Key words
|
| |
| Validation, Qualification, Fluid bed system, Mini Glatt. |
| |
Introduction
|
| |
| Validation is a concept that has been evolving continuously since its formal appearance in the United States in 1978 [1]. Validation is documented evidence that provides a high degree of assurance that a specific process will consistently produce a product that meets its predetermined specifications and quality attribute [2]. The concept of validation has expanded through the years to encompass a wide range of activities from analytical methods used for the quality control of drug substances and drug products, to equipments, facilities and process for the manufacture of drug substances and drug products to computerized systems for clinical trials, labeling or process control. Validation studies are essential part of Good Manufacturing Practice (GMP) and should be conducted in according with predefined protocols. A written report summarizing results and conclusions should be recorded, prepared and stored [3]. |
| |
| Validation should thus be considered in the following situations [4]: |
| |
| ? Totally new process |
| |
| ? New equipment |
| |
| ? Process and equipment which have been altered to suit changing priorities; and |
| |
| ? Process where the end-product test is poor and an unreliable indicator of product quality. |
| |
| The key elements of a validation programme are clearly defined and documented in a validation master plan (VMP) [5]. The PIC/S-document PI006 define VMP as “A document providing information on the company’s validation work programme. It should define details of and time scale for the validation work to be performed. Responsibilities relating to the plan should be stated” [6]. Validation of pharmaceutical process equipment involves the following four sequential phases [7]: |
| |
| Prequalification, Qualification, Process qualification and Process validation. Prequalification includes vendor specification, design and operation checkout. Qualification includes installation and operational check out including design qualification, installation qualification, operational qualification, performance qualification and maintenance qualification. Process validation is applicable to the manufacture of pharmaceutical dosage forms. It covers the initial validation of new processes, subsequent validation of modified processes and re-validation. Maintenance qualification is used to describe and document regular maintenance and repairs. |
| |
| Fluidizes bed processor [MINI- Capacity: 200ml (min.) to 750ml (max.); GLATT – Vendor: Glatt (India) Pharma Engineering Pvt. Ltd.] is a component laboratory equipment uses the principle of fluid bed system and is designed to perform drying, granulation and coating (Wurster process). A fluid bed system brings powdery or grained substances (bulk material) in a floating condition, with the carrying air flow enabled to exchange heat and substances with the floating material. The objective of the this validation was to establish documented evidence that Mini Glatt (fluidizes bed processor) is performing as per the manufacturer’s recommendations and process requirements and cGMP guidelines at Astron Research Ltd. Ahmedabad and it complies with the scope of supply. |
| |
|
User requirement specification (URS)
|
| |
| User requirement specifications are the basic document that covers all the needs of the manufacturing department. URS of Mini Glatt (fluidizes bed processor) was prepared by a coordinated approach among production, quality assurance, and engineering department of Astron Research Ltd. Ahmedabad, considering all the GMP requirements the machine has to fulfil. |
