Keywords
Diplococcic Streptococcus, Gram-positive bacteria, penicillin resistance, penicillin binding proteins, Xylitol
Introduction
Preventive effect of xylitol in reducing dental caries of children has helped us think a similar application of xylitol in respiratory diseases caused by the diplococcic mitis group Streptococcus. The 5-carbon sugar alcohol xylitol in combination with fluoride has also been applied but without the required knowledge of diplococcic bacterial growth curve [1]. However, use of xylitol alone is still acceptable but the use of fluoride without xylitol has a risk. How does the fluoride work? At the non-bactericidal concentration of fluoride, the diplococcic Streptococcci prevailing in chains fall apart resulting in the release of their individual units to initiate new multiplication. The xylitol is also not very effective in the presence of fructose in the growth environment and therefore it seems important to purify the xylitol phosphate or derivative(s) formed by the growth of diplococcic Gram- positive streptococci in the presence of xylitol (2% or more) alone or in the presence of both fluoride and xylitol prevent their reproduction (bacterial birth control) [2]. Because the fructose phosphotransferase system transporter is located in the membrane, the bacteria even in pre-competent phase are capable of taking in xylitol and forming xylitol phosphate to kill them even in the pre-competent phase [3]. In E.coli K-12 genetic linkage map, the fructose promoter dominates the expression of xylitol loci being negatively controlled by the fructose repressor [4,5]. If fructose promoter is necessary to express xylitol loci, then we have to determine the minimal concentration or conditions required to activate fructose promoter to read through the xylitol operon essential to form the ultimate product (xylitol phosphate?). This product has a serious effect on bacterial reproduction ability and even the diplococcic population in pre-competent phase can’t run away [5,6]. The mitis group bacteria silently prevailing in chains, if ruptured are immediately dissociated into their individuals of different sizes and shapes. Figure 1 shows a comparison between xylitol and fructose structures [6].
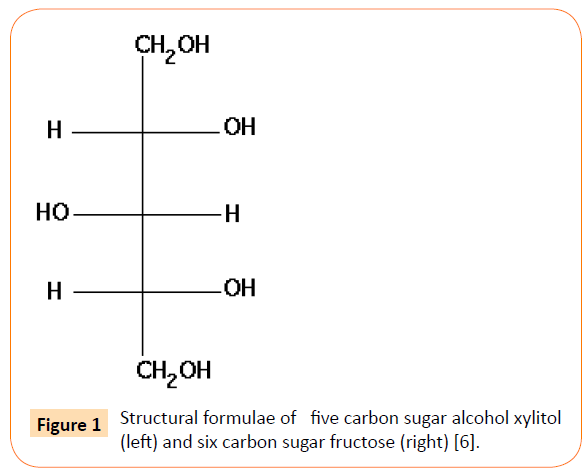
Figure 1: Structural formulae of five carbon sugar alcohol xylitol (left) and six carbon sugar fructose (right) [6].
Diplococcic streptococcus in pre-competent, competent and stationary phases (growth curve)
Growth curve of diplococcic streptococci remains to be explored. One reason for such delay is our bias towards the double stranded DNA transfer (segments) in the artificial transformation of Gram –negative E.coli K-12 which is far apart from the natural transformation of Gram positive S. pneumoniae. Based on our most recent data we claim that the life cycle of diplococcic Streptococcus is their gradual physiological changes: baby (small, round) grows to the young (oval, pre-competent), then the young becomes adult (competent and diplococcic) with an ability to produce pheromone and finally reach their latent or stationary phase (Figure 2). However, donor DNA in an eclipse phase has not been isolated and therefore we think there is no direct evidence of homologous recombination between the donor DNA of any length and the recipient chromosome [7,8].
