Keywords
Dementia; Neurodegenerative disorders; Cognitive; Elisa; Biomarkers; Parkinson's disease; DSM-IV
Introduction
Dementia in neurodegenerative disorders such as Parkinson’s disease (PD) was as an impairment in the structure and function of cerebral blood vessels and associated cells (neurovascular unit), and these effects are mediated by inflammation [1]. Systemic and central immune systems can communicate in the presence of an intact brain blood barrier (BBB). In AD (Alzheimer’s disease), where there is evidence of a breakdown in BBB permeability [2-5], there is an even greater likelihood of communication by means of inflammatory mediators between the CNS and periphery. Some studies showed that plasma levels of inflammatory markers such as Interleukin-6 (IL-6) and high sensitivity Creative protein (hs-CRP) increased in the early stages of PD and in the early stages of Cognitive decline leading to AD [6]. Recently there is an activated research field to an early role for inflammatory processes in disease onset and progression, accumulating research also focuses a more complicated picture, in which pro-inflammatory markers inconsistently and at times inversely relate to cognitive decline. Several large comprehensive studies declared no association between baseline inflammation and future risk of AD or non-AD dementia or have noted no independent effects over and above traditional vascular risk factors [7-9]. Due to the activated primary response of immune system, it has been observed that endothelial dysfunction markers such as soluble inter-cellular adhesion molecule (ICAM-1) and soluble vascular cell adhesion molecule (VCAM-1) increased in neurodegenerative disease [10]. However, other studies have reported no association [11,12] in identifying the diagnostic biomarker in dementia in Parkinson's patients help clinician that select right patients for appropriate treatment. The Present study designed to answer the question whether the inflammatory biomarkers have a suitable power in comparing to vascular biomarkers for diagnosis of dementia in PD, so, we investigate the serum concentration of hs-CRP and IL-6 as representative of systemic inflammation and ICAM-1 and VCAM-1 as vascular biomarkers.
Materials and Methods
Population
Seventy-five consecutive diagnosed PD patients seen in an outpatient primary care setting who presented movement symptoms were enrolled. PD patients were evaluated by trained neurologists and diagnosed based on the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnosis Criteria for Parkinson’s disease. We excluded patients with secondary causes of Parkinsonism (e.g. Wilson’s disease, neuroleptic drug use or psychiatric diseases) [13-15]. PD patients with a cerebrovascular disease or focal neurological signs of cerebral disease were also excluded. None of the subjects included in this study had a recent out- or inpatient history of recent infection, a history of surgery or trauma during the previous month, cardiovascular disease, and use of NSAIDs. Informed consent was obtained from the participants before entering the study. The study was approved by the local ethics committee of Islamic Azad University, Iran.
Measurements
Dementia was diagnosed by DSM-IV criteria [16] and by Mini-Mental state exam (MMSE) [17] scores adjusted for educational adjustment. In addition, we defined hypertension, diabetes mellitus, hypercholesterolemia, and cigarette smoking based on medical histories to examine relationships between these atherosclerosis risk factors and biomarkers levels. Hypertension was defined as a systolic blood pressure of >140 mmHg, a diastolic blood pressure of >90 mmHg, or the current use of antihypertensive medications. Diabetes mellitus (DM) was defined as a history of a fasting glucose>110 mg/dl or the current use of hypoglycemic agents. Hypercholesterolemia was defined as a total cholesterol concentration of >220 mg/dl or the current use of lipidlowering agents. Cigarette smoking was described as the patient stating any cigarette smoking during the previous 5 years.
Blood sampling and biological assays
Venous blood samples for biomarkers analysis were obtained from all 75 non-fasting subjects. Biomarker levels in PD patients were measured in the morning before antiparkinsonian therapy was administered. The serum samples were obtained within 1 hour of blood sampling by centrifugation at 3,000 rpm for 10 minutes. Serum samples were stored at -70°C until required for laboratory evaluations. Levels of ICAM-1 by Boster biological Technology – Human ICAM-1 ELISA kit, levels of VCAM-1 by Boster Biological Technology- Human VCAM-1 ELISA Kit, levels of hs-CRP by LDN –CRP – high sensitivity Human Elisa kit, levels of IL-6 by Orgenium IL-6 human Elisa kit.
Statistical analysis
Calculation was performed using the SPSS version 19.0 software. Group results are expressed as means ± standard deviations (SD). Pearson’s Chi-square analysis was used to compare group categorical variables, and independent t-tests were used to compare continuous variables. Binary logistic regression analysis was used to adjust for potential confounders. Statistical significance was determined using an error level of 5%.
