective, niosomes are attractive drug carriers from a
technical perspective since they have superior chemical stability
and don't have the numerous drawbacks of liposomes, such
as their high cost and issues with changeable phospholipid
purity [1-5]. Proniosomes have drawn a lot of attention from
researchers since the early 1980s due to the possibility of using
them as pharmacological targets and carriers. In comparison to
conventional medication delivery methods, these applications
have a number of benefits while avoiding disadvantages [6].
Benefits of Proniosomes
(I) Non-ionic surfactants and phospholipids can both help with medication diffusion and act as penetration enhancers in
proniosomes.
(II) Proniosomes have various advantages, including simpler
distribution, storage, and dosage. (III) They avoid problems
including leakage, aggregation, fusion, and physical instability
that are connected to one or more aqueous dispersions.
(IV) Proniosomes avoid the problems associated with liposomes,
such as oxidative or hydrolytic degradation, also decreased
potential for fusion, agglomeration, or deposition while being
stored. (V) Proniosomes not only offer a promising drug delivery
technique, but they may also hasten epidermal barrier restoration
[7-10].
Mechanism of Action
A dormant form of niosomes is called a proniosome that require
hydration in order to become their active forms. The two ways
to hydrate are: the first uses the skin's natural moisture, and
the second uses solvents like water or a buffer. Transdermal
medication delivery methods use a variety of skin penetration
strategies. Due to their deformable characteristics, among them,
like transfers, may get through the skin undamaged. Other types,
like ethosomes, disrupt the epidermis' dense structure as they
enter the body intact. Still other types, like proniosomes and
niosomes, utilize surfactants to improve penetration into the
skin. The SC and viable epidermis must first be crossed by the
topically administered molecule [11-15].
There are three different routes by which this might occur: the
path of appendages, the intercellular highway is used by cells
and lipids to pass via sebaceous glands and hair follicles, or cells
travel via the complex web of lipids to reach other cells [16]. Skin
appendages are not a significant conduit because they account
for only 0.1% of total skin surface. Multiple partitioning and
diffusion phases are necessary to cross SC via the Trans cellular
pathway. Drug molecules are thought to be transported mostly
via the intercellular pathway. The fluidity and permeability of
the SC are increased, which enhances drug penetration into the SC, and the highly reversible organisation of the highly thick
intercellular lipid lamellae matrix is disturbed. Proniosomes
moisturise the skin when they are applied to it, causing a gradient
of thermodynamic activity to emerge at the interface, increasing
the diffusion pressure for drug penetration through the SC [17-20].
In the vascular system, niosomes are endocytosis, and proteolytic
enzymes break down their membranes, releasing the medication
they carry. Proniosome activity involves penetration via the skin
and systemic absorption and might be dermal, intracellular, or
transdermal (Figure 1). In terms of physical characteristics, a drug
intended for transdermal administration should have a molecular
weight of less than 600 Da, optimum oil solubility, an ideal
partition coefficient, a low latent heat of fusion, and a log p-value
of 1-3. Molecular entities with a log p-value below one are too
hydrophilic to successfully diffuse into SC by passive diffusion.
If the log p-value of the particle is greater than 3, the particle's
hydrophobicity will lead it to get stuck in the lipid matrix [21-25].
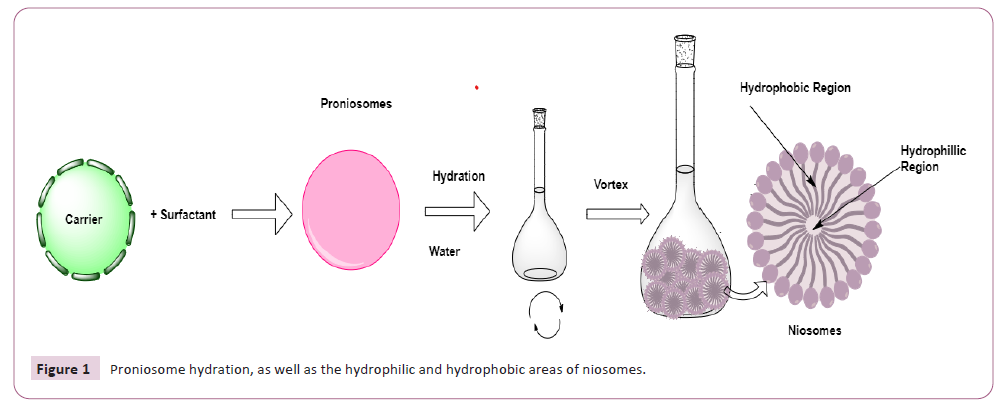
Figure 1: Proniosome hydration, as well as the hydrophilic and hydrophobic areas of niosomes.
Materials
The following are the various substances employed and how they
affected proniosomes preparation: (Table 1)
| Material |
Specification |
Action |
References |
| the span and tween |
Surfactants |
Maintains HLB level |
[36,37,39,42] |
|
1. Spans 20, 40, 60, and 80 2. Tween-20,60 |
|
|
|
3. Span 85 |
|
|
| cholesterol & lecithin |
stabilizers for membranes |
Cholesterol: Has an impact on the permeability and stability of vesicles. Lecithin is a penetration-enhancing substance. Keep the vesicles' stability, permeability, and integrity intact. Improves penetration |
|
|
|
|
[35,37,38,39,40,41] |
| Glucose monohydrate, Sucrose stearate, Lac, Mannitol, Polyols, and Maltose |
Carriers |
Holds the drug |
[35,37,38,39,40] |
| Methanol, chloroform, ethyl alcohol |
Organic solvents |
influence on the drug's vesicle size and penetration |
[35] |
Table-1. The various substances Employed and how they affected Proniosomes preparation.