Significantly, overnight culture, diluted 10,000-fold by the standard laboratory protocol shows the purple colored diplococcic population as a minority and the remaining population with their heterogeneity of shapes and sizes (Figure 2). The small, round shaped bacterial baby (0.2um) grows gradually to oval shape and then the size of the oval increases from 0.2um to its full size about 1.7um. Cleavage appears at the mid-cell position of the oval shaped bacterium as an index of their competence for reproduction. The diplococcic bacterium is just the shape during reproduction but never means two bacterial cells. Pre-competent (spherical to oval), competent (oval to diplococcic) and the incompetent (spheroplasts with thin cell wall or protoplasts without cell wall) representing their different phases of growth are clearly labelled in the micrograph (Figure 2). Some of them are stained partially many of them may even remain invisible because of their inability to adsorb crystal violet, an essential dye used in Gram staining technique. If we stabilize them by their growth in the presence of xylitol an unusual pattern of their heterogeneity of sizes and shapes can be visualized. Gram staining technique identifies these bacteria as Gram-positive purple with heterogeneity of sizes: baby (small, round) grows to oval (precompetent), pre-competent becomes competent (diplococcic) and finally reaches saturation (stationary phase). All prevail in a single chain but is stabilized by their growth to saturation in Xylitol (2% or higher) [2]. In the absence of such knowledge, the population prevailing in- chains are ruptured while growing in our microbiological rich nutrient broth, BHI or TSB, by shaking or by the shearing force induced by our laboratory dilution techniques. As an outcome, visualization by the standard Gram-staining technique has deprived many of our investigators from seeing the bacterial in vivo existence in heterogeneity (Figure 2).
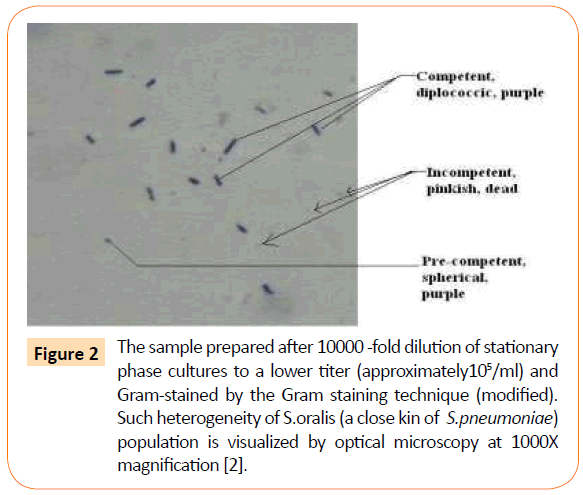
Figure 2: The sample prepared after 10000 -fold dilution of stationary phase cultures to a lower titer (approximately105/ml) and Gram-stained by the Gram staining technique (modified). Such heterogeneity of S.oralis (a close kin of S.pneumoniae) population is visualized by optical microscopy at 1000X magnification [2].
This explains why in our Gram stain preparation of the overnight culture diluted to 10,000-fold in fresh broth, diplococcic competent bacteria (purple) are not crowded but there are many others who are partially stained and also not stained at all. Even in stationary phase some pink colored bacterial clusters are seen, in addition to purple ones. In the absence of this knowledge we may wonder as if this Streptococcus culture or population is contaminated! Truth is that the old population prevailing in- chain but with diminishing cell wall thickness is either poorly stained or stained like Gram negative not stained at all. They appear pinkish compared with Gram negative pink E.coli K-12 (control) but their sizes are significantly diminished to spheroplasts, protoplasts and beyond.
Competent phase or reproduction
Competence means that the diplococcic Gram-positive bacteria reach their growth phase to re-produce their own kind. At this competent phase, a cleavage is apparently formed by the fusion of thick peptidoglycan cell wall with its membrane (?) and the oval shape changes to diplococcic shape (Figure 3).
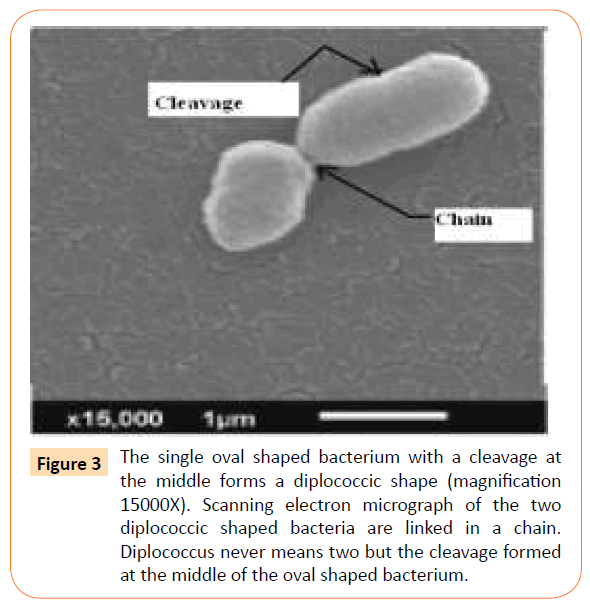
Figure 3: The single oval shaped bacterium with a cleavage at the middle forms a diplococcic shape (magnification 15000X). Scanning electron micrograph of the two diplococcic shaped bacteria are linked in a chain. Diplococcus never means two but the cleavage formed at the middle of the oval shaped bacterium.