Results
The demographic and baseline clinical features of the PDD patients and PD patients presented in Table 1.
| Characteristics |
PDD (n=20) |
PD (n=55) |
P value |
| Demographics |
| Male, n (%) |
6 (30) |
33 (60) |
0.43 |
| Age (year), mean ± SD* |
70/25 ± 9/04 |
61/03 ± 10/23 |
0/001 |
| Illiterate |
15 (75%) |
30 (54%) |
0/12 |
| MMSE Score* |
17/35 ± 4/17 |
27/07 ± 2/65 |
0/000 |
| Biomarker studies |
| hs-CRP (mg/dl), mean ±SD |
0/15 ± 0/19 |
0/10 ± 0/03 |
0/06 |
| IL-6 (ng/ml), mean ±SD |
78/61 ± 69/05 |
56/20 ± 50/15 |
0/13 |
| ICAM-1 (ng/l), mean ±SD |
779/57 ± 611/79 |
796/00 ± 612/94 |
0/92 |
| VCAM-1 (ng/ml), mean±SD* |
56/06 ± 58/16 |
30/43 ± 38/30 |
0/04 |
| Clinical features |
| Symptom duration (years), mean ±SD |
4/18 ± 3/84 |
5/98 ± 5/96 |
0/21 |
| H & Y stage |
2/001 ± 1/01 |
1/69 ± 0/82 |
0/203 |
| HO |
1 (5%) |
5 (9/09%) |
0/58 |
| Hypertension (%) |
10 (50) |
16 (29) |
0/09 |
| Diabetes mellitus (%) |
5 (25) |
8 (14/5) |
0/29 |
| Hypercholesterolemia (%) |
5 (25) |
8 (14/5) |
0/29 |
| Smoking (%) |
3 (15) |
7 (12/7) |
0/47 |
PDD: Parkinson disease with dementia, PD: idiopathic Parkinson's disease, hs-CRP (high sensitivity C-reactive protein), MMSE: Mini Mental Status Examination, H & Y stage: Hoehn and Yahr Stage, HO: Hypotension Orthostatic, Values was expressed as mean ± standard deviation, Gender was analyzed by Pearson chi-square test among 2 group, *P-value: PD vs. PDD <0.05
Table 1: Baseline characteristics of all subjects in each group.
There were no significant differences in gender or symptom duration, although PDD patients were older. When evaluating serum levels of hs-CRP and VCAM-1 between PDD and PD after adjustment for age, sex and atherosclerotic risk factors, binary logistic regression analysis demonstrated no significant association between hs-CRP level and dementia in PD (P=0.99), but VCAM-1 has a significant difference between two groups (P =0/04) (Table 2).
| Variables |
Cut off Point |
Specificity (%) |
Sensitivity (%) |
NPV (%) |
PPV (%) |
Under area |
| VCAM-1 (ng/ml) |
40.14 |
64 |
73 |
92.3 |
57.9 |
0/64 |
| IL-6 (ng/ml) |
38.12 |
49 |
73 |
90.4 |
26.3 |
0/62 |
| Hs-CRP (mg/dl) |
0.09 |
58 |
68 |
88.5 |
42.1 |
0/65 |
Negative Predictive Value (NPV) and Positive Predictive Value (PPV)
Table 2: Cut-off point for VCAM-1, IL-6 and hs-CRP for diagnosis of dementia in Parkinson's disease.
By binary logistic regression analysis, the odds ratio (with a 95% confidence interval) for PDD based on a VCAM-1 level was 1/01 (95% confidence interval=1/00–1/02, P= 0/04) (Table 2). Regarding association between VCAM-1 level and atherosclerosis risk factors, smoking had the largest effect on the VCAM-1 levels in PDD group, although this was not statistically significant (Table 3).
| Variables |
VCAM-1 in PDD (ng/ml) (no/yes) |
P-value |
| Hypertension |
51.92 ± 60.65/60.66 ± 58.55 |
0.75 |
| Diabetes mellitus |
62.36 ± 64.85/38.42 ± 32.07 |
0.44 |
| Hypercholesterolemia |
55.76 ± 65.51/57.18 ± 16.31 |
0.96 |
| Smoking |
45.76 ± 47.68/111.01 ± 89.54 |
0.07 |
*P value: positive vs. negative <0.05
Table 3: Comparison of VCAM-1 by atherosclerotic risk factors in PDD patients.
