Marta Rico Rodríguez1, María De-las Mercedes Calero Ruiz2* and Nelia Jiménez Valencia3
1Department of Clinical Analysis, Altollano Clinic, Leon, Spain
2Department of Clinical Analysis, Puerta del Mar University Hospital, Cadiz, Spain
3Department of Clinical Analysis, Riotinto Hospital, Huelva, Spain
Corresponding Author:
María De-las Mercedes Calero Ruiz
Area Specialist, Department of Clinical Analysis
Puerta del Mar University Hospital, Cadiz, Spain
Tel:+34636797997
E-mail: marta.rico.rodriguez@gmail.com
Received date: March 02, 2019; Accepted date: March 15, 2019; Published date: March 22, 2019
Citation:Rodríguez MR, Ruiz MDMC, Valencia NJ (2019) Validation of the ARK™ Methotrexate Reagent for the Determination of Methotrexate in Serum Samples. Ann Clin Lab Res Vol. 7 No. 1:298
Keywords
Validation; Methotrexate; MTX validation reagent
Introduction
Drug monitoring represents a very important chapter in clinical biochemistry, as it enables maintaining and adjusting the administration of certain types of drugs to a patient in accordance with their individual pharmacokinetic characteristics. This then ensures maximum efficiency of the drug both in regard to the choice of dosage as well as the possibility of association with other drugs and minimisation of the risk of toxicity [1].
Methotrexate (MTX) is an aminopterin analogue derived from folic acid which acts as its antimetabolite, competitively inhibiting the dihydrofolate reductase (DHFR) enzyme. This enzyme participates in the formation of the folinic acid necessary for the production of the thymidine nucleoside, required for the synthesis de DNA, RNA, thymidylates and proteins [2].
It was initially used in 1948 for the treatment of childhood lymphocytic leukaemia and since then has been used against numerous malignant diseases, including osteosarcomas, non- Hodgkin lymphoma, Hodgkin's disease, cutaneous T-cell lymphoma (mycosis fungoides), breast cancer and cancers of the head and neck [3]. It is also considered a first choice drug in the treatment of rheumatoid arthritis [4].
The monitoring of MTX, a routine clinical practice to identify patients at risk and adjust the dose of folinic acid, has managed to reduce the incidence of serious adverse effects as well as the number of deaths caused by high MTX concentrations [5].
Objective
To know the precision and accuracy of the ARK™ methotrexate reagent in the Cobas 6000 analyser from Roche Diagnostics® in the analytical process of our laboratory, ensuring the reliability of the results in the monitoring MTX levels in daily practice.
Materials and Methods
All samples sent to the Clinical Analysis Service of the Puerta del Mar University Hospital during the months of April and May 2015 for the determination of MTX levels. The analysis was performed in duplicate with both reagents described.
Instruments
The Cobas 6000 analyser from Roche Diagnostics® was used for the analysis of methotrexate.
Calibration
A complete calibration (6 points) was performed using ARK methotrexate calibrators A, B, C, D, E, and F (double test) and the curve then verified with three levels of quality control. A new calibration was performed for each new batch number of the reagent kit.
Quality control
Each day that patient samples were processed, the technique was tested with at least three levels of quality control (high, medium and low medical decision points). The results were reviewed and, if necessary, corrective measures taken before processing the test samples.
Reagents
• Previous Reagent: Siemens Dade methotrexate (Reference: 6L119 EMIT).
• Reagent to be evaluated: ARK™ methotrexate (Reference: 5026-0001).
Samples
• The analytical determination was performed on samples of serum or plasma, the extraction being made in agar tubes or with lithium heparin as anticoagulant respectively.
• The intervals between sample collection depend on the dose, the duration of the infusion, and the clinical condition of the patient; but as a general rule they were performed at 24, 36, 48, 54 and 72 hours.
• The samples were processed on the same day as the extraction and if the assay was not to be performed, they were frozen (-10°C). Freezing-thawing cycles were avoided.
Quantitation of MTX
The test for the determination of MTX levels is based on the competition between the drug present in the sample and the methotrexate marked with glucose-6 phosphate dehydrogenase (G6PDH) enzyme bonding to the reagent antibody. When the marked methotrexate bonds to the antibody, the enzymatic activity is reduced. In the presence of drug in the sample, the enzymatic activity increases and is directly proportional to the drug concentration. The active enzyme converts the nicotinamide adenine dinlcleotide coenzyme (NAD) into NADH whose level is then measured using spectrophotometry as it is in direct relationship with the difference in absorbance. The endogenous serum G6PDH does not interfere with the results because the NAD coenzyme only functions with the enzyme of bacterial origin used in the assay.
Assessment protocol
The following assessment protocol was applied:
Imprecision study: Three control samples were used with concentrations of 0.07, 0.4 and 0.8 μmol/L, respectively. The intra-series precision test analysed each of the control samples 10 times in a single series. The inter-series precision analysed the same control samples on 10 different days within the same month [6].
Linearity study: The range of linearity reported by the manufacturer was 0.04-1.2 μmol/L. This was corroborated by making serial dilutions (1:0, 3:1, 2:2, 1:3 and 0:1) of two different samples with values of 2.54 and 0.1μmol/L to obtain 5 MTX concentrations between both values. The samples were analysed in duplicate [7].
Study of the detection limit: Two series were performed of 10 replicates of calibrator A (0 μmol/L). The 95 percentile of the mean sensitivity of the two series analysed being considered the limit of detection [8].