Methods of preparation
There are several ways to create proniosomes, such as spraying
a non-ionic surfactant over water-soluble carrier particles,
employing a slurry method, and coacervation phase separation
[26], Provides an explanation of the preparation procedures and
their sequential processes (Table 2 and Figure 2)
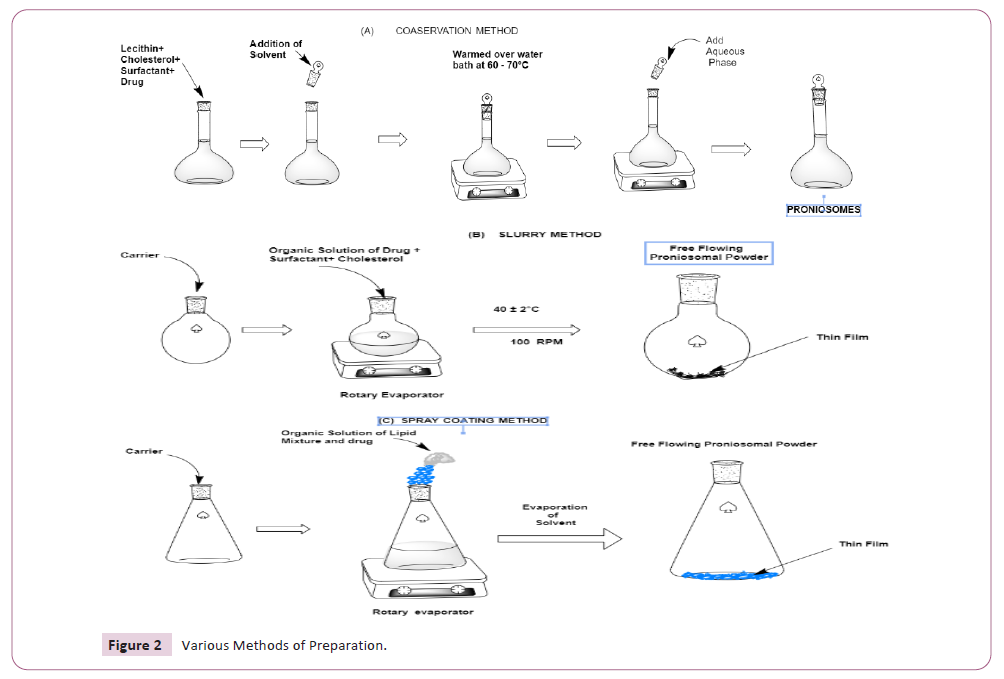
Figure 2: Various Methods of Preparation.
| Process of preparation |
Philosophy |
Product type |
References |
| Method of coacervation phase separation |
In order to create a translucent dispersion, lipids, a surfactant, and a medicine are mixed with a solvent and heated at 60 to 70 °C over a water bath. |
|
|
|
|
Transparent gel |
[18,19,20] |
| Slurry technique |
An organic solution, cholesterol, surfactants, and a medication are combined, and the resulting mixture is poured over a carrying medium to create slurry. To create proniosomes that flow freely, rotary evaporators should be used to evaporate the solvent. |
|
|
|
An organic solution, cholesterol, surfactants, and a medication are combined, and the resulting mixture is poured over a carrying medium to create slurry. To create proniosomes that flow freely, rotary evaporators should be used to evaporate the solvent. |
|
|
|
An organic solution, cholesterol, surfactants, and a medication are combined, and the resulting mixture is poured over a carrying medium to create slurry. To create proniosomes that flow freely, rotary evaporators should be used to evaporate the solvent. |
Powdered form |
[21–22] |
| Method of spray coating |
A spinning evaporator connected to a flask with a rounded bottom is used to spray organic cholesterol, surfactant, and medicine solutions one after the other onto a carrier material. |
|
|
|
|
|
|
|
|
Powdered form |
[23] |
Table-2. Explanation of the Preparation Procedures and their Sequential processes.
Study Design
Clinical epidemiology studies often concentrate on the
relationship between an exposure, such as a treatment or
environmental factor, and an outcome, like a disease or death.
Numerous study designs can be used to answer these research
topics. Both observational study types, such as cohort and
case-control studies, and randomised controlled trials (rcts) are
frequently employed in nephrology research [27] (Figure 3).
Figure 3: Analysis of System under Study
Types of experimental research design
The procedures used to collect data in experimental investigations
are referred to as experimental design in the classic sense.
Three sorts of experimental designs can be identified:
The following research designs are available:
• Design of the pre-experimental study
• True experimental or real-world experimental research
• Quasi-experimental.
The kind of research strategy to use depends on how you
categories study subjects depending on circumstances or
groupings.
1. Design of the pre-experimental study: An observational
group or groups are preserved after the application of causeand-
effect elements. If these groups need to be the topic of
additional research, this study will assist you in making that
decision.
Three sorts of preliminary analysis can be identified:
• Individual Case Studies
• One-group pre-post-test-test study design; comparison of
static groups.
1. Real-world experimental research methods: Statistics are
used in real experimental study to confirm or deny a notion.
It is therefore the most trustworthy sort of research. Only real design can demonstrate a cause-and-effect link within a
set of participants among the several forms of experimental
design. Three requirements must be met for an experiment
to be valid:
Two groups will participate in the study:
• Comparison of the experimental group, which will experience
changes, and the control group, which won't.
• A variable that a researcher can change
• Roughly distributed
This form of experimental inquiry is frequently employed in the
physical sciences [28, 29-35].
1. Design of a quasi-experimental study: "Quasi" means
"almost," which implies similarity. Though it is different
from an experimental design, a quasi-experimental design
is similar to one. Assigning a control group to each makes
the difference between the two. The individuals of a
group are not chosen at random; instead, an independent
variable is altered in this study. When random assignment is
unnecessary or unimportant, quasi-research is employed in
field situations (Table 3).
| Research Plan |
Essential Qualities |
Toughness |
Weakness |
| Case Report and Series of Cases |
one or several subjects. |
The earliest types of publications It is quick and affordable to produce hypotheses. |
There is very little chance of proving selection bias has a causal effect. |
|
Without a control group, a thorough description of (a) and (s) |
|
|
| Cross - sectional study /research |
Exposure and results are measured simultaneously. Comparison of subjects with and without results |
Quick and affordable hypothesis generation This is useful for describing the prevalence of illness. |
There is very little chance of establishing causes and effects. |
|
|
|
|
|
|
|
bias in favour of survival and selection |
|
|
|
|
|
|
|
|
| Case-control research |
In terms of exposure, cases—those who achieve the intended result—are compared to controls—those who don't. |
Efficient Suitable for researching uncommon consequences and numerous exposures, reasonably affordable for creating theories. |
There may be some opportunity to identify causes and effects, but All we can do is analyses One result Choosing a randomized controlled trial could be challenging. |
Table-3. Design of a quasi-experimental study.
RCT Subjects are randomly assigned to experimental or control
groups. The gold standard for determining causes in therapeutic
research suitable to investigate several interventions. It can be
very expensive and time-consuming. Unsuitable for studying
uncommon events may be improper Due to tight selection
criteria, generalizability is frequently low [36-45].
Observational Designs
Case Report and Series of Cases
Without using a control group, Case Report and Series of Cases
provide in-depth descriptions of cases. The probable association
between the observed outcome and a case report or small group
of patient histories and clinical evaluations are used to report a
specific exposure (case series). These research techniques could
be among the first to discover a brand-new disease or harmful
health consequence of an exposure. For example, the first case
reports on acute phosphate nephropathy-a type of acute renal
failure-following the use of oral sodium phosphate products for
stoma cleansing before colonoscopy were published in the English
language literature in 1985 [46-48]. Following this preliminary
research, numerous additional cases and series of instances were
published, which provided further evidence of this unusual and
important adverse event. Following the completion of these
investigations, the oral sodium phosphate shouldn't be given to
patients who have kidney disease, impaired kidney function or
reperfusion, dehydration, or uncorrected electrolyte imbalances,
according to an United states Food And drug caution [49].
