Keywords
Atomic force microscopy; Red blood cells; Ovarian cancer
Introduction
At present, the most complete data on the structure of red blood cells is obtained using atomic force microscopy (AFM) [1-3]. Study of samples fixed in the air eliminates the need for rigid fixation techniques (unlike electron microscopy) and thereby allows to injure cells or change the conformation of biomolecules minimally [4,5]. Thus, the main advantages of AFM in the study of biological objects are a high resolution, ease of sample preparation, determination of morphometric parameters, the study of objects in a native environment for them (in solution), the study of viscoelastic, charge, hydrophobic properties of objects.
Ovarian cancer (OC) takes one of the leading positions on the incidence and mortality of malignant tumors in women of reproductive age. Paraneoplastic syndromes, chemotherapy side effects and postoperative complications are topical problems in clinical oncology. Study of erythrocytes in the pathogenesis of tumor growth is important due to the fact that red blood cells are susceptible to the influence of biologically active substances released by a tumor, and by immune cells, to the action of cytokines, growth factors and reactive oxygen species. Hemolytic effects of such substances are due to their direct impact on basic protein and lipid components of the membrane, or to the influence on erythrocyte enzymes that regulate energy processes and synthesis of hemoglobin [6]. Evolving herewith, the oxidative haemolysis is accompanied by the accumulation of peroxide compounds. This leads to structural and functional changes in hemoglobin, irreversible changes in the lipid phase of the membranes of erythrocytes and inhibition of sulfhydryl groups activity. Most of the toxic substances form oxidizing derivatives only provided that the protective systems function in the organism.
These enzymes of antioxidant protection are catalase, peroxidase, glucose-6-phosphate dehydrogenase and glutathione system, which is an important biocatalyst to carry out protection of hemoglobin, and the sulfur-containing enzymes. Thus, acute intravascular hemolysis caused by AsH3, is caused not only by its direct action on the erythrocyte membrane but by inhibiting the activity of erythrocyte catalase and glutathione reductase [7]. It is shown that the average life span of red blood cells circulating in the blood of tumor-bearing organisms is reduced by 1.5-2 times. Occurring in this case, tissue hypoxia and anemia reduce the effectiveness of the therapy, and tumor biology changes.
Deformability of red blood cells determines blood flow properties. It (deformability) is characterized by a considerable variability of the shape of red blood cells while interacting with each other and depends on the three main factors: membrane elasticity, internal viscosity of the content in cells, ratio of cell surface to their size. Elasticity of the red blood cell membrane is determined by the condition of lipid components, hemoglobin concentration, ATP and ??2+ ions. Apart from membrane elasticity internal viscosity that depends on the calcium concentration is of great importance in determining mechanical properties [8]. Entering into reaction with intracellular proteins while binding to the membrane, calcium increases the red blood cell rigidity. The interest to the deformability study of red blood cells is associated with the fact that rigid red blood cells are capable of occluding a part of the capillary bed and thus, capable of blocking blood circulation in the system of microcirculation. The hypoxic state of the organism is considered the main cause of decreasing of the deformation capacity of red blood cells [9]. In patients with chronic hypoxia the shape of red blood cells proves to be significantly changed: the number of discocytes falls, the number of spherocytes increases, erythrocytes in the shape of a inflated burred disc-echinocytes. Impairment of the structure and function of membranes at chronic hypoxia is determined by activation of the lipid peroxidation system (LPS), which is a non-specific response to exogenic effects. Activation of lipid peroxidation leads to profound disorders of the membrane structure, to the formation of lipid-lipid and lipid-protein intermolecular «cross-links», that results in the change of physical and chemical properties of lipid matrix, the passive ion membrane permeability is transformed. At present the relationship between deformability, mechanical persistency, red blood cell life time and lipid peroxidation intensity has been proved [10].
Thus, it seems topical to study structural and functional red blood cell alterations by atomic force microscopy and spectrophotometry in assessment of redox-dependent processes in the dynamics of experimental ovarian tumor growth.