| |
|
Qualification protocol
|
| |
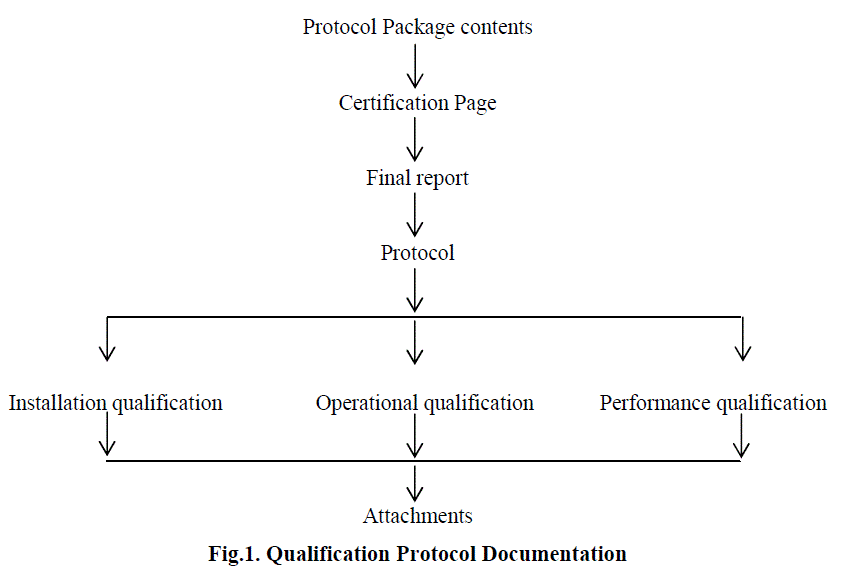
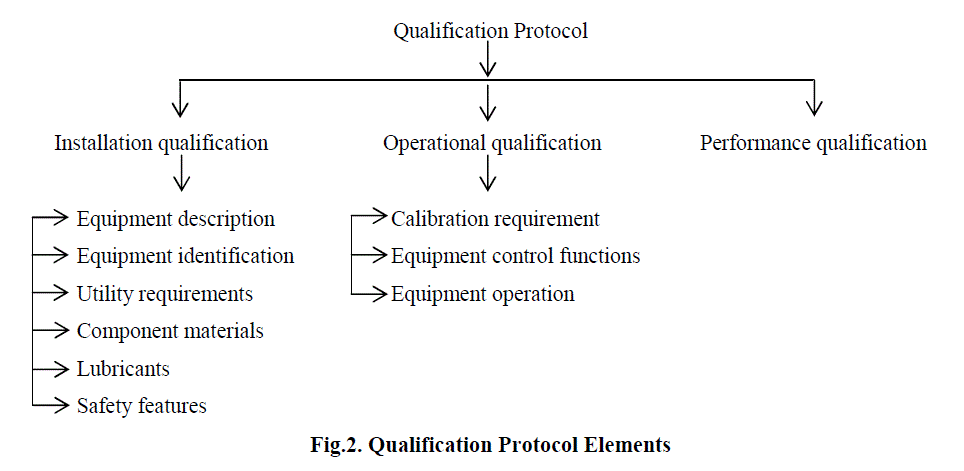
| The qualification protocol is the center of gravity of validation documentation process. The protocol is industry tool used to document the qualification process but it can take any form. Major elements of qualification protocol are given in Fig. 1 and Fig. 2 below [8]: |
| |
|
Machine description
|
| |
| Complete machine can be divided in following subsections: |
| |
| Inlet air system: It consists of compressed air supply, filtering and regulating unit, air flow meter, heating chamber consisting of heating element, process air control valve. |
| |
| Machine tower: It consists of lower plenum where the bottom spray gun fixing port is provided, product container to hold powders, expansion chamber with spray port, acrylic windows to view the process, filter housing to view the process and filter housing to fix product retaining cartridge filter. |
| |
| Spray system: Consists of peristaltic spray pump and spray gun with atomizing air connection. |
| |
| Electrical system: consists of electrical panel with contactors, terminals, SMPs and logic controls. |
| |
| Pneumatic system: consists of air reservoirs, pressure regulator and gauges. |
| |
| Indication and control elements: consists of temperature control/ indicator, operating switches, pressure regulators and indicators. |
| |
|
Qualification
|
| |
| An equipment validation program can be described in four sequential phases: Prequalification, Qualification, Process qualification and Process validation. Prequalification includes vendor specification, design and operation checkout. Qualification includes installation and operational check out including design qualification, installation qualification, operational qualification, performance qualification and maintenance qualification. |
| |
|
Design qualification (DQ)
|
| |
| It is the first element of the validation of new facilities, systems or equipment. The compliance of the design of Mini Glatt (fluidizes bed processor) with GMP was demonstrated and documented. The design qualification complies with the requirements and was accepted. |
| |
|
Installation qualification (IQ)
|
| |
| Installation qualification is performed on new or modified facilities, systems and equipment. IQ includes installation of equipment, piping, services, instrumentation checked to current engineering drawings and specifications, collection and collation of supplier operating and working instructions, calibration requirement and verification of materials of construction. All the above parameters were checked and installation qualification complies with the requirements and was accepted. |
| |
|
Operational qualification (OQ)
|
| |
| The operational qualification describes all aspects of the testing of the equipment in detail. Tests to include a condition or a set of conditions encompassing upper and lower operating limits, sometimes referred to as “worst case” conditions. Much of the qualification testing entails working with placebo batches. The completion of operational qualification allows finalisation of calibration, operating and cleaning procedures, operative training and preventative maintenance requirements. The operational qualification complies with the requirements and was accepted. |
| |
|
Performance qualification (PQ)
|
| |