Cross-Sectional Research
A specific outcome and the population's exposure status are
both looked into simultaneously in a cross-sectional assessment.
The likelihood and attributes of a result at a specific accent
might be thought of as being "snapped" by cross-sectional
investigations. Due to the simultaneous measurement of the
exposure and the consequence, since it is frequently impossible
to tell whether an exposure occurred before or after an event,
cause and effect relationships are ambiguous. The bulk of crosssectional
studies that have been published talk on the treatment
of certain patient populations or the prevalence of a disorder in
a population. A cross-sectional study is well-exemplified by Bello
et al. They looked into the prevalence of micro albuminuria in
family members of individuals suffering from chronic kidney
disease (CKD) in relation to the general population as part of a
population-based surveillance system, a form of cross-sectional
study. Researchers discovered that people with a CKD family
history had significantly more cases of micro albuminuria than
the sex- and age-comparison groups. It is evident in this case that knowing that there is a genetic predisposition of CKD happened
before the onset of micro albuminuria, in contrast to the majority
of cross-sectional research [50-59].
Case-control research
Finding probable contributing factors to a result is the aim of
case-control research. In this kind of study, participants are
chosen depending on the dependent variables and contrasted
with participants who do not have the condition (controls). The
patients and controls have already been compared with regard
to exposure. When analyzing unusual outcomes, case control
studies are very useful. End-stage renal disease is one illustration
of such an unusual result (ESRD). Ibanez et al. investigated if the
development of ESRD was associated with use of non-steroidal
anti-inflammatory medications, aspirin, and other analgesics
for an extended period of time (NSAIDs). They selected as cases
those patients with ESRD who registered in the neighbourhood
dialysis programme over a two-year period. They kept track of
when the medications were used in the past. The selection of
control individuals, who were hospitalized at the same hospital
as the cases and had a similar age and sex distribution, and their
drug use were also recorded. When the researchers compared
the two groups, neither NSAIDs nor non-aspirin an increased
risk of ESRD was linked to the use of analgesics. The researchers
contrasted the two groups; however, aspirin use on a regular
basis seems to be linked to a higher incidence of ESRD [60-64].
Cohort Studies/ Research
A study group (cohort) made up of individuals who are not
exposed to the intended outcome is chosen by the researcher
while conducting a cohort study. The goal of this study's design
is to identify the factors that contribute to the occurrence
of this result. Whether a subject was exposed or not before
the investigation relies on their occurrence status (controls).
Following that, People are observed over time to determine who
will experience the consequence and who won't. Researchers can
examine several results and widespread exposure variables in
cohort research. In cohort research, a researcher selects a study
group (cohort) made up of volunteers who are not exposed to
the desired result. Finding the factors that affect the appearance
of this outcome is the goal of the design of this study. The classification of subjects as exposed or unexposed depends on
their exposure status prior to the investigation (controls) [65-68].
RCTs
For evaluating treatment or other interventions, the RCT is
considered the gold standard. RCTs are capable of removing
selection bias and prognostic selection (often referred to as
confounding by indication), providing them a clear advantage
over observational studies in establishing a causal relationship
[69]. Randomization, in which patients are admonished at
random to either the experimental group (which would receive
the intervention under study) or the control group, is the main
concept. The relationship between the therapy recommended by
the doctor and the patient's prognosis is broken via randomization.
The outcome of the experimental and control groups are then
compared after being followed up on for a predetermined
amount of time. The ADEMEX trial [70] is a prime nephrology
RCT illustration. For this process, 965 Mexican peritoneal dialysis
patients were randomly split into two groups: to increase
peritoneal creatinine clearance, a modified prescription was given
to the experimental group, while the control group received their
regular peritoneal dialysis prescriptions. The two group’s initial
traits following randomization were comparable, with a few
minor exceptions, for example, a somewhat greater degree of
diabetes in the comparison group. But rather than being a result
of the investigator’s decision, this divergence was the result of
chance [71]. Following a comparison of death rates between the
two groups over the course of at least two years, the researchers
came to the conclusion that an increase in peritoneal smallsolute
clearance did not clearly improve survival. Despite the
fact that RCTs are useful tools, there are certain disadvantages
[72, 73]. They cost a lot more than observational studies, first
and foremost, however it will be impossible to evaluate every
healthcare intervention in an RCT due to the sheer quantity
of them. Additionally, it is frequently considered unethical
to subject patients to a treatment that is ostensibly (but not
yet demonstrably) superior to the standard of care. RCTs are
technically possible but are not the best method for detecting
adverse outcomes that are uncommon or take years to emerge
[70-74].
Factorial Designing
In factorial designs, input variables are purposefully and
simultaneously changed in accordance with an established
matrix of potential sequences of factor values. They vary most
from what is typically determined in this regard since each factor
can be altered independently of the others. A, B, C, and other
capital letters are frequently used to represent factors, while +1
and -1 stand for a factor's lower and upper levels, respectively. In
the event that there is a middle level, this is defined as (0). This
clearly represents the levels in a coded manner, but the following
equation shows that it also represents the real values of the
parameters:
Xcoded = (Xactual - Xmean)/ [(Xhigh – Xlow)/2]
All possible factor level configurations are included in full
factorials. The following equation indicates how many tests are necessary:
Number of experiments = Levels Factors
For instance, 23=8 experiments are needed to complete a twolevel
factorial with three elements. Straightforward two-level
factorial models for factors with two and three are shown in
(Figure 3).
Partial factorial designs, which are a portion of the pertinent
full design, often, represent half or a fourth of the complete
factorial. They are typically used for screening purposes if
there are more than 4 criteria. As was already established,
confusion, or the aliasing of key effects and interactions, is their
fundamental problem. Resolution refers to a design's ability to
accurately assess impacts and interactions absent any potentially
confusing factors. These resolution rating are the most widely
used. Two-factor interactions can be used to alias main effects;
however, Resolution III designs state that while some two-factor
interactions may be achieving to other main effects, others are
not. When there are significant two-factor interactions that
influence it, the answer can be inaccurate.
Three-factor interactions can be utilized to alias primary
effects, according to Resolution IV designs, as opposed to other
significant effects or two-factor interactions. Additionally, there is
overlap between two-factor interactions. They are an appropriate
screening option since the primary impacts won't reveal any twofactor
interactions [75-78].
Resolution V (or greater) models effectively cut down on the
number of experiments required while still offering performance
that is almost on par with full factorials. These results show that
no aliased major implications or two-factor interactions with
the other key variables or two-factor interactions exist. One
possible name for the latter is interactions among three factors.
All significant effect and two-factor correlations can be roughly
predicted if 3 (and above) interactions are neither statistically
significant nor improbable to occur. Evidently, the interpretation
of the findings is made more difficult whenever the design
resolution is lowered. Figure 4 shows a two-level factorial with
a half-fraction for four factors. Each of the cubes at the fourth
element's two levels, D, stands in for one of the three first
components, A, B, or C (Figure 4).