The MMSE in general neuropsychological tests showed significant difference related to age, VCAM-1 and hs-CRP at determined levels of cognitive function in Parkinson's disease because there was significant difference among normal cognitive function and mild cognitive impairment as follow: (P=0/01, P=0/04) and ICAM-1 levels was significant among mild and severe Cognitive Impairment.
The fluctuation of IL-6 based on cognitive function from Normal to sever dysfunction showed in Figure 1. Used by ROC, the best cut-off point for differentiating dementia in PD from PDD was VCAM-1 levels equal to or greater than as 40/14 ng/ml. At this cut–off point, the sensitivity and specificity were 73% and 64%, The cut-off point calculated for IL-6 and hs-CRP levels equal to or greater than as 38/12 ng/ml and 0/09 with sensitivity and specificity were (73%, 49%, 68%, 58%) (Table 4 and Figure 1). In the group of patients with a cut-off value of VCAM-1 (40/14 ng⁄ml) according to our results, the PDD patients with positive value, the average age was 71/81 ± 8/09 vs.71/81 ± 8/09 years.
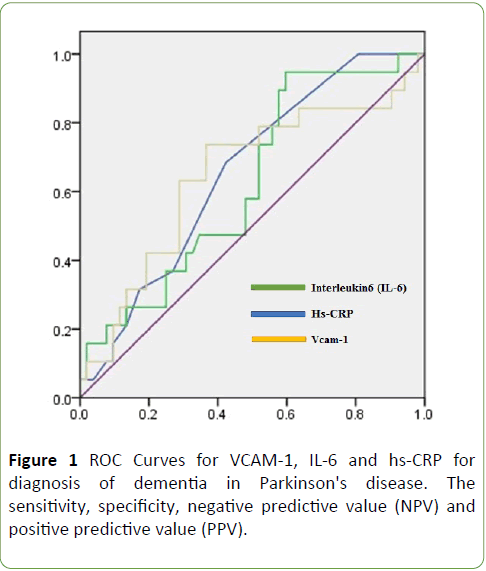
Figure 1: ROC Curves for VCAM-1, IL-6 and hs-CRP for diagnosis of dementia in Parkinson's disease. The sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV).
| Variables |
Odds ratio |
P value |
95% CI |
| Hypertension Gender |
1.20 1.29 |
0.78 0.71 |
0.35-5.00 0.38-6.34 |
| AGE * |
1.09 |
0.01 |
1.02-1.18 |
| Hs-CRP |
2.33 |
0.99 |
0.00-1.70 |
| VCAM-1 * |
1.01 |
0.04 |
0.99-1.02 |
PDD group, PD normal MMSE result, hs-CRP high-sensitivity C-reactive protein, CI confidence interval, P value by binary logistic regression analysis
Table 4: hs-CRP and VCAM-1 comparisons between PDD and PD after adjustment for risk factors.
Duration of symptoms disease and the score of H & Y was 4/51 ± 3/93 years and 2/29 ± 0/96, only the MMSE score (16/07 ± 4/39) has a significant difference according to positive vs. negative values of VCAM-1 (P=0/03) (Table 5).
| Characteristics |
VCAM-1 in PDD (cut point=40/14 ng/ml) |
P value |
| Negative |
Positive |
| Age (Mean ± SD) |
69/80 ± 11/94 |
71/81 ± 8/09 |
0/85 |
| Sex (Male, N (%)) |
4 (80%) |
10 (66/6%) |
0/70 |
| Duration of symptoms (Mean ± SD) |
2/30 ± 2/28 |
4/51 ± 3/93 |
0/25 |
| H & Y stage (Mean ± SD) |
1/51 ± 1/00 |
2/29 ± 0/96 |
0/14 |
| MMSE Score (Mean ± SD) |
20/60 ± 0/54 |
16/07 ± 4/39 |
0/03 |
| VCAM-1 (Ng/ml) (Mean ± SD) |
5/24 ± 2/88 |
80/94 ± 63/84 |
0/01 |
*P value: positive vs. negative <0.05
Table 5: Comparison of the clinical profiles by VCAM-1 cut point for the PDD patients.
Discussion
To answer the question whether the inflammatory biomarkers have suitable power in comparing to vascular biomarkers for diagnosis dementia in Parkinson disease, our first finding that the higher serum levels of VCAM-1 appears to play role in PDD. Serum levels of IL-6, hs-CRP and ICAM-1 did not show any significant differences among PDD and PD. PDD is a multifactorial illness that the vascular pathologic interaction with the neuroinflammatory changes progresses to dementia. More knowledge about the mechanism of dementia in PD result to control modifiable risk factors. Our results proposed VCAM-1 as a modifiable vascular risk factor for PDD. This is supported by Zuliani et al. [2] showed that the levels of VCAM-1 was elevated among Alzheimer's disease than healthy controls, the differences was not statistically significant, while VCAM-1 positively correlated with age (and alcohol consumption and negatively associated with HDL-C levels). The level of VCAM-1 in presence of smoking is significantly increasing in PDD patients. For comparison between mechanisms of dementia in Alzheimer's disease and Vascular dementia, Engelhart et al. showed no changes in the plasma levels of VCAM-1 and ICAM-1 in Dementia, AD and VD [5], also, Hochstrasser did not reveal that VCAM- affected in monocytes of AD and MCI patients [17,18]. ICAM-1 can be induced by different cytokines, such as, e.g. interleukin-1. It has been shown that ICAM-1's plasma levels were increased in AD but another study shown that ICAM-1/CD14 has not changed in MCI and AD [5]. Our study find that the IL-6 level as a proinflammatory cytokine has no significant correlation with PDD, other factors may be involve to induce adhesion molecules in this regard. Kravitz et al. proposed inflammation is typically thought as a risk factor for AD, so, CRP as a systemic inflammatory biomarker was in association with the plaque and tangle pathology in AD brain [19]. Doung et al. showed that the concentrations of CRP similar to those detected in the serum of AD patients are neurotoxic in vitro [20]. CRP may also contribute to atherosclerotic processes used by increase the expression of adhesion molecules such as vascular cell adhesion molecule (VCAM-1) and intercellular adhesion molecule (ICAM-1) in vascular endothelial cells [21-23], the relationship between CRP and atherosclerotic processes which can underlie VD may account for the relationship between this inflammatory marker and VD [24,25].
Conclusion
Previous studies have further suggested that the association between CRP and dementia in VD is stronger than AD [20,26]. While cerebrovascular dysfunction and neurodegeneration exist independently, both conditions can impact cognition, cerebrovascular dysfunction directly impacts dementia, a broad clinical determination for specific impairments. Importantly, global evaluation of vascular pathologies allows for greater attention to prevention of dementia and AD.
Limitations
Our study has several limitations: (1) the present study is a cross-sectional study; therefore, we cannot draw any conclusions how the markers will change when disease progresses, or in the case of MCI remains stable or reverses. (2) The number of patients in this study is small and the differences are very small. (3) We extracted proteins by sonication of cells, thus we analyzed soluble cytoplasmic proteins. We do not know how membrane-bound proteins are changed. (4) The stability of the protein extracts was not tested. (5) Clinical diagnosis of dementia is made through specific impairments, meaning cognitive testing does not always have the sensitivity to discriminate specific underlying pathological conditions. It seems the serum level of VCAM1 may be a useful marker for diagnosis of PDD, However, it is unclear whether VCAM-1 can predict the risk of progression from mild cognitive impairment to dementia in Parkinson’s disease. The role of blood brain barrier dysregulation mediated by VCAM-1 in progression of Parkinson’s disease with dementia should be investigated in the future.
Acknowledgements
This research was supported by (Islamic Azad University, Najaf Abad branch, school of medicine, Isfahan, Iran). We thank our colleagues from Esfahan University of medical science and Dr. Rejaly as the head of Shariaty Hospital who provided insight and expertise that greatly assisted the research, although they may not agree with all of the interpretations of this paper. We thank from Professor Roy Alcalay and his research assistant Chris Liong from Columbia University, Neurology Department, and School of medicine for assistance for comments that greatly improved the manuscript. We would also like to show our gratitude to the 3 “anonymous” reviewers for their so-called insights.
Conflict of Interest
We have no conflicts of interest in this study.
Author Contributions
Homa Ebrahimi, Mohammad Saadatnia and Ahmad Sobhani conceived and designed the experiments.
Homa Ebrahimi, Ali Ebrahimi and Heshamtallah Orooji performed the experiments.
Homa Ebrahimi and Ali Ebrahimi analyzed the data.
Ahmad Chitsaz contributed the reagents/ materials/analysis tools.
Homa Ebrahimi and Mohammad Saadatnia wrote the paper.
21764
References
- Smith JA, Das A, Ray SK, Banik NL (2012) Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 87: 10-20.
- Zuliani G, Cavalieri M, Galvani M, Passaro A, Munari MR, et al. (2008) Markers of endothelial dysfunction in older subjects with late onset Alzheimer's disease or vascular dementia. J Neurol Sci 272: 164-170.
- Ewers M, Mielke MM, Hampel H (2010) Blood-based biomarkers of microvascular pathology in Alzheimer’s disease. Exp gerontol 45: 75-79.
- Rentzos, M, Michalopoulou M, Nikolaou C, Cambouri C, Rombos A, et al. (2004) Serum levels of soluble intercellular adhesion molecule-1 and soluble endothelial leukocyte adhesion molecule-1 in Alzheimer’s disease. J Geriatr Psychiatry Neurol 17: 225-231.
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, et al. (2004) Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol 61: 668-672.
- Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, et al. (2007) Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging 28: 1810-1820.
- Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, et al. (2012) Non-motor symptoms in patients with Parkinson’s disease–correlations with inflammatory cytokines in serum. PloS one 7: e47387.
- Song IU, Chung SW, Kim YD, Maeng LS (2015) Relationship between the hs-CRP as non-specific biomarker and Alzheimer's disease according to aging process. Int J Med Sci 12: 613.
- Eriksson UK, Pedersen NL, Reynolds CA, Hong MG, Prince JA, et al. (2011) Associations of gene sequence variation and serum levels of C-reactive protein and interleukin-6 with Alzheimer's disease and dementia. J Alzheimer's Dis 23: 361-369.
- Song IU, Chung SW, Kim JS, Lee KS (2011) Association between high-sensitivity C-reactive protein and risk of early idiopathic Parkinson’s disease. Neurol Sci 32: 31-34.
- Luca M, Luca A, Calandra C (2015) The role of oxidative damage in the pathogenesis and progression of alzheimer’s disease and vascular dementia. Oxid Med Cell Longev.
- Schapira AH, Jenner P (2011) Etiology and pathogenesis of Parkinson's disease. Mov Disord 26: 1049-1055.
- Beal MF (2005) Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58: 495-505.
- Cockrell JR, Folstein MF (2002) Mini-mental state examination. Principles and practice of geriatric psychiatry. p: 140-141.
- Prince MJ, Llibre de Rodriguez J, Noriega L, Lopez A, Acosta D, et al. (2008) The 10/66 Dementia Research Group's fully operationalised DSM-IV dementia computerized diagnostic algorithm, compared with the 10/66 dementia algorithm and a clinician diagnosis: A population validation study. BMC public health 8: 1.
- Dik, MG, Jonker C, Hack CE, Smit JH, Comijs HC, et al. (2005) Serum inflammatory proteins and cognitive decline in older persons. Neurology 64: 1371-1377.
- Hochstrasser, T, Weiss E, Marksteiner J, Humpel C (2010) Soluble cell adhesion molecules in monocytes of Alzheimer’s disease and mild cognitive impairment. Exp Gerontol 45: 70-74.
- Mayo L, Quintana FJ, Weiner HL (2012) The innate immune system in demyelinating disease. Immunol reviews 248: 170-187.
- Kravitz BA, Corrada MM, Kawas CH (2009) Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old. Alzheimer's & Dementia 5: 318-323.
- Duong T, Acton PJ, Johnson RA (1998) The in vitro neuronal toxicity of pentraxins associated with Alzheimer's disease brain lesions. Brain research 813: 303-312.
- Ferri C, Croce G, Cofini V, De Berardinis G, Grassi D, et al. (2007) C-reactive protein: interaction with the vascular endothelium and possible role in human atherosclerosis. Current pharmaceutical design 13: 1631-1645.
- Jialal I, Devaraj S, Singh U (2006) C-reactive protein and the vascular endothelium: implications for plaque instability. J Am College Cardiol 47: 1379-1381.
- Uchikado, H, Akiyama H, Kondo H, Ikeda K, Tsuchiya K, et al. (2004) Activation of vascular endothelial cells and perivascular cells by systemic inflammation-an immuno-histochemical study of postmortem human brain tissues. Acta neuropathologica 107: 341-351.
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, et al. (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21: 383-421.
- Blauw, G, Bollen EL, Van Buchem MA, Westendorp RG (2001) Dementia at old age: A clinical end-point of atherosclerotic disease. Eur Heart J 3: N16-19.
- Schmidt, R, Schmidt H, Curb JD, Masaki K, White LR, et al. (2002) Early inflammation and dementia: A 25 up of the Honolulu-Asia aging study. Annals of neurology 52: 168-174.