Recently, the regulatory role of two –component signaling system is explored but our bias towards E.coli K-12 gene cloning by double stranded DNA transfer is still interfering in our accepting the difference between artificial transformation (in vitro double stranded DNA transfer) in Gram negative E.coli K-12 and the natural transformation of Gram-positive diplococcic streptococci. In the natural phase of competence the pneumococcus involves too many loci starting with com A, com B, com C, com D , com E , com X and late com genes [9,10]. Briefly, com A and com B transporters help excrete com C product CSP (17 amino acids peptide) to sense the external environment including the presence of our modern medicines. Recently, reviews have been published on these com loci, and therefore we are not expanding. What is the role of exogenous DNA segment even in a single stranded form with 3-prime OH end in the mutation of PBP genes by point mutations? We think that the progeny with point mutations arise by the lack of fidelity in bacterial chromosome or DNA duplication. These PBP mutants are selected mostly by the human medicines (antibiotics), being in their growth environment. Antigenic variations may similarly arise by the presence of vaccines, mono-valent or polyvalent and therefore the currently available vaccines are not producing always very positive effect. We conclude that the natural transformation means pneumococcal physiological changes (phenotypes) from birth to death. However bacterial genotypes responsible for the continuity of their kind do not change. Best example is that the S.pneumoniae has been continuing as S.pneumoniae with their ability to cause different diseases like pneumonia, otitis media, meningitis, bacteremia (differentiation of diseases) [11,12]. Its chromosome duplication and equi-distribution of newly synthesized chromosome into the progeny is also necessary but this knowledge is still in infancy in the published articles or text books.
Bio-signaling and penicillin resistance crisis
The cleavage is formed at the middle of the oval shaped bacterium but in the membrane. The PBP2X is the most important of the five PBPs of high molecular weight, because of its location in the cleavage site plays an indispensable role in bacterial cell division or cell multiplication. Disappearance of such cleavage by the thinning or unfolding of peptidoglycan layers initiated at the membrane-cell wall junction by the xylitol phosphate (xylitol intermediate product), the protoplasts are thus formed with the dislodgement of Ser/Thr protein kinase StkP and PBP2X [13,14]. Even these protoplasts form xylitol intermediate product (Palchaudhuri, S .unpublished data).
It has already been demonstrated that xylitol (5-carbon sugar alcohol) in combination with fluoride inhibits the reproduction of these diplococcic Streptococcal population even in the presence of dextrose (6-carbon sugar, a preferred carbon source for several bacteria) [2]. Fortunately, the Pneumococci are genetically capable of metabolizing xylitol and forming an insoluble xylitol 5-phosphate during their transport via fructose phosphotransferase system. This Xylitol 5 phosphate produces toxic effect on their biological continuity because signalling is affected.
These diplococcic bacteria live silently in a stationary phase or latent phase in the nasopharynx of children. Silent phase means bacterial metabolism is completely turned off by the bacteria. Usually these conditional non-growing bacteria in their latent phase are usually resistant to all beta- lactam drug like penicillin, but question remains how the growing bacteria survive in our beta lactam drugs. Penicilin resistant viridans group streptococci including pneumococci and the dental pathogen S.mutans, have altered PBPs by the selection of the mutants pre-existing in streptococcal population. The beta- lactamases are not known to occur in Streptococcal species, but they become resistant to penicillin by point mutations in their genes of penicillin binding proteins (PBPs) [14]. Question remains how these PBPs are mutated? Unlike Gram-negative bacteria, an involvement of any extra-chromosomal DNA elements is a remote possibility but direct alterations of the progeny chromosome, is a possibility. Of course, abuse of penicillin has a role not in the alterations of PBPs but probably in the selection of rare mutants which usually arise during error prone DNA replication. These rare mutants are apparently cross –fed by the nutrients released by the bactericidal effect of penicillin. We are using penicillin to kill them but it is helping the resistant mutants to flourish! Sensing of penicillin in our immediate environment by their one or two-component communication system, the fidelity of DNA duplication is compromised but the few with the altered PBPs survive. The few with altered PBPs, mostly with high molecular weight (DNA which encodes these proteins are the genes, larger target for the alterations by point mutations). The high-molecular weight PBPs in Streptococcus pneumoniae are PBP1 (resolved into PBPs 1a and 1b, which appear functionally interchangeable and PBP2 (actually three proteins 2a, 2b and 2x) which are essential with high penicillin-binding affinity [15]. We must recognize that these proteins play important role in bacterial cell division as well as in the maintenance of cell wall ultra-structure [12]. The Pbp2x mutants cannot be isolated, because it initiates reproduction cycle. Even in S.pneumoniae, location of the replicating chromosome (~length 2200Kb) has not yet been confirmed. If bacterial cell division begins at the cleavage site, then it seems logical that the chromosome remains attached to the bacterial cell membrane but proximal to their cleavage and chromosome replication and segregation of the duplicated chromosomes into their daughters are the simultaneous events. One or two-component system (bio-communication) appears to be regulating bacterial growth cycle even in adversity (e.g. presence of bactericidal penicillin) but the details may differ.