Methods
The study design
The experiment was conducted on white outbred rats with the body weight of 180-200 gr. with grafted ascitic ovarian tumor (R?RC named after N.N. Blokhin, Moscow). The progress of this tumor type goes through 3 stages: logarithmic (from 4 days after grafting), stationary (from 8 days after grafting), and terminal (from 13 days after grafting). After decapitation under ether anaesthesia, heparinized blood was separated into plasma and red blood cells. Erythrocytes were washed 3 times with cold normal saline. To conduct the biochemical analysis, red blood cell hemolysate was prepared with distilled water in the ratio of 1:10. The experimental group was made up of rats in the stationary stage (n=22) and terminal stage of the tumor growth (n=22). The control group consisted of healthy sexually mature animals (n=24).
Biochemical investigations: In plasma and erythrocytes the level of protein oxidative modification (POM) was measured by E. V. Dubinina’s method [11]. To analyze the spontaneous OMP level, 1 ml of 10 mM 2,4-dinitrophenylhydrazine solution in 2 M solution of HCl was added to 0.1 ml erythrocyte hemolysate. After incubation for 1 hour at room temperature, the proteins were precipitated by adding 20% trichloroacetic acid, centrifuged at 3000 rpm, the supernatant was removed and the precipitate was washed three times with a mixture (1:1) of ethanol and ethyl acetate to remove unreacted 2,4 DNPH and lipid impurities. The dried precipitate was dissolved in 3 ml of 8 M urea. The quantity of the 2,4- dinitrophenylhydrazones of basic and neutral character formed was recorded at 346, 370, 430, 530 nm. The obtained optical density results of the solution were recounted on the amount of protein determined by the Bradford method.
The intensity of lipid peroxidation was evaluated by the level of diene conjugates (DC), ketodienes (KD), schiff bases (SB) by the absorption intensity at wavelengths of 220, 232, 278 and 400 nm in a heptane extract of erythrocytes by Volchegorsky method. Isopropyl alcohol and heptane in a ratio of 3:5 were added in 0.1 ml of biomaterial, lipids were extracted with active shaking for 15 minutes. Then, 1 ml of 1 M HCl was added to clearly separate the phases, and the upper heptane phase was photometred at 220, 232, 278, 400 nm. Results were expressed in OD units/ml.
The content of malondialdehyde (MDA) was determined by reaction with 2-thiobarbituric acid (TBA), which results in a formation of a colored complex with an absorption maximum at a wavelength of 535 nm. 3ml of a solution of orthofosforic acid and 1 ml of a TBA solution were added to 0.3 ml of the supernatant of red blood cells. Samples were incubated for 45 minutes at 100°C. Then the cooled mixture was added 4 ml of butanol. Separation of the aqueous and organic phases was achieved by centrifugation at 3000 rpm for 10 minutes. Then fluorescence of butanol extracts was measured at wavelength of 535 nm be Andreeva method. The results were expressed in micromol/liter.
Activity of superoxide dismutase (SOD) in erythrocyte hemolysate was determined using the system of nitrobluetetrazolium reduction by method of Nishikimi and Dubinina. The erythrocytes were diluted with cold Tris-HClbuffer at a ratio of 1:9 with the addition of potassium dihydrogen phosphate (KH2PO4) and mixture of alcohol:chloroform to precipitate hemoglobin. After centrifugation at 5000 g for 20 minutes, 100 mul were collected for analysis. The incubation mixture consisted of nitrobluetetrazolium (NBT), phenazinemetasulfate (PMS) and EDTA in phosphate buffer (pH 7.8). Forming reaction of superoxide anion in the HBT-PMS system was initiated by adding 10 mM NAD*H. After 10 min incubation at 37°C the optical density of the solution was remeasured. Calculation of SOD activity was performed according to the formulas: T (%)=(Eo-Ek)/Eink* 100%. A=(100-T)/T* 50. SOD activity was expressed in arbitrary units (1 conv-50% inhibition of NBT reduction reaction) [2].
Catalase activity in the erythrocyte hemolysate was assessed by the Karpishchenko method, counted by the decrease of hydrogen peroxide in the incubation mixture. The control and experimental samples were added 200 mul of biomaterial, 2.5 ml 0.3% hydrogen peroxide, then the reaction in the control test tube was immediately stopped with the addition of 50% trichloroacetic acid, in the experimental test tube-after 10 minutes of incubation at room temperature. After centrifugation (10 minutes, 3000 rpm) the solution was spectrophotometred at 260 nm (absorption maximum of the hydrogen peroxide). The degree of dilution, the volume of biomaterial used, the change in optical density for 1 minute, the molar extinction coefficient of H2O2 (0.071) were used in the calculation. The enzyme activity was expressed in mmol/min/L. [3].
Determination of glutathione-S-transferase (GST) was made with Habig, method. The reaction was carried out in a spectrophotometer cuvette, to which was added 1.2 ml of 2 mM reduced glutathione (GSH) and 0.1 ml of hemolysate. The reaction was run by adding 1.2 ml of 2 mM 1-chloro-2,4- dinitrobenzene. The absorbance of the sample solution was recorded at 340 nm for 3 minutes after the reaction initiation. The calculation was carried out using a molar extinction coefficient of the resulting product 9.6*103* M-1 cm-1, the incubation time and the dilution degree. GST activity was expressed in micromoles/min/l [12].
The levels of GSH and oxidized (GSSG) glutathione were determined using dithio-bis-nitrobenzoic acid (DTNB) according to the method of Karpishchenko AI (1999). To 0.6 ml of erythrocyte hemolysate was added 0.2 ml of a 20% solution of sulfosalicylic acid. Samples were mixed and centrifuged for 10 min at 3000 rpm. 0.2 ml of the supernatant was transferred to tubes containing 2.55 ml of 0.1 M Tris-HCl buffer containing 0.01% EDTA. To this mixture was added 25 ul solution of DTNB. Enzyme activity was expressed in micromoles/liter. The GSSG in the experiment was initially reduced by glutathione reductase to GSH. It was then determined by reaction with DTNB [13].
Morphological investigations by AFM. The examination of cytoarchitectonics, cross section, rigidity of erythrocytes in experimental and control groups was carried out with the use of the atomic force microscope SolverPro (NT-MDT, Zelenograd, Russia) in the tapping mode. Branded silicon probes were used with stiffness of 0.2 N/m, the radius of the probe tip round was approximately 10 n?. The membrane rigidity was estimated according to Young’s modulus that was calculated in accordance with Hertz’s theory. The probe tip was used as an instrument to determine local viscous and elastic membrane properties of biological objects. The method principle consists in various deviations of a cantilever upon contact with a hard and soft sample. Herewith, the deviation curve of the cantilever depending on the piezoscanner move is necessary to transform into the curve of amount of the surface deflection under the needle–the force applied to probe F.
Structural and functional characteristics of erythrocyte membranes were evaluated by Kazintsa et al. (1984) classification, according to which the cells with echinocitary transformation signs (discocytes, discocytes with one protuberance, discocytes with a comb, discocytes with multiple growths, red blood cells in the form of mulberry) include to reversibly deformed, and the dome-shaped red blood cells, smooth surface spherocytes, spherocytes with spines on the surface, erythrocytes in the form of "a deflated ball," degenerative forms of red blood cells-to irreversibly deformed shapes. Changes in surface architectonics of erythrocytes had number of indices: IT-the index of transformation-quantification of the ratio of normal and abnormal forms of erythrocytes: IT=(RD%+ID%)/D%, where D %=percentage of discocytes, OD%-the percentage of reversibly deformed red blood cells, ND% -the percentage of irreversibly deformed red blood cells; IRT-index of reversible transformation: IRT=RD%/D%; IIT–index of irreversible transformation: IIT=IT%/D%.
To assess the significance of differences in levels of rigidity and LPO-AO parameters at different stages of tumor process, paired t-test was used. Correlation between parameters was evaluated by Spearman method. Statistical analysis was carried out in Stata 6.0. Differences were considered statistically significant at p ≤ 0.05 level from the control group.
Results
Superoxide dismutase (SOD) is a key enzyme of antiradical defense of cells, inactivating superoxide anion-radical and functioning in a cell in the cascade of enzymes that are capable of decomposing hydrogen peroxide-catalase and glutathioneperoxidase. The specific feature of SOD functioning is that in the presence of excessive amount of ?2?2 it can form reactive hydroxyl radical which attacks a SOD protein molecule, resulting in its fragmentation and activity loss. Superoxide dismutation into ?2?2 with the help of SOD is often called a primary defense, because this enzyme prevents further formation of free radicals. Literature data shows that the increase of mitotic activity in malignant tumours can be accompanied by the increase of superoxide radical output. The due elimination of activated oxygen metabolites does not take place in tumor cells. It was also established that SOD activity is getting lower in the organism of animals, beginning with early stages of tumor growth and reaching minimum values till the time of death.
In our research we detected a tendency for SOD activation increase in the stationary and terminal phases of tumor growth (Table 1). However, these changes are not statistically significant.
Phases
Indexes |
Stationary phase(n=22) |
Terminal phase (n=22) |
| MDA |
1.864 (95% CI 0.67-3.05)* |
2.23 (95% CI 0.33-4.13) |
| DC |
0.07 (95% CI 0.027-0.11)* |
0,057CI 0.022-0.9)* |
| KD |
0.002 (95% CI 0-0.008) |
0,0056 (95% CI 0.001-0.103)* |
| SB |
-0.0005 995% CI-0.003-0.002)* |
0.0023 (95% CI0.00026-0.0043)* |
| SOD |
0.037 (95% CI -0.12-0.19) |
0.18 (95% CI-0.12-0.48) |
| Catalase |
4.97 (95% CI 1.8-8.13)* |
2.83 (95% CI-0,35-6) |
| GT |
0.022 (95% CI 0.01-0.035)* |
0.013 (95% CI-0.004-0.23) |
Note * – р ≤0.05; data, significantly differing from control
Table 1: The dynamics of the activity of antioxidant enzymes and lipid peroxidation products (compared to control) in different phases of development of AOC, paired t-test.
The erythrocyte defense from the action of ROS is also carried out by the glutathione system in the form of reduced glutathione (GSH) and enzymes of its metabolism. Glutathione-S-transferase (GST) belongs to the family of enzymes that neutralize the toxic impact of various hydrophobic and electrophilic compounds by their conjugation with reduced glutathione.
GST is capable of reactivating hydroperoxygroups of oxidized phospholipids directly in membranes without their preliminary phospholipid hydrolysis by free fatty acids. This enzyme conjugates toxic LPO products with glutathione and thereby contributes to their excretion from the organism. Thus, GT is an important component of antioxidant defense, from endogenic metabolites formed during oxidative stress especially.
We established a statistically significant increase of catalase and GT levels in erythrocytes in the stationary and terminal phases (Table 1) in comparison with the control group.
Up to 70% of the aldehydes formed in the cell is metabolized by conjugation with glutathione. The main antioxidant effect has glutathione in reduced form due to the reactive SH group. The decrease in intracellular redox potential of GSH/GSSG may indicate the occurrence of oxidative stress. We have established progressive reduction of GSH level and GSH/GSSG ratio in erythrocytes in dynamics of experimental ovarian cancer (Figure 1).
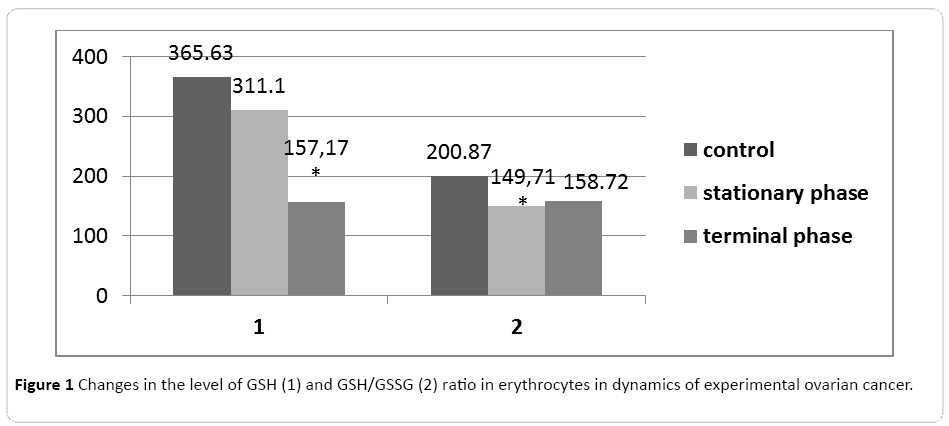
Figure 1: Changes in the level of GSH (1) and GSH/GSSG (2) ratio in erythrocytes in dynamics of experimental ovarian cancer.
