Shraddha Goswami1 and Mukesh Dube2*
1Department of Nephrology, Sri Aurobindo Medical College and P.G. Institute, Indore, Madhya Pradesh, India
2Department of Neurology, Clara Swain Mission Hospital, Bareilly, Uttar Pradesh, India
Corresponding Author:
Dr. Mukesh Dube
Department of Neurology, Clara Swain Mission Hospital, Civil Lines, Bareilly, Uttar Pradesh, India
Tel: +91-581-2500000
E-mail: dube_mukesh@yahoo.com
Rec Date: November 05, 2018; Acc Date: December 18, 2018; Pub Date: December 22, 2018
Citation: Goswami S, Dube M (2018) Vitamin D Deficiency: A Cause of Secondary Lumbar Canal Stenosis - A Case Report. J Neurol Neurosci Vol. 9 No.6:280. doi: 10.21767/2171-6625.1000280.
Keywords
Degenerative lumbar; Vitamin deficiency; Lumbosacral scan; Hypertrophy
Abbreviations
LCS: Lumbar Canal Stenosis; BMI: Body Mass Index; ESR: Erythrocyte Sedimentation Rate; PTH: Parathormone; MMPs: Matrix Metalloproteinases; TIMPs: Tissue Inhibitors of Matrix Metalloproteinase; TGF: Transforming Growth Factor
Introduction
Chronic low backache is a feature of lumbar canal stenosis (LCS). Degenerative LCS can lead to central canal, lateral recess and foraminal stenosis. Ligamentum flavum hypertrophy is an important cause of central canal stenosis. Vitamin D deficiency can lead to ligamentum flavum hypertrophy and manifest as LCS. Obese females residing in north India are predisposed to vitamin D deficiency and have symptomatic or asymptomatic vitamin D deficient state.
Case Presentation
32-years-old rich married right handed obese female resident of north India came with history of chronic low mild backache in lumbosacral region, in paravertebral location and occasionally in her legs since last 6 months. The backache was pronounced on extending backwards and turning sideways. No history of neurological claudication, trauma, aggravation of pain at night while sleeping or with bending forwards, no history of tuberculosis or chronic illness, muscle pains or muscle weakness, connective tissue disorder, coagulation disorders, diabetes mellitus, no use of regular drug intake, no history of malabsorption, no intestinal surgery, no inadequate exposure to sun.
There was no family history suggestive of similar complaints in any first and second-degree relatives.
Detailed general examination revealed obesity with BMI and neurological examination was normal. Lower back examination reduced mobility, with extension more limited than flexion.
In the given clinical context of chronic low backache, the possibilities considered were:
1) Secondary lumbar canal stenosis.
2) Paraspinal muscle spasm.
Haematological profile was normal, with normal ESR and normal thyroid functions. Normal serum calcium (9.1 mg/dl) and phosphorus (3.6 mg/dl) with raised serum parathormone (PTH) (143 pg/ml, normal range 15-68 pg/ml). Deficient serum 25-OH Vitamin D total levels (8.33 ng/ml, deficiency levels <20 ng/ml) were found. (No report of Vitamin D levels prior to the onset of symptoms), HLA B27 and Rheumatoid factor negative. Her MRI lumbosacral scan revealed grade I spondylolisthesis with spondylolysis seen at L4 over L5. Ligamentum flavum and facetal hypertrophy was seen at multiple levels causing bilateral lateral recess stenosis. Diffuse disc bulge causing thecal sac compression and bilateral foraminal nerve root compression seen at L4-L5 level (Figure 1).