If the bio-communication via signaling is affected then growth cycle should be affected. In consequence they should be irreversibly stabilized in-chains or even in protoplasts by growth in the presence of xylitol at an optimal concentration. The thinning of cell wall thickness of the viridans group streptococci supports an elongation of the bacteria, oval to rod, when they are grown in the TSA solid agar medium with or without sheep blood for 24 hrs or longer at 37°C [16]. Similarly, thinning occurs even the experiment is repeated in nutrient broth (TSB or BHI) with xylitol. It remains to confirm if it is due to unfolding of peptidoglycan layers or the destruction of cross-peptides. Significantly, bacterial colony sizes differ: they grow much slower with xylitol than their growth without xylitol (colony sizes considerably differ). Initially bacterial colony sizes are reduced and if these colonies are transferred to the fresh medium with xylitol they don’t grow to visible colonies even after few days of incubation [16]. We have demonstrated in our most recent publications that the gradual thinning of cell wall thickness or forming protoplasts by growth in xylitol renders the recipient bacteria inactive in their formation of cleavage (shape). We think loss of cleavage displaces these pbp2x and StkP(Serine /threonine kinaseP) and therefore streptococcal growth and multiplication are inhibited. Displacement of these proteins should also block their bio-signaling pathway [17].
This should support how their genetic ability to select point mutants in PBP not PBP 2X arising during their DNA replication error (change of penicillin sensitivity patterns and antigenic variations). Signal based bio-communication helps us to think of a regulation to co-ordinate biological growth phases (precompetent phase to competent phase) (bacterial puberty). Then differential expression of genetic characters including differentiation of diseases via si RNA is known but our knowledge about regulatory role of these siRNAs appears to be growing [10,16]. In order to prevent breakage of these chains during laboratory manipulations we have added sucrose (6.5%) and then visualized by scanning electron microscopy. It shows an unusual distribution of the population. They appear clustered around old parents but still in-chains. This is an ideal orientation of families, bacteria or humans, for signal- based communication! The mitis group bacteria including S.pneumoniae, are all serious human pathogens and the natural transformation is really their growth curve. Without the knowledge of this growth curve the inhibitory effect of xylitol on bacterial reproduction can’t be appreciated [3,17]. Metabolism of xylitol by the diplococcic Streptococcus causes thinning of cell wall which in turn eliminates cleavage (septal growth) and results in the formation of spheroplasts or protoplasts, but still all are contained in the same chain. Biochemically these protoplasts are capable of taking in xylitol by their PEP-dependent phosphotransferase system (PEP-PTS) [18,19]. We are in full agreement with Beliharz et al. that the control of cell division in S.pneumoniae cells mutated for Ser/Thr protein kinase StkP plays an important role in regulating cell wall biosynthesis and controls septum formation [12]. PASTA domains of StkP appear to bind PG subunits via StkP and PBP2X and their bio-communication center all are located in the cleavage formed at the mid cell position [20].
Concomitant use of Xylitol and Fluoride in Dentistry
Maehara et al. have shown a synergism between fluoride and xylitol on glycolysis without the knowledge of diplococcic S.mutans growth curve [1]. In this article we have presented their growth curve and therefore at any particular time all the members in the chain are not competent to uptake xylitol. These bacteria usually grow in- chains (asynchronous) and therefore 100% bacteria will not be in growth phase and the presence of fluoride together with xylitol (nutrients?) may dissociate them into individuals to initiate growth and the uptake of xylitol.