Diene conjugates (DC) that is the primary products of lipid peroxidation (LP) are referred to toxic metabolites, which have damaging action on lipoproteins, proteins, enzymes and nucleic acids. Schiff bases (SB) are formed as a result of a reversible reaction between carbonyl group of aldehyde or acetone with free amino group. Continuous accumulation of Schiff bases destabilizes membranes and promotes cell destruction. The study of the lipid peroxidation intensity in erythrocytes of intact animals showed the content increase of DC, KD, SB during the stationary and terminal phases of tumor growth. The most significant change is found during the stationary phase in the group of primary products-DC (Table 1). Malondialdehyde (MDA) is a secondary lipid peroxidation product, the increase of which provokes intoxication syndrome. MDA crosslinks lipid molecules and reduces membrane fluidity (due to this, the membrane becomes more brittle, the processes connected with the membrane surface changes are interrupted: phagocytosis, pinocytosis, cell shift. We found MDA level increase in erythrocytes in the stationary phase of tumor growth in comparison with the control (Table 1).
The method of AFM enables us to examine cell parameters without using an extended and complicated fixation, thereby distorting the obtained information to a lesser extent. AFM makes it possible to measure elastic properties of cell surfaces. AFM also makes it possible to get a 3D image of the surface. Topology, profile side section, and 3D images of erythrocytes of healthy animals and tumour-bearing animals are presented on Figures 1-3. Study of red blood cells of tumor-bearing animals cytoarchitectonics showed a decrease in the number of biconcave discocytes and increase in the proportion of morphologically altered red blood cells compared to control. Echinocyte is considered reversibly deformable until the stage of membrane material loss [8]. It is believed that the change in the geometry of the figure of rotation makes the red blood cell defective and prepares it for destruction. [9] In accordance with this, we considered red blood cells cytoarchitectonics changes, detected by AFM, irreversible. There was a significant increase in IT, IRT and IIT (Table 2).
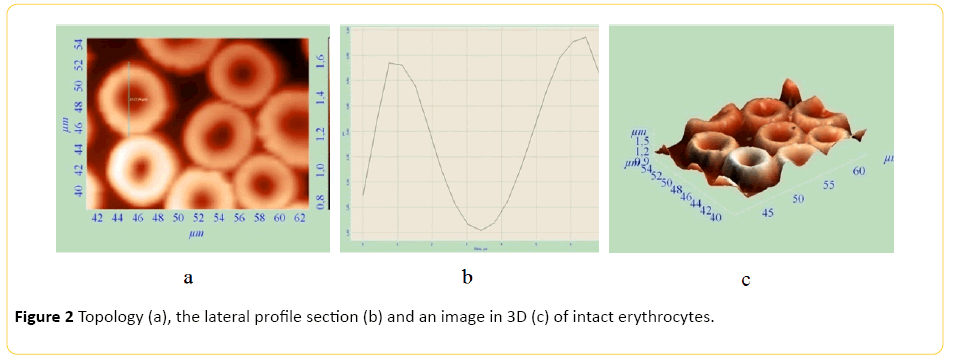
Figure 2: Topology (a), the lateral profile section (b) and an image in 3D (c) of intact erythrocytes.
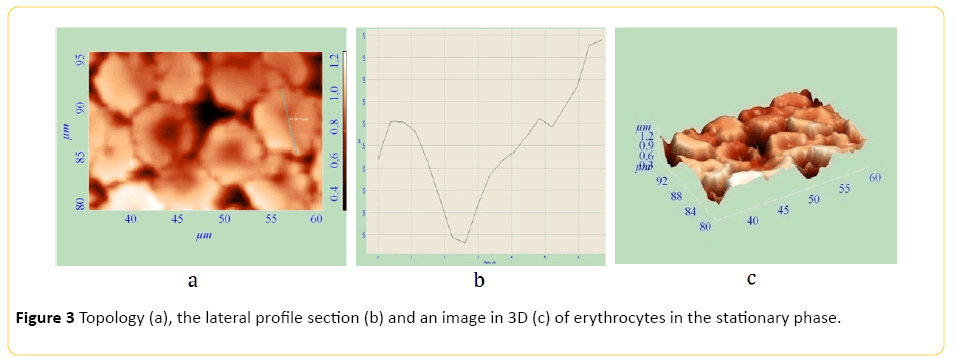
Figure 3: Topology (a), the lateral profile section (b) and an image in 3D (c) of erythrocytes in the stationary phase.
