Maria De Las Mercedes Calero Ruiz*, Marta Rico Rodriguez, Olga Diz Mellado, Ana Isabel Mangano Armada, Maria Angeles Bailén Garcia and Rafael Torrejon Cardoso
Hospital Universitario Puerta del Mar, Cádiz,Spain
*Corresponding Author:
Ruiz MDLMC
Hospital Universitario Puerta del Mar. Ana
de Viya 21 11009 Cadiz
Spain.
Tel: 956002069
E-mail: mercaru767@gmail.com
Received date: May 10, 2018; Accepted date: May 23, 2018; Published date: May 26, 2018
Citation: Ruiz MDLMC, Rodriguez MR, Mellado OD, Armada AIM, Garcia MAB, et al. (2018) Validation of the Reagent Hormone Anti-Mullerian Plus (Roche Diagnostics) for the Dosage of FSH Recombinant Delta (Ferring Pharmaceuticals) in Patients Submitted to Techniques of Assisted Reproduction. Ann Clin Lab Res. Vol.6 No.2: 236. doi: 10.21767/2386-5180.100236
Keywords
AMH; Reactive validation; Assisted reproduction
Introduction
Ovarian stimulation is one of the fundamental pillars on which the treatment of in vitro fertilization is based. However, it is not yet fully clear what parameters can help us achieve an adequate ovarian response [1-3]. The anti-Mülleriana hormone (AMH) has been proposed by several authors as a useful indicator when predicting the ovarian response, as well as being a marker that can guide as to the dose of gonadotropins is concerned [4].
In this sense, the biopharmaceutical company Ferring has proposed the posology of recombinant FSH in an individualized manner for each patient referred be Nyboe et al., and Buur et al., [5,6] with the aim of obtaining an ovarian response associated with a favorable efficacy/safety profile, managing to recover an adequate number of oocytes and reducing the necessary interventions to prevent ovarian hyperstimulation syndrome (OHSS).
The safety and efficacy of such treatment with recombinant follicle-stimulating hormone (Follitropin delta from Ferring Pharmaceuticals-REKOVELLE®) is based on the accuracy of the results of the Elecsys AMH Plus immunoassay [7].
Objective
To know the precision and accuracy of the AMH Plus test of Roche Diagnostics in the analytical process of our laboratory, ensuring the reliability of the results and its possible applicability in the individualized dosage of recombinant FSH gonadotropin.
Materials and Methods
The cobas 6000 analyzer (Roche Diagnostics) uses a sandwich test with a total duration of 18 minutes, consisting of:
a) A first incubation of 50 µL of sample with an anti-AMH biotinylated monoclonal antibody and an anti-AMH monoclonal antibody marked with ruthenium kalate forming a sandwich complex.
b) A second incubation, in which after incorporating the streptavidin-coated microparticles, the complex formed is fixed to the solid phase by interaction between biotin and streptavidin.
c) The reaction mixture is transferred to the measuring cell where, by magnetism, the microparticles are fixed to the surface of the electrode. Applying a defined electric current produces a chemiluminescent reaction whose emission of light is measured with a photomultiplier and
d) The results are determined by a calibration curve generated specifically for the instrument from a calibration at 2 points and a master curve provided by the bar code of the reagent or the electronic barcode.
The reagent to be evaluated is AMH plus from Roche Diagnostics. It is composed of streptavidin-coated microparticles (M), mouse anti-AMH monoclonal biotinylated antibodies (R1) and mouse anti-mouse AMAM monoclonal antibody labeled with ruthenium (R2) chelate.
In our study, we performed the measurement of 2 control samples (PC1 and PC2 from Roche Diagnostics) in duplicate for 10 consecutive days and after two calibrations within the period of the experiment (day 1 and day 5). To this end, a control of each level was reconstituted and aliquoted for daily measurement, keeping said aliquots at -20°C until processing. The results obtained are expressed in nanograms per milliliter. Subsequently, we calculated the mean (X [ng/mL]) and standard deviation (SD [ng/mL]) for PC1 and PC2, relative bias (oRB [%]) and the imprecision (oCV [%]) of the immunoassay performance Elecsys AMH Plus 3.
Relative bias is calculated using the formula:
(Average value PC1 or PC2 - Target value PC1)/Target value PC1 or PC2=oRB% PC1 or PC2.
The inaccuracy is calculated using the formula:
(SD PC1 or PC2/Average value PC1 or PC2) × 100=oCV% PC1 or PC2.
Results
The results of the daily measurements are shown in Table 1.
| Valor tarjet |
PC nivel 1
(ng/mL) |
PC nivel 2
(ng/mL) |
| Day 1 |
Run 1 |
1.01 |
5.07 |
| Run 2 |
1.02 |
5.11 |
| Day 2 |
Run 1 |
1.03 |
5.11 |
| Run 2 |
1.01 |
5.06 |
| Day 3 |
Run 1 |
1.02 |
5.09 |
| Run 2 |
1.03 |
5.10 |
| Day4 |
Run 1 |
0.99 |
4.99 |
| Run 2 |
0.99 |
4.96 |
| Day 5 |
Run 1 |
1.01 |
5.12 |
| Run 2 |
1.02 |
5.16 |
| Day 6 |
Run 1 |
1.00 |
5.04 |
| Run 2 |
1.00 |
4.96 |
| Day 7 |
Run 1 |
1.00 |
5.07 |
| Run 2 |
1.01 |
5.04 |
| Day 8 |
Run 1 |
0.98 |
4.95 |
| Run 2 |
0.98 |
4.95 |
| Day 9 |
Run 1 |
0.98 |
4.92 |
| Run 2 |
0.98 |
4.91 |
| Day 10 |
Run 1 |
0.98 |
4.94 |
| Run 2 |
0.99 |
4.95 |
Table 1: Results daily measurements PC1 and PC2 CH4+O2+M (M=Fe, Co, Ni, Ru, Rh, Pd, Os, Ir, Pt) compounds.