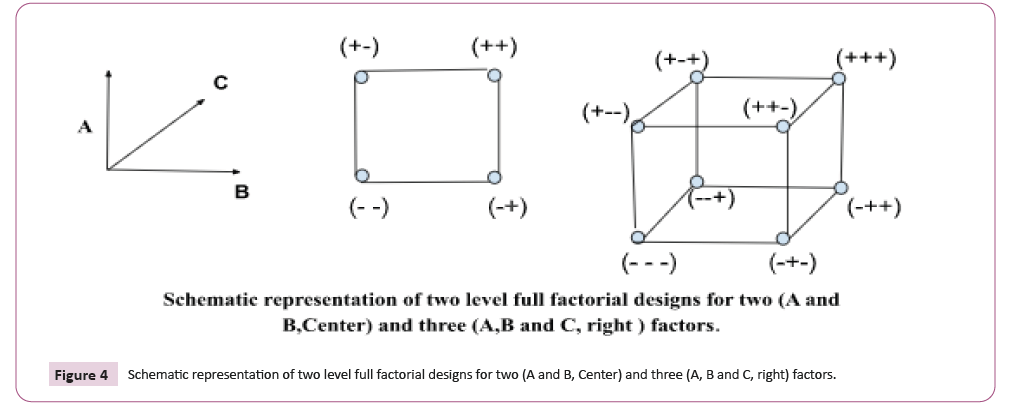
Figure 4: Schematic representation of two level full factorial designs for two (A and B, Center) and three (A, B and C, right) factors.
It is obvious that just eight of the appropriate full factorials total
16 points—or roughly half of the design—is used in this instance
(circled points). A DoE's primary goal is, as was previously said,
to develop mathematical equations that connect the causes to
the effects. Both the direct impacts of the constituents and their
interactions are necessary for the latter. The mean difference in
reaction that occurs as a component rises from a low to a very
high level is what matters most in terms of an effect. It can be
calculated by contrasting the general trend at the higher factor
level with the typical reaction at the relatively low level for the
identical factor. Interactions between the elements are frequently
observed in addition to the principal impacts and should be
carefully taken into account. The degree to which two factors A
and B interact, denoted by the letters AB, and determines how
much of an impact factor A has on the solution. In this instance,
factor B's low and high ratings for component are used to calculate the interaction as the variation in response between them [79].
These are the most approach in favour schemes.
• Three-level factorial arrangements.
• Design Center for Composites (CCDs): Since cubic or higher
models are incredibly rare in practice, they are the most
typical choice to represent the operations under investigation
(Figure 5).
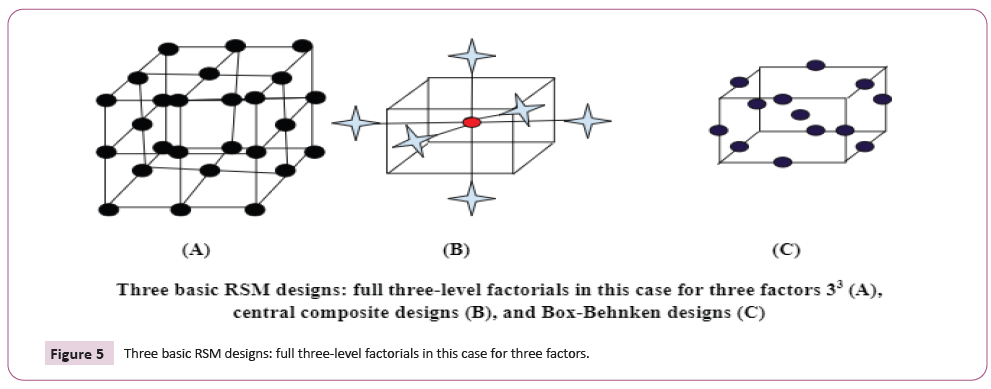
Figure 5: Three basic RSM designs: full three-level factorials in this case for three factors.
Instead of employing cognitive subtraction, the experiment's
design might be modified to process the cognitive circumstances
in a factorial approach, allowing assessments of interactions
between the various elements [80]. To properly pinpoint the
task components, this strategy relies on neuropsychological data
and, if available, supplementary behavioral data. The goal is to
get the subject to do a function in which the cognitive elements
(or dimensions) are combined in certain situations and divided in
others (Figure 6).
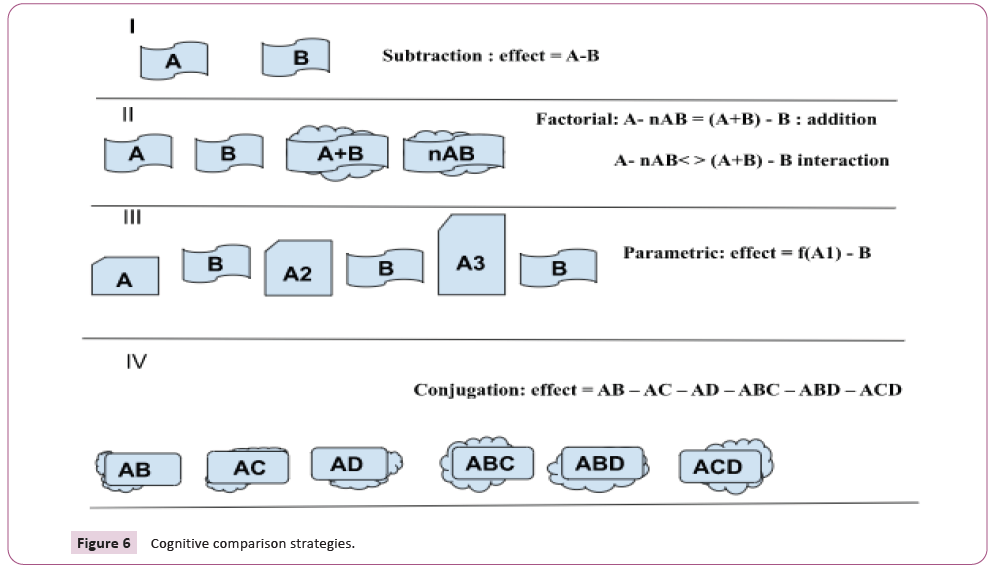
Figure 6: Cognitive comparison strategies.
The strategy is based on the premise that the BOLD responses
resulting from the circumstances are linear, even though a nonlinear
approach is possible. Otherwise, unexpected interactions
can contaminate some of the results. However, this method is
quite beneficial for analyzing cognitive interactions [81].
Characterization of Proniosomal
Transdermal Drug delivery system:
(Figure-7)
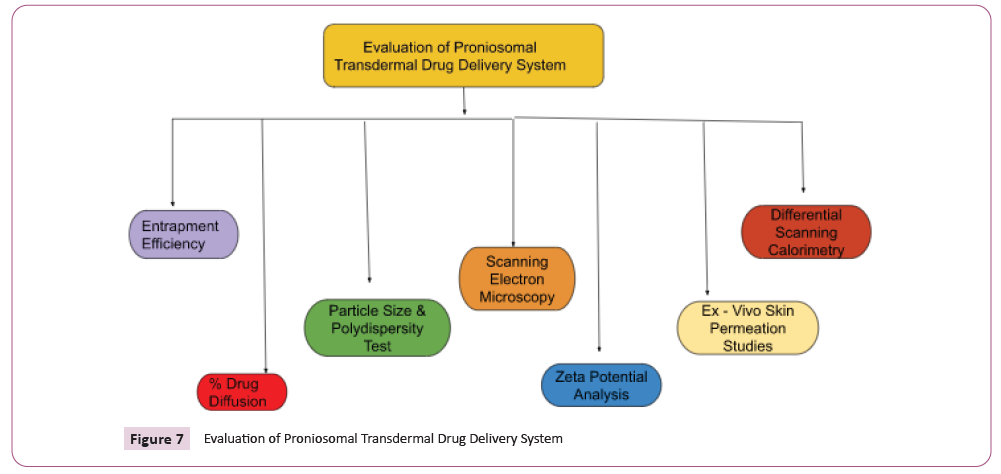
Figure 7: Evaluation of Proniosomal Transdermal Drug Delivery System
Entrapment efficiency
Proniosomal gel weighing in a glass tube was mixed with
prepared phosphate buffer having a pH 7.4 and 0.1 g. Then
aqueous suspension was sonicated by using an ultrasonicator
for five minutes. Centrifugation at 9000 rpm for 45 minutes was
used to separate the produced niosomes carrying lornoxicam
from entrapped medication. Using a UV spectrophotometer
(Shimadzu), Spectrophotometric analysis of the supernatant was performed at 375 nm, against the solution. Following equation
determine the drug's level of entrapment:
Entrapment effectiveness (%) = Entrapped Drug x100/ Drugs
added in total
Proportion of drug diffusion
Diffusion investigations were performed in a Franz cell. The
diffusion cell was equipped with a dialysis membrane. On one
part of the dialysis membrane, proniosomal gel was placed in a
precise amount. Phosphate buffered saline with a pH of 7.4 was
present in the receptor compartment (PBS) in 10 ml. The fluid in
the donor compartment was continually swirled using a Tefloncoated
magnetic bead at a speed of 100 rpm. Every 60 minutes, 1
ml of sample should be taken from the sample cell and continue
for 24 hours from the starting time. The sample that was collected
will be analyzed at 375 nm in an UV-visible spectrophotometer.
Replace the same amount of receptor compartment sample with
fresh 7.4 pH phosphate - buffered saline [82-88].
Size of the particles and PDI index
After being hydrated with PBS pH 7.4, drug-loaded proniosomal
gel's average particle size and size distribution were measured.
Using photon correlation spectroscopy (PCS) on Nanophox at
room temperature, to avoid multicasting activities, Water that
had been filtered and twice-distilled was used to homogenize the
produced noisome dispersion. Using the following equations, the
PDI revealed the range of the size distribution.
PID ¼ ð Þ X90 – X10 X50
Scanning Electron Microscopy
Following soaking using a pH 7.4 buffer, the proniosomes'
morphology examined five minutes of gold ion coating and
scanning electron microscopy [89].
Zeta potential analysis
Potential on the surface of drug-loaded compartments was found using a Zeta potential analyzer. (Brookhaven Instrument
Corporation). PH 7.4 after PBS resuscitation, at 25 °C, the typical
proniosomes preparation zeta gradient and charges were
determined across three runs. The analysis time was set to 60
seconds [90].
Differential Scanning Calorimetry
By means of a calorimeter with differential scanning, evaluated
the Proniosomes' thermal properties after being hydrated with
phosphate-buffer saline pH 7.4, one milligram proniosomal gel
samples encapsulated in common aluminium pans were used
for the study. A plot of proniosomes and bulk medication was obtained using a scan range of 10 °C/min and a mean temperature
between 30 and 300 °C [91].
Diffraction of X-rays
After being hydrated with PBS, lornoxicam proniosomes were
examined using X-ray diffraction analysis to identify their solidstate
properties. Using an X-ray diffractometer and an X-ray
producer operating at a 40 kV voltage and 20 mA current, drugloaded
proniosomal disperse were scanned at a scanning speed
of 2 °/min [92].
Skin permeation research in ex vivo
By using Franz diffusion cell for studies of ex vivo skin penetration
was carried out. A male albino with rat abdominal skin is used
for the test. The test was conducted on a Wistar rat weighing
250–20 g. The clamping method brought the skin's dermal side
into interface with the receptor medium. Receptor medium was
placed inside the receptor chamber, which has a cross-sectional
size of 4.32 cm2. After the rat's membrane on the dorsal surface
had been evenly coated with gel, a donor chamber was attached.
With a tolerance of 0.5°C at 100 rpm, at 37 C, the temperature
held steady. During specified 18-hour periods, a sample of 1 ml
was gathered, and the amount of medication which has migrated
from the formulation into the receptor was measured using UV
spectrophotometer assessment at 375 nm [93].
Conclusion
Proniosomes are water soluble carrier particles that are coated
with a surfactant and can be hydrated immediately before used
to yield to aqueous noisome dispersion. They are more stable
than the noisome and liposomes. They can incorporate both
lipophilic as well as hydrophilic drugs. They have emerged as
challenging carriers for drug delivery via transdermal route. It has
become useful dosages form for transdermal drug delivery due
to the simple and cost-effective scale up production procedure.
Proniosomes have enabled to overcome all the stabilities
problems associated with a noisome and liposomes such as
fusion, aggregation on storage.
References
- Radha GV, Rani TS, Sarvani B (2013) a review on proniosomal drug delivery system for targeted drug action. J Basic Clin Pharm 4: 42-48.
Indexed at, Google Scholar, Crossref
- Arunothayanun P, Bernard MS, Craig DQ, Uchegbu IF, Florence AT et al (2000) the effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether. Int J Pharm 201: 7-14.
Indexed at, Google Scholar, Crossref
- Mittal T, Chaudhary S, Chaudhary A, Kumar A, Ankit S et al (2020) Proniosomes: the effective and efficient drug-carrier system. Therapeutic Delivery 2019: 65–68.
Indexed at, Google Scholar, Crossref
- Paolino D, Cosco D, Cilurzo F (2012) Improved in vitro and in vivo collagen biosynthesis by asiaticoside-loaded ultradeformable vesicles. J. Control Rel 162: 143-151.
Indexed at, Google Scholar, Crossref
- Celia C, Cilurzo F, Trapasso E (2011) Ethosomes and transfersomes containing linoleic acid: physicochemical and technological features of topical drug delivery carriers for the potential treatment of melasma disorders. Biomed. Microdevices 14: 119-130.