Xylitol inhibits phosphoglucose isomerase and phosphofructokinase in the form of Xylitol -5- phosphate and inhibits bacterial growth. The effects of fluoride on streptococcal cells are partly described to the inhibition of enolase, one of the series of glycolytic enzymes (Figure 4). This inhibition decreases the intracellular level of Phosphoenol pyruvate (PEP), and thus decreases the bacterial sugar uptake via PEP-dependent phosphotransferase system (PEP-PTS). Such starvation may break the bacteria in -chains and release the young but the old with thinning of the cell wall are ruptured. The nutrients thus released may feed the young and help them to regrow. In addition, fluoride can directly inhibit bacterial proton translocating ATPase (H+- ATPase) that is considered to partly contribute to the excretion of proton out of the cells, leading to increase of extracellular pH. In turn, bacterial chain is ruptured initiating the growth phase and xylitol uptake. These bacteria grow in chain and the chain is stabilized by growth in the presence of xylitol. The combination of fluoride and xylitol inhibit the acid production (as a result of glycolytic pathway inhibition) more efficiently than fluoride or xylitol alone. Xylitol inhibits the upper portion of glycolytic pathway and fluoride inhibits the lower portion of the glycolytic pathway (Figure 4). However, fluoride without xylitol breaks S.mutans population growing in-chain, helps them to dissociate into individual members and initiates new growth necessary for the expression of pathogenesis. It simply means dental problem but such pathogenesis can be contained in their chains of inheritance if xylitol is simultaneously present with fluoride in the bacterial growth environment. Our recommendation is that fluoride should not be applied without xylitol.
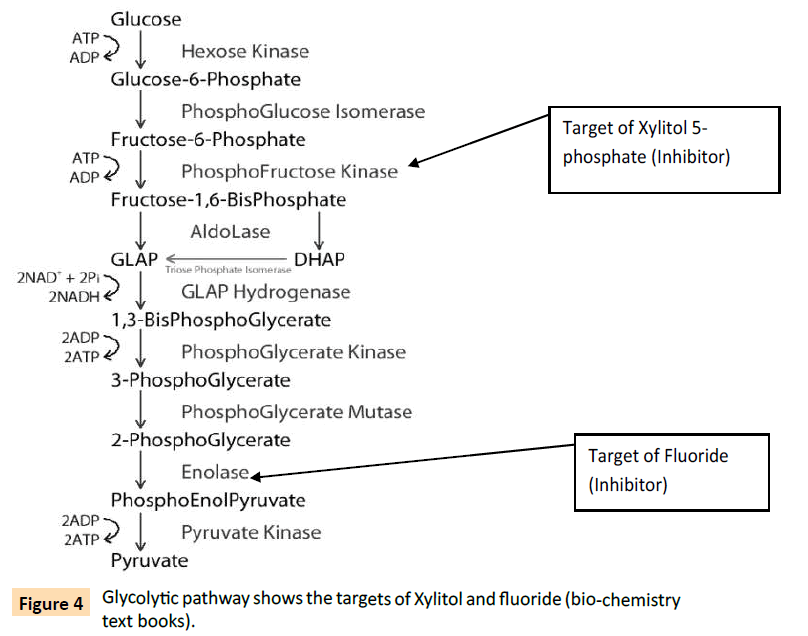
Figure 4: Glycolytic pathway shows the targets of Xylitol and fluoride (bio-chemistry text books).
The above glycolysis pathway, leads to a conclusion that fructose may compete with xylitol and therefore an optimal concentration of xylitol is necessary to overcome such competition [5]. We think that such competition between xylitol and fructose is a good possibility but appropriate concentration of xylitol in combination with fluoride should solve this problem.
Revisiting Dr Griffith’s Rough colony (stationary phase /opaque) and smooth colony (growth phase /transparent)
In 1928 Dr Fred Griffith has recorded in his pathology report that the two kinds of colonies are seen in microbiological solid blood agar media, the contour of smooth colony becomes uneven, termed rough colony after 24 hours or longer incubation of growth [21]. He has previously shown that severity of disease varies with different colony morphology, smooth (growth phase) and rough (stationary phase) on blood agar medium. He has streaked his clinical samples after collecting from patients with bacterial lobar pneumonia. We have repeated this observation not only with S.pneumoniae but also with a pure culture of S.orlis belonging to the mitis group and dental pathogen S.mutans [2]. Our data now establishes that these smooth and rough colonies are representatives of the bacterial physiological states of growth (phenotypes) but there is no good reason to think of any genotype difference between these colonies [22]. In stationary phase, the heterogeneity of the diplococcic streptococcus population prevails in-a chain as evidenced by scanning electron microscopy (Figure 5a and 5b with expanded legends).