The average obtained is 1.00 ng/mL for PC1 and 5.03 ng/mL for PC2. The observed standard deviation is 0.02 ng/mL and 0.08 ng/ mL, respectively.
The relative bias obtained is 0.99% for PC1 and 0.79% for PC2 while the coefficient of variation is 2.00% and 1.59%, respectively (Table 2 and Figure 1).
| Result |
Valor tarjet
(ng/mL) |
Valor medium ± DS
(ng/mL) |
| PC nivel 1 |
1.01 |
1.00 ± 0.02 |
| PC nivel 2 |
5.07 |
5.03 ± 0.08 |
| Relative bias |
PC1 (%) |
PC2 (%) |
| 0.99 |
0.79 |
| Coefficient of variation |
PC1 (%) |
PC2 (%) |
| 2.00 |
1.59 |
Table 2: Total results PC1 and PC2.
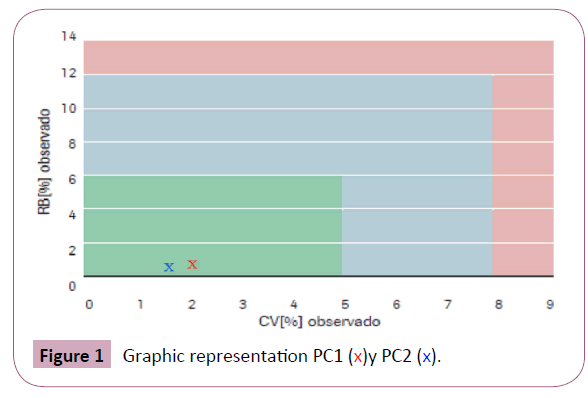
Figure 1: Graphic representation PC1 (x)y PC2 (x).
Discussion
REKOVELLE® is the first recombinant FSH (rFSH) derived from a human cell line and is the first treatment that is administered with an individualized dosage regimen [5,6,8,9]. The individualized dosing regimen is based on the levels of the woman's serum antibilirubin hormone and her body weight. With these parameters, a specific daily dose is determined for each patient from the beginning of the assisted reproduction cycle.
CE approval of REKOVELLE® is based on a comprehensive clinical data package that includes the results of the ESTHER Phase 3 trials (evidence-based stimulation test with human rFSH in Europe and Rest of the world). The data show that the individualized treatment with REKOVELLE® (follitropin delta), compared with the conventional rFSH treatment (follitropin alfa), had similar results in terms of pregnancy rates and implantation rates in progress. Patients receiving REKOVELLE® also achieved optimal oocyte performance (8-14 oocytes) more frequently than those receiving conventional rFSH treatment, with few clinically relevant cases of poor and excessive ovarian response. In addition, ovarian hyperstimulation syndrome (OHSS) and/or preventive OHS interventions occurred less frequently (p< 0.05) in women who received REKOVELLE® than women who received conventional rFSH treatment.
Conclusion
In our study it was demonstrated that the Elecsys AMH Plus immunoassay from Roche [7] provides an accurate, reliable and robust measurement of AMH levels. After using the basic implementation instructions, it is observed that the performance of the test within our laboratory is within the green section; that is, oRB [%] less than 6% and oCV [%] less than 5%. Therefore, we conclude that this trial is appropriate for routine clinical use, helping to establish the individual daily dose of FSH delta in combination with body weight in women who undergo a program of assisted reproduction techniques.
22663
References
- Pankhurst MW, Kelley RL, Sanders RL, Woodcock SR, Oorschot DE, et al. (2018) Anti-Müllerian hormone overexpression restricts preantral ovarian follicle survival. J Endocrinol 237: 153-163.
- Iwase A, Osuka S, Goto M, Murase T, Nakamura T, et al. (2018) Clinical application of serum anti-Müllerian hormone as an ovarian reserve marker: A review of recent studies. J Obstet Gynaecol Res 2: 1.
- Pilsgaard F, Grynnerup AG, Løssl K, Bungum L, Pinborg A (2018) The use of anti-Müllerian hormone for controlled ovarian stimulation in assisted reproductive technology, fertility assessment and -counseling. Acta Obstet Gynecol Scand 2: 1.
- Anderson RA, Anckaert E, Bosch E, Dewailly D, Dunlop CE, et al. (2015) Prospective study into the value of the automated Elecsys antimüllerian hormone assay for the assessment of the ovarian growing follicle pool. Fertil Steril 103: 1074-1080.
- Nyboe A, Nelson SM, Fauser BC, Garcia-Velasco JA, Klein BM, et al. (2016) Efficacy and safety of follitropin delta in an individualised dosing regimen: A randomised, assessor-blind, controlled phase 3 trial in IVF/ICSI patients (ESTHER-1). Human Reproduction 31: 324.
- Buur A, Nelson SM, Fauser BC, Garcia-Velasco JA, Klein BM, et al. (2016) Low immunogenicity potential of follitropin delta, a recombinant FSH preparation produced from a human cell line: Results from phase 3 trials (ESTHER-1 and ESTHER-2). Human Reproduction 31: 385.
- Roche Diagnostics (2015) Elecsys® AMH (anti-Mullerian hormone): Method sheet. https://pim-eservices.roche.com.
- GuíaCLSI-EP5-A2: https://yeec.com/uploadimages1/forum/20132/201321114424770758.pdf.
- Arce JC, Nyboe A, Fernandez SM, Visnova H, Bosch E, et al. (2014) Ovarian response to recombinant human follicle-stimulating hormone: A randomized, antimullerian hormone–stratified, dose–response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril 102: 1633–1640.