| Group Index |
IT,% |
IRT,% |
IIT, % |
| Control n=24 |
0.108 |
0.097 |
0.011 |
| Stationary phase AOC n=22 |
7.308* |
3.635* |
3.673* |
| Terminal phase AOC n=22 |
6.938* |
3.538* |
3.400* |
Note: * The value is significantly different from that in the control
Table 2: Indices of transformation of rats red blood cells in the dynamics of the AOC progression.
Echinocyte formes from discocyte. Rough growths appear on the cell surface (Figures 2 and 3). Thereafter echinocyte acquires a spherical shape. After irreversible transformation of a discocyte into a spherocyte, plasmolemma outgrowths turn into arbitrary microspherules (Figure 4).
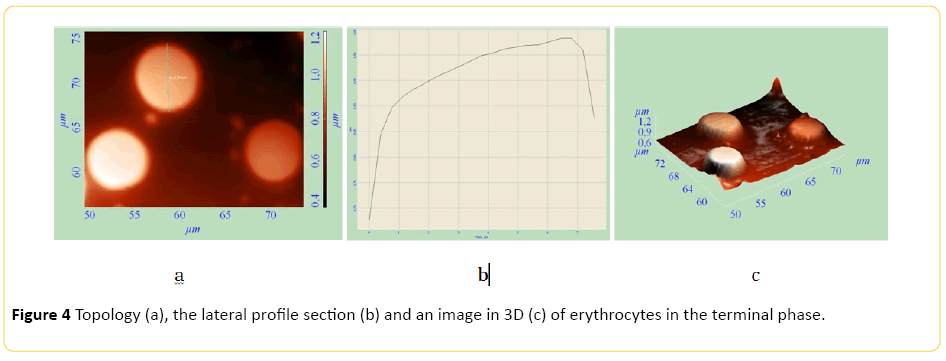
Figure 4: Topology (a), the lateral profile section (b) and an image in 3D (c) of erythrocytes in the terminal phase.
As a result of determining the rigidity we found that the rigidity of the erythrocyte membrane in the tumor-bearing organism increases in dynamics of tumor progression and is 133.1 ± 1.7 kPa for the stationary phase, 136 ± 2.2 kPa for the terminal phase vs. 101.1 ± 4.4 kPa in the control (p<0.05). In the literature, a model of the elastic properties of the erythrocyte membrane is presented, allowing, from the point of view of the author [12], to use the coefficients of rigidity for various forms of red blood cells simulating elastic properties of its membrane. In assessing the Spearman correlation of the parameters of the lipid peroxidation-antioxidant system with the indexes of transformation of red blood cells, we found that the coefficient of correlation of IT in the terminal phase of ovarian cancer with high levels of DC is highly positive (r=0.6910, p ≤ 0.05). The correlation of IT with the activity of GT and GSH/GSSG ratio in the stationary OC phase is negatively high (r=-0.8365, r ≤ 0.01 and r=-0.6916, P<0.05), respectively. A high positive correlation coefficient has the rigidity with the level of GSH and GSSG (Table 3) in both phases of the experimental ovarian cancer.
| Indices |
MDA |
DC |
KD |
SB |
SOD |
GT |
Catalase |
GSH |
GSH / GSSG |
| Group |
| Stationary phase |
IT |
r
p |
0.2340
≤0.02 |
0.100
≤0.05 |
0.2883
≤0.01 |
0.1576
≤0.02 |
-0.0748
≤0.05 |
-0.8365
≤0.05 |
-0.3571
≤0.05 |
0.3819
≤0.01 |
-0.1261
≤0.02 |
| Rigidity |
r
p |
0.1790
≤0.02 |
0.4040
≤0.05 |
0.2150
≤0.01 |
0.1670
≤0.02 |
0.4190
≤0.05 |
0.3330
≤0.01 |
0.1430
≤0.05 |
0.5450
≤0.02 |
0.1000
≤0.05 |
| Terminal phase |
IT |
r
p |
0.2910
≤0.02 |
0.6910
≤0.02 |
0.4954
≤0.01 |
0.3084
≤0.02 |
-0.3333
≤0.05 |
0.3455
≤0.01 |
-0.1273
≤0.05 |
0.0805 |
-0.6916
≤0.02 |
| Rigidity |
r
p |
0.3130
≤0.02 |
0.1070
≤0.05 |
0.1560
≤0.01 |
0.2170
≤0.02 |
0.2060
≤0.01 |
0.3470
≤0.02 |
0.3710
≤0.02 |
0.3190
≤0.01 |
0.5380
≤0.05 |
Table 3: Indicators of Spearman correlations between the parameters of lipid peroxidation-antioxidants, IT and rigidity of
erythrocyte membrane in rats with experimental ovarian cancer.