| Performance qualification represents the actual studies conducted to show that all systems, subsystems or unit operations of a manufacturing process perform as intended. This verifies that the system is repeatable and is consistently producing a quality product [9].Sometimes termed process validation, this is the stage at which equipment is used in earnest. There are two basic approaches to the validation of the process itself (apart from the qualification of equipment used in production, the calibration of control and measurement instruments, the evaluation of environmental factors, etc). These are the experimental approach and the approach based on the analysis of historical data. The experimental approach, which is applicable to both prospective and concurrent validation, may involve [10] : |
| |
| ? Extensive product testing, |
| |
| ? Simulation process trials, |
| |
| ? challenge/worst case trials, and |
| |
| ? Control of process parameters (mostly physical). |
| |
| Process controls include raw materials inspection, in-process controls and targets for final product. The purpose is to monitor the on-line and off-line performance of the manufacturing process and then validate it. Even after the manufacturing process is validated, current good manufacturing practice also requires that a well-written procedure for process controls is established to monitor its performance [11]. Prior to the equipment being used on routine production, representatives from Production, Engineering and Quality Assurance approve the validation documentation. |
| |
|
Maintenance qualification (MQ)
|
| |
| Maintenance qualification is used to describe and document regular maintenance and repairs. As a result, this helps ensure that the equipment can be continuously operated to avoid downtime. Maintenance qualification covers all necessary measures entailing cleaning, maintenance and repair. This work is documented in a service logbook. |
| |
|
Performance test
|
| |
|
Test run (Top Spray) Granulation
|
| |
| Test run of the equipment for granulation (Top spray) was done for both minimum and maximum load of the equipment. Equipment was run as per the Standard Operating Procedure(SOP) and monitoring all the parameters of coating like; inlet air temperature, bed temperature, spray rate/gun, no. of gun, process air flow, atomizing air pressure, peristaltic pump speed and purging time were recorded. After completion of the process samples were withdrawn (as per sampling plan) and were analysed for % loss on drying, bulk density, tap density, particle size distribution and assay. All the samples analysed complies with the requirements and are with in acceptable limits. |
| |
|
Test run (Bottom Spray) Wurster coating
|
| |
| Test run of the equipment for wurster coating (Bottom spray) was done for both minimum and maximum load of the equipment using non peril seeds. Equipment was run as per the SOP and monitoring all the parameters of coating like; inlet air temperature, bed temperature, spray rate/gun, no. of gun, process air flow, atomizing air pressure, peristaltic pump speed and purging time. After completion of the process samples were withdrawn (as per sampling plan) and were analysed for % loss on drying, % yield, weight build up and assay. All the samples analysed complies with the requirements and are with in acceptable limits. |
| |
Conclusion
|
| |
| The equipment qualification for Design, Installation, Operation and Performance was successfully done. No deviation was observed at any stage of qualification. The study revealed that all the functions and parameters were complying with specifications set and required. All acceptance criteria set are meeting the requirements, hence the equipment is qualified. |
| |
Conflict of Interest
|
| |
| : None |
| |
Source of Support
|
| |
| Nil |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |