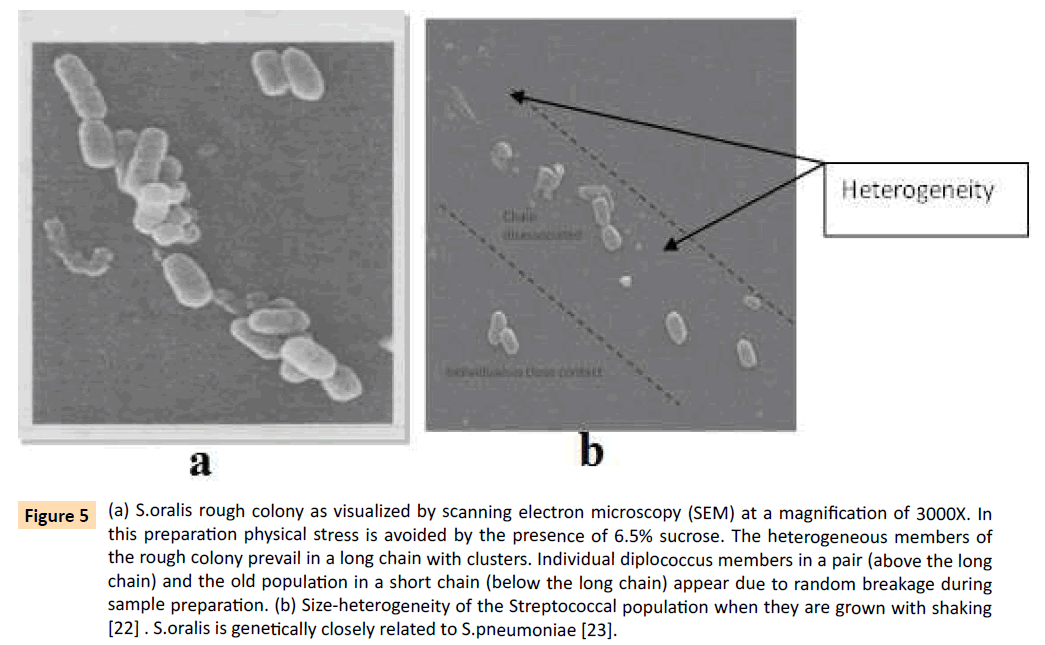
Figure 5: (a) S.oralis rough colony as visualized by scanning electron microscopy (SEM) at a magnification of 3000X. In this preparation physical stress is avoided by the presence of 6.5% sucrose. The heterogeneous members of the rough colony prevail in a long chain with clusters. Individual diplococcus members in a pair (above the long chain) and the old population in a short chain (below the long chain) appear due to random breakage during sample preparation. (b) Size-heterogeneity of the Streptococcal population when they are grown with shaking [22] . S.oralis is genetically closely related to S.pneumoniae [23].
The stationary phase in liquid broth is equivalent to rough colony on solid blood agar medium but these bacteria while in precompetent phase can’t multiply and therefore their OD and cfu may differ. For the same reason, classification by Gram staining technique may fail to identify the Gram positive pathogens even genetically pure because of their difference in cell wall thickness. Culturing in rich broth although it consumes time but there is no option.
Expression of the xylitol loci in the presence of fructose
We have already demonstrated that Xylitol metabolism by bacterial loci inhibits bacterial reproduction even in the presence of dextrose but not in the presence of fructose. The uptake of xylitol into S.mutans cells is known to be mediated by a specific fructose phosphotransferase system [5]. In the presence of fructose (how much), Xylitol may not show growth inhibition because the fructose phosphotransferase system is dependent on fructose for its affinity. In the low concentration of fructose, xylitol may not have such competition with other sugars. It remains to be studied how this Gram-positive species of bacteria has inherited their xylitol operon, partially or fully; but the Gram-negative E.coli C differs from E.coli K-12 by their xylitol loci or operon [2]. The E.coli C is sensitive to a single stranded DNA containing phage ΦX 174 but E.coli K-12 is resistant to the same phage. What is more, fructose operon becomes constitutively dominant when E.coli K12 and E.coli C forms a recombinant termed E.coli HF4714 which gradually becomes resistant to the phage because of constitutive expression of xylitol operon following the inactivation of fructose repressor and hence their shape (Palchaudhuri S, unpublished data).
Xylitol resistance should not arise
Like antibiotic resistance crisis xylitol resistance may also be a possibility. The bacterium S.pneumoniae responsible for the increasingly high mortality rate of children from the disease Pneumonia has become resistant to penicillin and their derivatives. In addition to antibiotic resistance crisis, the recently developed vaccines against the same pathogen and newer expensive antibiotics are no longer reducing the mortality rate of children [24]. In the existing literature it shows that long term use of xylitol produces xylitol resistant S.mutans [25,26]. S.mutans grows well at acidic pH but S.pneumoniae prefers neutral pH. The dental pathogens grow under anaerobic condition and acidic pH but S.mitis group bacteria belong to the facultative group and therefore they may grow equally well regardless of anerobic and aerobic conditions.
Glycolytic pathway produces NADH+ H+. To continue this pathway NADH+H+ should be reverted back to NAD+. This conversion occur under two different conditions. In presence of oxygen this reversion occurs via electron transport chain and oxidative phosphorylation but in absence of oxygen this reversion occurs via fermentation with production of lactic acid resulting in pH reduction.
As Xylitol blocks glycolytic pathway, so lactate production decreases. They have shown that in Xylitol- resistant ones lactate production from pyruvate by the enzyme lactate dehydrogenase increases. In Xylitol resistant strain Xylitol-5-phosthate is not produced. This is due to lack of constitutive fructose PTS activity, and without the Fructose-PTS Xylitol cannot be metabolized. Since various preliminary results suggest that Xylitol-resistant mutants may be less virulent than the sensitive ones. So one question remains that the Xylitol resistant ones have developed growth problem and in competition sensitive ones should outgrow, if the xylitol resistant derivatives overgrow the xylitol sensitive ones. Under our conditions, xylitol phosphate formed interferes in bacterial reproductive system and penicillin added together with xylitol to kill the population still growing.
Conclusions
1) Natural transformation in S. pneumoniae does neither involve any extra-chromosomal genetic elements nor Streptococcal donor DNA but defines their physiological states of growth.
2) After 16 years of Dr. Griffith’s great observation, we prematurely recognized his observation in 1944. Prior to 1971, we have never isolated a DNA fragment of the size required to contain genetic character and the exogenous double stranded DNA (small or large) uptake by the stationary –phase bacteria.
3) S.pneumoniae chromosome (double stranded DNA biomolecule) contains total genetic information necessary for biological continuity with the expression of its pathogenesis. The S.pneumoniae of 1928 is still maintaining its microbiological identity as pneumococcus.
4) On the basis of total information we have defined the pneumococcal cleavage as a physiological index for bacterial reproduction for sending and receiving bio-signals via StkP and PBP2x.
5) Bio-communication by the two component signal transduction has already been confirmed and blockage of this bio-communication by the elimination of cleavage via xylitol phosphate (or xylitol derivative) provides us an idea of an alternative therapy to combat antibiotics resistance crisis.
6) Significantly, the same S.pneumoniae causes few different diseases (small- RNA mediated differential expression of pathogenesis) and therefore the inhibition of biocommunication of this bacterium should stop the morbidity and mortality of children and the elderly from the diseases like Pneumonia, otitis media and meningitis.
7) We have been feeding our cattle and chicks the beta-lactum antibiotics to make them grow fast but not to cure their diseases ! Use of penicillin in animal farms can still be allowed if we recognize and develop this alternative preventive measure which is equally effective on superbugs.
8) Antibiotics crisis will bring the death to humanity soon, but our work on preventive use of Xylitol as a low cost home therapy has high potential to be equally effective on penicillin resistant superbugs and all their antigenic variants.
9) Morbidity and mortality from TB can also be defeated because xylitol seems to block a specific bio communication which appears to be common to both Pneumococcus and TB !!!
Acknowledgement
We would like to acknowledge Dr. Anubha Palchaudhuri, M.D., Ph.D for the financial support.
7471
References
- Maehara H, Iwami Y, Mayanagi H, Takahashi N (2005) Synergistic inhibition by combination of fluoride and xylitol on glycolysis by mutans streptococci and its biochemical mechanism. Caries Res 39: 521-528.
- Palchaudhuri S, Rehse SJ, Hamasha K, Syed T, Kurtovic E, et al. (2011) Raman spectroscopy of xylitol uptake and metabolism in Gram-positive and Gram-negative bacteria. Appl Environ Microbiol 77: 131-137.
- Palchaudhuri S, Palchaudhuri A, Chatterjee B (2015) Diplococcic Streptococcal growth in three phases :pre-competent, competent and latent J.Bacteriol (in revision)
- Berlyn MKB, Low BK, Rudd KE (1996) E.coli and Salmonella (ed FC Neidhardt), 1715-1902, ASM press Washington DC.
- Kornberg HL (2001) Routes for fructose utilization by Escherichia coli. J MolMicrobiolBiotechnol 3: 355-359.
- Uhari M, Tapiainen T, Kontiokari T (2000) Xylitol in preventing acute otitis media. Vaccine 19 Suppl 1: S144-147.
- Trahan L, Bareil M, Gauthier L, Vadeboncoeur C (1985) Transport and phosphorylation of xylitol by a fructose phosphotransferase system in Streptococcus mutans. Caries Res 19: 53-63.
- Bergé MJ, Kamgoué A, Martin B, Polard P, Campo N, et al. (2013) Midcell recruitment of the DNA uptake and virulence nuclease, EndA, for pneumococcal transformation. PLoSPathog 9: e1003596.
- Morrison DA, Mannarelli B (1979) Transformation in pneumococcus: nuclease resistance of deoxyribonucleic acid in the eclipse complex. J Bacteriol 140: 655-665.
- Beilharz K, Nováková L, Fadda D, Branny P, Massidda O, et al. (2012) Control of cell division in Streptococcus pneumoniae by the conserved Ser/Thr protein kinase StkP. ProcNatlAcadSci U S A 109: E905-913.
- Straume D, Stamsås GA, Håvarstein LS (2015) Natural transformation and genome evolution in Streptococcus pneumoniae. Infect Genet Evol 33: 371-380.
- Håvarstein LS, Coomaraswamy G, Morrison DA (1995) An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. ProcNatlAcadSci U S A 92: 11140-11144.
- Brantl S, Brückner R (2014) Small regulatory RNAs from low-GC Gram-positive bacteria. RNA Biol 11: 443-456.
- Marx P, Nuhn M, Kovács M, Hakenbeck R, Brückner R (2010) Identification of genes for small non-coding RNAs that belong to the regulon of the two-component regulatory system CiaRH in Streptococcus. BMC Genomics 11: 661.
- Ruggiero A, De Simone P, Smaldone G, Squeglia F, Berisio R (2012) Bacterial cell division regulation by Ser/Thr kinases: a structural perspective. Curr Protein PeptSci 13: 756-766.
- Morlot C, Bayle L, Jacq M, Fleurie A, Tourcier G, et al. (2013) Interaction of Penicillin-Binding Protein 2x and Ser/Thr protein kinase StkP, two key players in Streptococcus pneumoniae R6 morphogenesis. MolMicrobiol 90: 88-102.
- Zapun A, Contreras-Martel C, Vernet T (2008) Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev 32: 361-385.
- Bergmann C, Fang C, Rachid S,Hakenbeck R(2004) Mechanisms for penicillin resistance in S.pneumoniae. In The pneumococcus (ed .Tuomanen E I, Mitchell T J, Morrison D A and Spratt B G) ASM Press, Washington DC: 339-349
- Czarnecki G, Dissanayake P, Palchaudhuri S (2013) Gram-Positive S.mitis become sensitive to colistin and nalidixic acid when grown in xylitol. J Mol Genet Med 7:4172-4147
- Palchaudhuri S, Dissanayake P, Palchaudhuri A (2013) Blocking the two-component signal transduction pathway of diplococcic Gram-positive pathogens by xylitol cloud as visualized by SEM. Intl. Conference on Electron Microscopy. Kolkata SINP, India: P43
- Griffith F (1928) The Significance of Pneumococcal Types. J Hyg (Lond) 27: 113-159.
- Palchaudhuri S, Kurtovic E, Kurtovic E, Dissanayake P, Zei Mei, et al. (2015) Xylitol blocks Streptococcal signal transduction pathway to reduce children mortality rate from pneumonia. Intl Journal of Research 1:2394-2397
- Fischer W (2000) Phosphocholine of pneumococcal teichoic acids: role in bacterial physiology and pneumococcal infection. Res Microbiol 151: 421-427.
- Henriques-Normark B, Tuomanen EI (2013) The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring HarbPerspect Med 3.
- Marttinen AM,Ruas-Madiedo P, Hidalgo-Cantabrana C, Saari MA, Ihalin RA, et al. (2012) Effects of xylitol on xylitol-sensitive versus xylitol-resistant Streptococcus mutans strains in a three-species in vitro biofilm. CurrMicrobiol 65: 237-243.
- Benchabane H, Lortie LA, Buckley ND, Trahan L, Frenette M (2002) Inactivation of the Streptococcus mutansfxpC gene confers resistance to xylitol, a ca ries-preventive natural carbohydrate sweetener. J Dent Res 81: 380-386.