Indexed at, Google Scholar, Crossref
- Paolino D, Celia C, Trapasso E (2012) Paclitaxel-loaded ethosomes: potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratoses. Eur J Pharma Biopharm 81: 102-112.
Indexed at, Google Scholar, Crossref
- Carafa M, Marianecci C, Rinaldi F (2014) Ammonium glycyrrhizinate-loaded niosomes as a potential nanotherapeutic system for anti-inflammatory activity in murine models. Int. J. Nanomed 9: 635-651.
Indexed at, Google Scholar, Crossref
- Di Marzio L, Marianecci C, Cinque B (2008) pH-sensitive non-phospholipid vesicle and macrophage-like cells: binding, uptake and endocytotic pathway. Biochim Biophys Acta 1778: 2749-2756.
Indexed at, Google Scholar, Crossref
- Donnelly RF (2017) how can micro needles overcome challenges facing transdermal drug delivery? Ther Deliv 8: 725-728.
Indexed at, Google Scholar, Crossref
- Brambilla D, Luciani P, Leroux J (2014) Breakthrough discoveries in drug delivery technologies: the next 30 years. J Control Rel 190: 9-14.
Indexed at, Google Scholar, Crossref
- McCrudden MT, Singh TR, Migalska K, Donnelly RF (2013) Strategies for enhanced peptide and protein delivery. Ther Deliv 4: 593-614.
Indexed at, Google Scholar, Crossref
- Muzzalupo R, Tavano L (2015) Niosomal drug delivery for transdermal targeting: recent advances. Res. Rep. Transdermal Drug Delivery 4: 23-33.
Indexed at, Google Scholar, Crossref
- Maryam K, Fakhar Ud D, Shefaat Ullah S, Naz D, Ahmad N et al (2017). Proniosomes derived niosomes: recent advancements in drug delivery and targeting. Drug Delivery 24: 56-69.
Indexed at, Google Scholar, Crossref
- Yasam VR, Jakki SL, Natarajan J, Kuppusamy G (2014) A review on novel vesicular drug delivery: proniosomes. Drug Deliv 21: 243-249.
Indexed at, Google Scholar, Crossref
- Ammar H, Ghorab M, EL-Nahhas S, Higazy I (2011) Proniosomes as a carrier system for transdermal delivery of tenoxicam. Int J Pharm 405: 142-152.
Indexed at, Google Scholar, Crossref
- Ahmad MZ, Mohammed AA, Mokhtar Ibrahim M (2017) Technology overview and drug delivery application of proniosome. Pharm Dev Technol 22: 302-311.
Indexed at, Google Scholar, Crossref
- Mujoriya RZ, Bodla R (2011). Niosomes–challenge in preparation for pharmaceutical scientist. Int J App Pharm 3:11-15.
Indexed at, Google Scholar, Crossref
- Mujoriya RZ, Bodla R (2011) Niosomes–challenge in preparation for pharmaceutical scientist. Int J App Pharm 3: 11–15.
Indexed at, Google Scholar, Crossref
- Noordzi J, Dekker M, Friedo W, Zoccali C, Kitty J et al (2009) Study Designs in Clinical Research. Nephron Clinical Practice 113: 218-221.
Indexed at, Google Scholar, Crossref
- Biberstein M, Parker BA (1985) Enema-induced hyperphosphatemia. Am J Med 79: 645-646.
Indexed at, Google Scholar, Crossref
- Rohack JJ, Mehta BR, Subramanyam K (1985) Hyperphosphatemia and hypocalcemic coma associated with phosphate enema. South Med J 78: 1241-1242.
Indexed at, Google Scholar, Crossref
- Bello AK, Peters J, Wight J, de Zeeuw D, El Nahas M et al (2008) A population-based screening for microalbuminuria among relatives of CKD patients: the Kidney Evaluation and Awareness Program in Sheffield (KEAPS). Am J Kidney Dis 52: 434-443.
Indexed at, Google Scholar, Crossref
- Ibanez L, Morlans M, Vidal X, Martinez MJ, Laporte JR et al (2005) Case-control study of regular analgesic and nonsteroidal anti-inflammatory use and end-stage renal disease. Kidney Int 67: 2393-2398.
Indexed at, Google Scholar, Crossref
- Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA et al (2003) Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 64: 339-349.
Indexed at, Google Scholar, Crossref
- Stel VS, Jager KJ, Zoccali C, Wanner C, Dekker FW et al (2007) The randomized clinical trial: an unbeatable standard in clinical research? Kidney Int 72: 539-542.
Indexed at, Google Scholar, Crossref
- Tripepi G, Jager KJ, Dekker FW, Wanner C, Zoccali C et al (2008) Bias in clinical research. Kidney Int 73:148-153.
Indexed at, Google Scholar, Crossref
- Paniagua R, Amato D, Vonesh E, CorreaRotter R, Ramos A (2002) Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13: 1307-1320.
Indexed at, Google Scholar, Crossref
- Jager KJ, Stel VS, Wanner C, Zoccali C, Dekker FW et al (2007) the valuable contribution of observational studies to nephrology. Kidney Int 72: 671-67.
Indexed at, Google Scholar, Crossref
- Jyotsana R, Nitesh P, Kamal D (2016).Formulation and evaluation of proniosomes containing lornoxicam. Drug Delivery and Translational Research 6: 511-518.
Indexed at, Google Scholar, Crossref
- Radha GV, Rani TS, Sarvani B (2013) a review on proniosomal drug delivery system for targeted drug action. J Basic Clin Pharm 4:42-8.
Indexed at, Google Scholar, Crossref
- Ahmad MZ, Mohammed AA, Mokhtar Ibrahim M (2017) Technology overview and drug delivery application of proniosome. Pharm Dev Technol 22: 302-311.
Indexed at, Google Scholar, Crossref
- Abd-Elbary A, El-laithy HM, Tadros MI (2008) Sucrose stearate-based proniosome-derived niosomes for the nebulisable delivery of cromolyn sodium. Int J Pharm 357: 189-198.
Indexed at, Google Scholar, Crossref
- Fang JY, Yu SY, Wu PC, Huang YB, Tsai YH (2001) In-vitro skin permeation of estradiol from various proniosome formulations. Int J Pharm 215: 91-99.
Indexed at, Google Scholar, Crossref
- Gupta A, Prajapati SK, Balamurugan M, Singh M, Bhatia D et al (2007) Design and development of a proniosomal transdermal drug delivery system for captopril. Trop J Pharm Res 6: 687-693.
Indexed at, Google Scholar, Crossref
- Solanki AB, Parikh JR, Parikh RH (2007) Formulation and optimization of piroxicam proniosomes by 3-factor, 3-level Box–Behnken design. AAPS PharmSciTech 8: 86.