Study of the functional sensitivity: An analysis was made of 10 replicates of the same series of a sample with 0.20 μmol/L of MTX, as this was de decisive cut-off value for clinical decisions from the haematological point of view (reduce the risk of toxicity) [8].
Study of the correlation: MTX levels using both reagents were determined in 62 test samples obtained during this period. The plasma concentrations varied between 0.03 μmol/L and 2.54 μmol/L. The results obtained were analysed using the linear regression method [9].
The results obtained are expressed as means (μmol/L), standard deviations (SD) and coefficients of variation (%CV), with their 95% confidence intervals (CI).
Results
The results of the study of intra- and inter-series imprecision are shown in Table 1.
| Study |
Control 1(µmol/L) |
Control 2(µmol/L) |
Control 3(µmol/L) |
| Intra-series |
Inter-series |
Intra-series |
Inter-series |
Intra-series |
Inter-series |
| Theoretical mean |
0.07 |
0.04 |
0.08 |
| Experimental mean |
0.078 |
0.08 |
0.374 |
0.408 |
0.772 |
0.859 |
| SD |
0.004 |
0.008 |
0.005 |
0.030 |
0.014 |
0.072 |
| CV (%) |
5.7% |
10.2% |
1.5% |
7.5% |
1.9% |
8.3% |
| *SD: Standard Deviation; CV: Coefficient of Variation. |
Table 1 Study of intra- and inter-series imprecision.
The linearity study resulted in the following equation for the straight line: y= 0.996x+0.148, with a coefficient of correlation (r2) of 0.989 (0.983-0.994). The resulting linearity is shown in Figure 1.
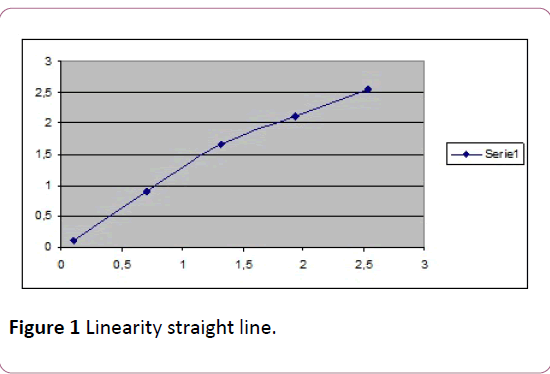
Figure 1: Linearity straight line.
The detection limits and functional sensitivity were 0.02 μmol/L (SD=0.009, CV=47.1%) and 0.184 μmol/L (SD=0.005; CV=2.8%), respectively.
The correlation study resulted in a straight line y=0.960x +0.016, with an r2 of 0.980 (0.967-0.988).
Discussion
There are currently no clearly established CV limits for this drug. Clinical experience has shown that CV levels >10% should not be accepted because of the importance of the negative effects associated with this anticancer drug. Our study shows that both the intra-series CV (controls 1, 2 and 3) and interseries CV (controls 2 and 3) meet this objective and the coefficient only exceeds 10% in the event of very low values (control 1). This has no important clinical implication as monitoring is no longer required at levels above this value [10], as the risk of toxicity and noxious effects on health reduce drastically.
This study confirms the range of linearity reported by the manufacturer of between 0.04 and 1.2 μmol/L, this means that higher concentrations require diluting the sample so that the level is within the range of linearity.
It is possible to state that the limit of detection for this reagent is 0.02 μmol/L, and so values at or below this value cannot be accurately reported.
In order to establish the behaviour of the technique at critical levels, a sample with a MTX level of 0.20μmol/L was used as this is the decisive cut-off value for clinical decisionmaking regarding finalization of the cycle and its monitoring [10]. In this regard the sensitivity detected and the coefficient of variation are both excellent (CV<5%).
The concordance between both reagents is good as the coefficient of correlation is close to 0.99, therefore being interchangeable and acceptable for monitoring this drug.
Conclusion
In our study was demonstrate that the ARK MTX reagent in the Cobas 6000 analyser from Roche Diagnostics® provides an accurate, reliable and robust measurement of MTX levels and complies with requirements for monitoring this drug from the technical point of view and therefore it can be used and implemented in routine clinical laboratory practice.
24212
References
- Calvo MV, García MJ, Martínez J, Fernández MM (2009) Farmacocinética clínica en España. Farmacia Hospitalaria 33: 1.
- Puig L (2014) Metotrexato: Novedades terapéuticas. Novedades En Dermatología. 105: 583-589.
- Castro RO, Contreras AE, Gutierrez JC, Villegas MC (2013) Optimal use of methotrexate. Seminars of the Spanish Foundation of Rheumatology 14: 1-28.
- Relling MV, Fairclough D, Ayers D, Crom WR, Rodman JH, et al. (2016) Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol 12: 1667-1672.
- Carey RN, Anderson FP, George H, Hartmann AE, Janzen VK, et al. (2006) User verification of performance for precision and trueness. Approved Guideline 2nd Edn. Clinical and Laboratory Standards Institute 21: 25.
- Guidelines EP6-A: https://shop.clsi.org/site/Sample_pdf/EP06A_sample.pdf.
- https://www.seqc.es/es/Varios/7/41/Modulo_3:_Control_de_la_calidad_analitica_y_evaluacion_de_metodos_analiticos/
- Guidelines EP9-A2: https://shop.clsi.org/site/Sample_pdf/EP9A2_sample.pdf.
- Serra IB (2014) Tratamiento de la Leucemia Aguda Linfoblástica de Nuevo Diagnóstico. SEHOP/PETHEMA pp: 1-261.