Thus, the results obtained allow us to conclude the following. Morphofunctional state of erythrocytes in circulating blood significantly change in the dynamics of experimental ovarian cancer. Herewith, strengthening of LPO together with the depletion of glutathione takes place.
Conclusion
AFM use allows to adequately assess the paraneoplastic changes in red blood cells in the dynamics of the progression of experimental ovarian tumor and can be recommended for testing in the clinic.
Conflict of Interest
The authors declare that they have no conflict of interest.
8787
References
- Dulinska I, Targosz M, Strojny W, Lekka M, Czuba P, et al. (2006) Stiffness of normal and pathological erythrocytes studied by means of atomic force microscopy. J. Biochem. Biophys. Methods 66: 1-11.
- Nowakowski R, Luckham P (2002) Imaging the surface details of red blood cells with atomic force microscopy. Surface and Interface Analysis 33: 118–121.
- Zaitsev BN, Durymanov AG, Generalov VM (2002) Atomic force microscopy of the interaction of erythrocyte membrane and virus particles. Scanning Probe Microscopy–2002: Proc. Intern. Workshop. Nizhny Novgorod 211–213.
- Pleskova SN, Gushchina YY, Zvonkova MB, Khomutov AE (2006) Study of morphology and rigidity of neutrophilic granulocyte membrane in the real time mode by scanning probe microscopy [Article in English, Russian], Bull Exp Biol Med 141: 760-762.
- Carvalho FA, Santos NC (2012) Atomic force microscopy-based force spectroscopy--biological and biomedical applications. IUBMB Life 64: 465-472.
- Xiang W, Weisbach V, Sticht H, Seebahn A, Bussmann J, et al. (2013) Oxidative stress-induced posttranslational modifications of human hemoglobin in erythrocytes. Arch Biochem Biophys 529: 34-44.
- Winski SL, Barber DS, Rael LT, Carter DE (1997) Sequence of toxic events in arsine-induced hemolysis in vitro: implications for the mechanism of toxicity in human erythrocytes. Fundam Appl Toxicol 38: 123-128.
- Liu F, Mizukami H, Sarnaik S, Ostafin A (2005) Calcium-dependent human erythrocyte cytoskeleton stability analysis through atomic force microscopy. J Struct Biol 150: 200-210
- Hakim TS, Macek AS (1988) Effect of hypoxia on erythrocyte deformability in different species. Biorheology 25: 857-868.
- Voronova O, Gening T, Abakumova T, Sysolyatin A, Zolotovskiy I, et al. (2014) The effect of picosecond laser pulses on redox-dependent processes in mice red blood cells studied in vivo. Progress in Biomedical Optics and Imaging-Proceedings of SPIE, Optical Interactions with Tissue and Cells XXV; and Terahertz for Biomedical Applications; San Francisco, CA; United States; 2 February 2014 through 4 February 2014; 2014. Volume 8941.
- Koziniec GI, Simovart Yu (1984) Superficial architectonics of peripheral blood cells, Tallinn.
- Nagornov YS (2013) Modeling of erythrocyte morphology and membrane stiffness after femtosecond laser irradiation. Russian Journal of Biomechanics 17: 112-121.
- Stachowicz-Stencel T, Synakiewicz A, Owczarzak A, Aleksandrowicz-Wrona E, Sliwinska A, et al. (2011) Pediatr Blood Cancer. 57: 561-568.