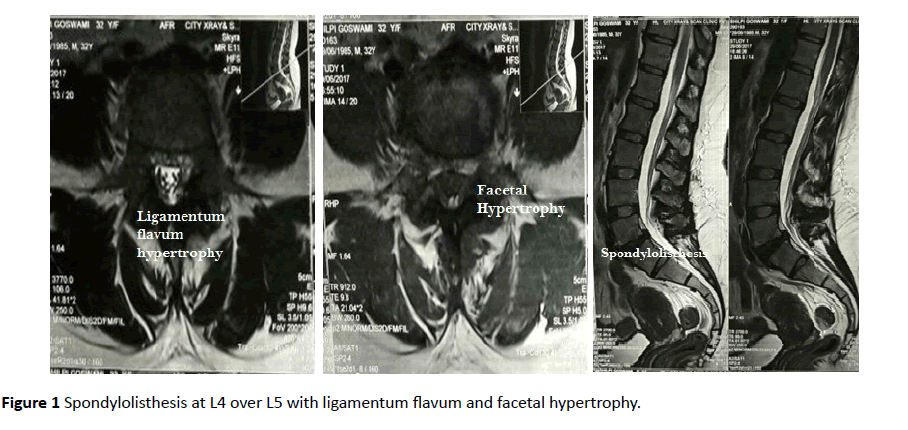
Figure 1 Spondylolisthesis at L4 over L5 with ligamentum flavum and facetal hypertrophy.
Hence a diagnosis chronic low backache secondary to LCS due to secondary hyperparathyroidism due to vitamin D deficiency was made.
She was treated with Vitamin D3 50,000 IU orally weekly for 8 weeks, followed by 800 IU daily, along with oral calcium supplementation 1000 mg daily. The patient had minimal improvement in pain in the initial first month of treatment, no further Vitamin D levels were done.
Discussion and Conclusion
Degenerative LSS anatomically involves the central canal, lateral recess, foramina or any combination of these locations. A decrease in the anteroposterior, transversal or combined diameter secondary to loss of disc height with or without bulging of the intervertebral disc, hypertrophy of the facet joints and the ligamentum flavum lead to central canal stenosis. Mechanical stress is the contributing factor for the fibrosis of ligamentum flavum hypertrophy. The lateral recess stenosis occurs due to, decreased disc height, facet joint hypertrophy (with or without spondylolisthesis) and/or vertebral endplate osteophytosis [1]. Foraminal stenosis can be either anteroposterior resulting from a combination of herniated disc that compresses the nerve root against the superior pedicle and/or vertical resulting from posterolateral osteophytes from the vertebral endplates protruding into the foramen along with a laterally bulging annulus fibrosis disc space narrowing and overgrowth of structures anterior to the facet joint capsule [1]. Lumbar spine stenosis has an important dynamic component. The central canal space decreases in loading and extension and increases in axial distraction and flexion. The same dynamics also affect the foramen with flexion causing a 12% increase, and extension a 15% decrease, in surface area [1].
Matrix metalloproteinases (MMPs) are a family of more than twenty enzymes that digest proteins in the extracellular matrix. The inhibitors of MMPs, are called tissue inhibitors of matrix metalloproteinase (TIMPs), and it is believed that, together, MMPs and TIMPs regulate the integrity and homeostasis of the extracellular matrix. There are four known TIMPs (TIMP-1 through 4) that suppress extracellular matrix degradation by forming an inhibitory 1:1 complex with the MMPs [2]. Elevated expressions of TIMP-1 and TIMP-2 have been implicated in the increased fibrosis found in a wide variety of human organs, including the liver, kidney, lung, and heart. TIMPs are also known to increase cellular proliferation and inhibit programmed cell death (apoptosis) in a wide range of cell types [2]. TIMP-1 and TIMP-2 were detected in the cytoplasm of ligamentum flavum fibroblasts. TIMP-1 and TIMP-2 concentrations were associated with hypertrophy of the ligamentum flavum [2]. In an in vitro study, Nakatani et al. found that mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum tissues through increased Transforming growth factor (TGF)-β 1 production [3]. (TGF)-β released by the endothelial cells may stimulate fibrosis, especially during the early phase of hypertrophy [1]. Many of the enzymes are produced as zymogens, and 1α,25(OH)2D3 increases zymogen activation [4]. 1α,25(OH)2D3 has been shown to regulate the levels of mineral ions that are required for metalloproteinase activity [4]. In addition, 1α,25(OH)2D3 modulates transcription of their mRNA [4]. Because regulation of matrix metalloproteinase (MMP) activity is critical, chondrocytes produce inhibitors like TIMP-1 and TIMP-2) and 1α,25(OH)2D3 modulates levels of these inhibitors as well [4]. 1α,25(OH)2D3 increased MMP activity suggested that 1α,25(OH)2D3 might also regulate availability of TGF-β1 by controlling activation of the latent growth factor. Hence Vitamin D deficiency leads to altered expression of MMPs and TIMPs, leading to fibrosis and hypertrophy of the ligamentum flavum.
Several studies have demonstrated low serum 25- hydroxyvitamin D [25(OH) D] levels in people living in various regions of across India. In North India, 94.3% of adults were found to have low vitamin D levels [5]. Obesity is associated with low levels of serum 25(OH) D. Association of obesity with low levels of serum 25(OH) D7 may be attributed to decreased exposure to sunlight because of limited mobility of obese people and negative feedback from elevated 1,25- dihydroxyvitamin D and parathyroid hormone levels on hepatic synthesis of 25(OH) D [5]. In obese urban Asian Indians without diabetes, higher values of total abdominal fat at the L2-L3 intervertebral level were associated with low 25(OH) D levels [5].
Our patient had a history of chronic low backache with obesity, belonging to north India. She fulfilled the criteria of secondary LCS due to secondary hyperparathyroidism due to vitamin D deficiency.
For the management of chronic low backache NSAIDs are the initial treatment for pain as they provide analgesic as well as anti-inflammatory effect. There is no consensus gold standard treatment yet [6]. Indians as have brown skin have more melatonin which acts as a natural sunscreen thereby decreased production of vitamin D. Thus, for Indian skin tone, minimum “direct sun exposure” required daily is more than 45 min to bare face, arms and legs to sun’s UV rays (wavelength 290-310 nm) [7]. For correcting vitamin D deficiency, cholecalciferol 50000 IU orally once a week for 6-8 weeks, followed by 800 IU daily is the regimen commonly practiced [8].
A comprehensive rehabilitation program for lumbar canal stenosis comprises of manual therapy, stretching, and strengthening exercises for the lumbar spine and hip region have been advocated for those with LSS [6]. The importance of endurance exercises to retard the deleterious consequences of inactivity and deconditioning is also emphasized.
23864
References
- Genevay S, Atlas SJ (2010) Lumbar spinal stenosis. Best practice & research Clinical rheumatology 24: 253-265.
- Park JB, Lee JK, Park SJ, Riew KD (2005) Hypertrophy of ligamentum flavum in lumbar spinal stenosis associated with increased proteinase inhibitor concentration. JBJS 87: 2750-2757.
- Nakatani T, Marui T, Hitora T, Doita M, Nishida K, et al. (2002) Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta 1. J Orthop Res 20: 1380-1386.
- Boyan BD, Schwartz Z (2009) 1, 25-Dihydroxy vitamin D3 is an autocrine regulator of extracellular matrix turnover and growth factor release via ERp60-activated matrix vesicle matrix metalloproteinases. Cells Tissues Organs 189: 70-74.
- Bhatt SP, Misra A, Sharma M, Guleria R, Pandey RM, et al. (2014) Vitamin D insufficiency is associated with abdominal obesity in urban Asian Indians without diabetes in North India. Diabetes Technology & Therapeutics 16: 392-397.
- Middleton K, Fish DE (2009) Lumbar spondylosis: Clinical presentation and treatment approaches. Current Reviews in Musculoskeletal Medicine 2: 94-104.
- Gupta A (2014) Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients 6: 729-775.
- Lhamo Y, Chugh PK, Gautam SR, Tripathi CD (2017) Epidemic of Vitamin D deficiency and its management: Awareness among Indian medical undergraduates. Journal of Environmental and Public Health.