Indexed at, Google Scholar, Crossref
- Vora B, Khopade AJ, Jain NK (1998) Proniosome based transdermal delivery of levonorgestrel for effective contraception. J Control Release 54: 149-165.
Indexed at, Google Scholar, Crossref
- Politis S, Colombo P, Colombo G, Rekkas D (2017) Design of experiments (DoE) in pharmaceutical development. Drug Dev Ind Pharm 43: 889-901.
Indexed at, Google Scholar, Crossref
- Amaro E, Barker GJ (2006) Study design in fMRI: basic principles. Brain Cogn 60: 220-232.
Indexed at, Google Scholar, Crossref
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RS et al (1996) the trouble with cognitive subtraction. NeuroImage 4:97-104.
Indexed at, Google Scholar, Crossref
- Hall DA, Haggard MP, Akeroyd MA, Summerfield AQ, Palmer AR, et al (2000) Modulation and task eVects in auditory processing measured using fMRI. Hum Brain Mapp 10: 107-119.
Indexed at, Google Scholar, Crossref
- Stark CE, Squire LR (2001) when zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A 98: 12760-12766.
Indexed at, Google Scholar, Crossref
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles Ket al (2002) Posterior parietal cortex is implicated in continuous switching between verbal Xuency tasks: an fMRI study with clinical implications. Brain 125:1024-1038.
Indexed at, Google Scholar, Crossref
- Florence AT (1993) Non-ionic surfactant vesicles: preparation and characterization. In: Gregoriadis G, editor. Boca Raton, FL: Liposome Technology. CRC Press 19: 157-176.
Indexed at, Google Scholar, Crossref
- Donnelly RF (2017) how can micro needles overcome challenges facing transdermal drug delivery? Ther Deliv 8: 725-728.
Indexed at, Google Scholar, Crossref
- Danaei M, Dehghankhold M, Ataei S, Davarani F, Javanmard R et al (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10: 1-17.
Indexed at, Google Scholar, Crossref
- Mokale VJ, Patil HI, Patil AP (2015) Formulation and optimisation of famotidine proniosomes: anin vitro and ex vivo study. J Exp Nanosci 11: 97-110.
Indexed at, Google Scholar, Crossref
- Madni A, Rahim MA, Mahmood MA, Jabar A, Rehman M et al (2018) Enhancement of dissolution and skin permeability of pentazocine by proniosomes and niosomal gel. AAPS PharmSciTech 19: 1544-1553.
Indexed at, Google Scholar, Crossref
- Mir M, Ishtiaq S, Rabia S, Khatoon M, Zeb A et al (2017) Nanotechnology: from in vivo imaging system to controlled drug delivery. Nanoscale Res Lett 12: 500.
Indexed at, Google Scholar, Crossref
- Caddeo C, Pons R, Carbone C, Fernàndez-Busquets X, Cardia MC et al (2017) Physico-chemical characterization of succinyl chitosan-stabilized liposomes for the oral co-delivery of quercetin and resveratrol. Carbohydr Polym 157: 1853-1861.
Indexed at, Google Scholar, Crossref
- Din FU, Choi JY, Kim DW, Mustapha O, Kim DS et al (2017) Irinotecan-encapsulated doublereverse thermosensitive nanocarrier system for rectal administration. Drug Deliv 24: 502-510.
Indexed at, Google Scholar, Crossref
- Verma P, Prajapati SK, Yadav R, Senyschyn D, Shea PR (2016) Single intravenous dose of novel flurbiprofen-loaded proniosome formulations provides prolonged systemic exposure and anti-inflammatory effect. Mol Pharm 13: 3688-3699.
Indexed at, Google Scholar, Crossref
- Tripepi G, Jager KJ, Dekker FW, Wanner C, Zoccali C (2008) Bias in clinical research. Kidney Int 73: 148-53.
Indexed at, Google Scholar, Crossref
- Stel VS, Jager KJ, Zoccali C, Wanner C, Dekker FW (2007) The randomized clinical trial: an unbeatable standard in clinical research? Kidney Int 72(5): 539-542.
Indexed at, Google Scholar, Crossref
- Goud BA, Raju J, Rambhau D (2012) Improved oral absorption of carbamazepine from sorbiton monolaurate based proniosome systems containing charged surface ligands. Int J Biol Pharm Res 3: 37-42.
Indexed at, Google Scholar, Crossref
- Akhilesh D, Bini KB, Kamath JV (2002) Comparative study of carriers used in proniosomes. Int J Pharm Chem Sci 3: 6-12.
Indexed at, Google Scholar, Crossref
- Kish-Trier E, Hill CP (2013) Structural biology of the proteasome. Annu Rev Biophys 42: 29-49.
Indexed at, Google Scholar, Crossref
- Rattanapak T, Young K, Rades T, Hook S (2012) Comparative study of liposomes, transfersomes, ethosomes and cubosomes for transcutaneous immunisation: characterisation and in vitro skin penetration. J Pharm Pharmacol 64: 1560-1569.
Indexed at Google Scholar, Crossref
- Najlah M, Hidayat K, Omer HK (2015) facile approach to manufacturing non-ionic surfactant nanodipsersions using proniosome technology and high-pressure homogenization. J liposome Resources 25: 32-37.
Indexed at, Google Scholar, Crossref
- Abdelbary GA, Aburahma MH (2015) Oro-dental mucoadhesive proniosomal gel formulation loaded with lornoxicam for management of dental pain. J Liposome Res 25: 107-121.
Indexed at, Google Scholar, Crossref
- Imam SS, Aqil M, Akhtar M, Sultana Y, Ali A (2015) Formulation by design-based proniosome for accentuated transdermal delivery of risperidone: in vitro characterization and in vivo pharmacokinetic study. Drug Deliv 22: 1059-1070.
Indexed at, Google Scholar, Crossref
- Shruthi PA, Pushpadass HA, Magdaline Eljeeva Emerald F, Surendra Nath B, Laxmana Naik N et al (2021) Formulation and characterization of catechin-loaded proniosomes for food fortification. J Sci Food Agric 101: 2439-2448.
Indexed at, Google Scholar, Crossref
- Farooqui NA, Kar M, Singh RP, Jain S (2017) Development of Proniosomal Gel: in-vitro, ex-vivo and in-vivo Characterization. Indian J Pharm Educ Res 51:758-764.
Indexed at, Google Scholar, Crossref
- Mel MMRD, Gunathilake KDPP, Fernando CAN (2020) Formulation of microencapsulated rutin and evaluation of bioactivity and stability upon in vitro digestive and dialysis conditions. Int J Biol Macromol 159: 316-323.
Indexed at, Google Scholar, Crossref
- Rahman Z, Zidan AS, Khan MA (2010) Non-destructive methods of characterization of risperidone solid lipid nanoparticles. Eur J Pharm Biopharm 76: 127-137.
Indexed at, Google Scholar, Crossref
- Aboelwafa AA, El-Setouhy DA, Elmeshad AN (2010) Comparative study on the effects of some polyoxyethylene alkyl ether and sorbitan fatty acid ester surfactants on the performance of transdermal carvedilol proniosomal gel using experimental design. AAPS PharmSciTech 11: 1591-1602.
Indexed at, Google Scholar, Crossref
- Schlich M, Lai F, Pireddu R, Pini E, Ailuno G et al (2020) Resveratrol proniosomes as a convenient nanoingredient for functional food. Food Chem 310: 125-950.
Indexed at, Google Scholar, Crossref
- García-Manrique P, Machado ND, Fernández MA, Blanco-López MC, Matos M et al (2020) Effect of drug molecular weight on niosomes size and encapsulation efficiency. Colloids Surf B Biointerfaces 186: 110-711.
Indexed at, Google Scholar, Crossref
- Minakshee G, Bhushan R, Wrushali A, Ashish B, Jagdish V et al (2021) An overview of characterizations and applications of proniosomal drug delivery system. GSC Adv Res Rev 7: 025-34.
Indexed at, Google Scholar, Crossref
- Abdul NK, Thimmaraju DR (2019) Proniosomes: innovative vesicular drug delivery system: a review. Int J Pharm Sci Rev Res 59: 44-51.
Indexed at, Google Scholar, Crossref
- Sammour RMF, Taher M, Chatterjee B, Shahiwala A, Mahmood S (2019) Optimization of aceclofenac proniosomes by using different carriers, part 1: Development and characterization. Pharmaceutics 11:7.
Indexed at, Google Scholar, Crossref
- Soujanya C, Satya L, Navyas Y (2020) A review on novel vesicular drug delivery system: proniosomes. Manipal J Pharm Sci 6: 94-100.
Indexed at, Google Scholar, Crossref
- Upadhye S, Rafik IN (2020) Proniosomes: A novel vesicular drug delivery system. Am J PharmTech res 10: 260-273.
Indexed at, Google Scholar, Crossref
- Goud BA, Raju J, Rambhau D (2012) Improved oral absorption of carbamazepine from sorbiton monolaurate based proniosome systems containing charged surface ligands. Int J Biol Pharm Res 3: 37-42.
Indexed at, Google Scholar, Crossref
- Lankalapalli S, Sphingosomes DM (2012) Applications in targeted drug delivery. Int J Pharm Chem Biol Sci 2: 507-516.
Indexed at, Google Scholar, Crossref
- Zidan AS, Rahman Z, Habib MJ, Khan MA (2010) Spectral and spatial characterization of protein loaded PLGA nanoparticles. J Pharm Sci 99: 1180-1192.
Indexed at, Google Scholar, Crossref
- Ravaghi M, Sinico C, Razavi SH, Mousavi SM, Pini E (2017) Proniosomal powders of natural canthaxanthin: preparation and characterization. Food Chem 220: 233-241.
Indexed at, Google Scholar, Crossref
- Lai F, Schlich M, Pireddu R, Fadda AM, Sinico C et al (2018) Nanocrystals as effective Delivery 18 systems of poorly water-soluble natural molecules. Curr Med Chem 23: 56-59.
Indexed at, Google Scholar, Crossref
- Singh SK, Makadia V, Sharma S, Rashid M, Shahi S et al (2017) Preparation and in-vitro/in-vivo characterization of trans-resveratrol nanocrystals for oral administration. Drug Deliv Transl Res 7: 395-407.
Indexed at, Google Scholar, Crossref
- Ge X, Wei M, He S, Yuan WE (2019) Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 11: 55.
Indexed at, Google Scholar, Crossref
- Debnath A, Kumar A (2015) Structural and functional significance of niosome and proniosome in drug delivery system. Int J Pharm Eng 3: 621-637.
Indexed at, Google Scholar, Crossref
- Bomma G, Harika SM, Babu AM, Bakshi V (2017) Formulation development and evaluation of proniosomal powder of candesartan. Anaal Chem Lett 7: 567-577.
Indexed at, Google Scholar, Crossref
- Lohumi A (2012) a novel drug delivery system: niosomes review. J Drug Deliv Ther 2: 5.
Indexed at, Google Scholar, Crossref
- Veerareddy PR, Bobbala SKR (2013) Enhanced oral bioavailability of isradipine via proniosomal systems. Drug Dev Ind Pharm 39: 909-917.
Indexed at, Google Scholar, Crossref
- Barry BW (2001) Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci 14: 101-114.
Indexed at, Google Scholar, Crossref
- Bayindir ZS, Yuksel N (2010) Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci 99: 2049-2060.
Indexed at, Google Scholar, Crossref
- Karatzas AA, Politis SN, Rekkas DM (2017) Development of rapidly dissolving pellets within the Quality by Design Approach. Drug Dev Ind Pharm 43: 770-779.
Indexed at, Google Scholar, Crossref
- Belotti S, Rossi A, Colombo P, Bettini R, Rekkas D et al (2015) Spray-dried amikacin sulphate powder for inhalation in cystic fibrosis patients: the role of ethanol in particle formation. Eur J Pharm Biopharm 93:165-172.
Indexed at, Google Scholar, Crossref
- Wurth C, Demeule B, Mahler HC, Adler M (2016) Quality by design approaches to formulation robustness – an antibody case study. J Pharm Sci 105: 1667-75.
Indexed at, Google Scholar, Crossref
- Abdelbary GA, Aburahma MH (2015) Oro-dental mucoadhesive proniosomal gel formulation loaded with lornoxicam for management of dental pain. J Liposome Res 25: 107-121.
Indexed at, Google Scholar, Crossref
- Balakrishnan P, Shanmugam S, Lee WS, Lee WM, Kim JO et al (2009) Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm 377: 1-8.
Indexed at, Google Scholar, Crossref
- Rinaldi F, Hanieh PN, Chan LKN, Angeloni L, Passeri D et al (2018) Chitosan glutamate-coated niosomes A proposal for nose-to-brain delivery. Pharmaceutics 10: 38.
Indexed at, Google Scholar, Crossref
- Obeid MA, Khadra I, Mullen AB, Tate RJ, Ferro VA et al (2017) The effects of hydration media on the characteristics of non-ionic surfactant vesicles (NISV) prepared by microfluidics. Int J Pharm 516: 52-60.
Indexed at, Google Scholar, Crossref
- Salem HF, Kharshoum RM, Abo El-Ela FI, F AG, Abdellatif KRA et al (2018) Evaluation and optimization of pH-responsive niosomes as a carrier for efficient treatment of breast cancer. Drug Deliv Transl Res 8: 633-644.
Indexed at, Google Scholar, Crossref
Citation: Sharma RB, Kashyap D, Thakur H
(2023) Updated Review on Proniosomal
Transdermal Drug Delivery System. Int J
Drug Dev Res J, Vol. 15 No. 1: 989.













