Keywords
|
| Zonisamide, C6 glioma cells, Anticancer, Antioxidant |
Introduction
|
| Tumor-associated seizures (TAS) by lipid production and peroxidation included: enzyme disturbances and cell membrane changes are frequently seen in patients with brain cancer. TAS are partially controlled with multiple and high doses of antiepileptic drugs [1] such as acetazolamide, phenobarbital, valproic acid, perampanel, zonisamide, gabapentin and levetiracetam [2,3]. Concerning metastatic tumors, treatment of levetiracetam reduced seizure frequency to more than 50% in all 13 patients [4]. Similar results were obtained from the use of valproic acid. It was reported by Van Bremen et al., that 79.3% in 99 glioma patients with secondary epilepsy remained entirely seizure free after treatment with valproic acid [5]. Zonisamide, due to its antioxidant and with a low level of anti-cancer drugs interaction properties, is also associated with tumor-associated seizures [6,7]. |
| The best way to treat seizures in patients with cancer could be use of anti-epileptic drugs that have anti-cancer activity. Up to the present, only valproic acid has been associated with anticancer activity [8]. Valproic acid has been shown to be associated with several cellular pathways such as focal adhesion pathways, cell cycle regulation, Wnt signaling and apoptosis pathways [8]. Valproic acid induces neuronal differentiation, growth arrest and suppression of cell migration in C6 glioma cells [9]. Furthermore, in malignant neuroblastoma cells, valproic acid has a synergy antitumor effects with interferon-α and the combine use of valproic acid and interferon-α increased caspase-8 promoter activity [10]. |
| Since valproic acid’s anticancer activity has been documented in a number of solid tumors, another anti-epileptic drug of zonisamide may show a similar effect on C6 glioma tumor cells. Therefore, the cell viability, measurement of DNA synthesis, superoxide dismutase (SOD) activity, the measurement of glutathione (GSH) assay were used to investigate the possible effects of zonisamide on cell proliferation and an antioxidant enzyme levels of rat C6 glioma cells in vitro. |
Material and Methods
|
|
Cell culture
|
| C6 glioma cell line was cultured in Dulbecco’s Modified Eagle’s medium (DMEM) and F-12 Ham’s medium (1:1) supplemented with 10% fetal bovine serum (FBS) and antibiotics in a humidified incubator at 37°C with 5% CO2. |
|
Cell viability (MTT reduction)
|
| Cell viability was determined by measuring the metabolism of a tetrazolium substrate, 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT). For this study, 1 x 104 cells were plated in 96-well culture plate. After 24- or 48-h exposure to zonisamide (ZNS) (0.78, 1.56, 3.12, 6.25, 12.50, 25, 50, 100, 200 μM dissolved in DMSO), the cells were treated with 20 μl MTT (5 mg/mL, Sigma) and were incubated for 2 h at 37 °C. Then, the medium was removed and 200 μl of DMSO were added to each well. Cells were incubated at 25°C for further 10 min. The absorbance was measured at 540 nm by ELISA microplate reader. The values of the blank wells were subtracted from each well of treated and control cells, the percentage viability was determined as formulated below: |
| Cell viability % = (The absorbance of the treated cells) - (The absorbance of the blank) / (The absorbance of the control) - (The absorbance of the blank) × 100 |
| IC50 value was calculated as the concentration that shows 50% inhibition of cell on the tested cell line. |
|
Quantitation of DNA synthesis
|
| The rate of DNA synthesis in treated and untreated cells (control) was estimated by means of measurement of bromodeoxyuridine (BrdU) incorporation into growing DNA strands using the Cell Proliferation ELISA kit, (BrdU calorimetric, Roche). Various concentrations of ZNS treated cells in 200 μl mediums were seeded in 96-well plates, with the first column of wells representing blank. Cells were incubated at 37°C and in 5% CO2 for 24, 48, and 72 h. Next, cells were treated according to the protocol supplied by manufacturer. After the end of incubation time, 20 μl BrdU was added and incubated at 37°C for 2 h. After that, the medium was removed and the cells were fixed (30 min, RT) and labeled with anti-BrdU. The substrate solution was added and the cells were incubated at RT for 5-30 min. The absorbance was measured at 490 nm using an ELISA microplate reader (Elx808-IU Bio-Tek). |
|
Determination of SOD activity
|
| SOD activity was measured after 24-h exposure to ZNS using OxiSelect™ Superoxide Dismutase Activity Assay Kit (Cell Biolabs, Inc). Briefly, after exposure to ZNS, cells were washed with icecold PBS and placed on ice. Cold 1×lysis buffer (10 mMTris, pH 7.5, 150 mM NaCl, 0.1 mM EDTA and 0.5% Triton-100) was added and incubated on ice for 10 min. Cells/debris was collected with a rubber policeman. The cell extract was transferred to a microfuge tube and centrifuge at 12, 000 × g for 10 min. Then the cell lysate supernatant was collected. Samples were prepared, including a blank in a 96-well Microtiter plate according to the manufacturer’s instructions. The absorbance was measured at 490 nm on a microplate reader (Elx808-IU Bio-Tek). |
| SOD Activity (inhibition%) = (ODblank-ODsample) /(ODblank) x 100 |
|
Measurement of intracellular GSH
|
| Intracellular GSH contents were measured using the Glutathione Detection Kit (Chemicon International). In brief, 1 × 106 cells were washed with ice-cold 1× wash buffer and placed on ice. 1 ml of cold 1×lysis buffer was added. After 10 min. Incubation on ice cells/debris was collected with a rubber policeman. Cell extract was centrifuged at 12, 000 × g for 10 min. The supernatant solution was used for GSH measurement by using a 380/460 nm filter in a fluorometer. |
Data Analyses
|
| Statistical differences were obtained using one-way ANOVA followed by Dunnett’s or Tukey’s tests for all studies. The statistical analyses were carried out using GraphPad Prism version 5.0. The results were expressed as the mean ± S.E.M. to show variation in groups. Differences were considered significant when P ≤ 0. 05. |
Results
|
|
Cell viability
|
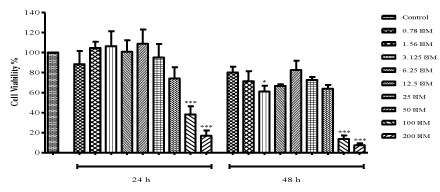
| Treatment with ZNS dramatically decreased cell viability from 95.11% to 16.81% starting with 25 μM concentration after 24 h (100 and 200 μM: P<0.001) as seen in Figure 1. C6 glioma cells were observed to be affected more at 48 h treatment. However, lower concentrations of ZNS showed irregular effects both at 24 and 48 h. ZNS at the doses of 3.125, 100 and 200 μM significantly decreased cell viability (P<0.05, P<0.001 and P<0.001, respectively) when compared to control cells at 48 h. Inhibitory concentration (IC50) value of ZNS was derived from the dose-response curve as 80 μM which was used in further experiments along with the concentrations that shows 95% (25 μM) and 75% (50 μM) cell viability. |
|
Quantitation of DNA synthesis
|
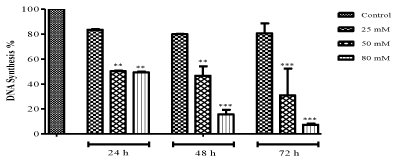
| The effect of ZNS on DNA synthesis in C6 glioma cells was estimated by using a thymine analog BrdU which gets incorporated during DNA synthesis and the results presented in Figure 2. IC50 value, 80 μM and 50 μM ZNS showed a decrease in DNA synthesis (49.77 and 48.92%, respectively) and also they were inhibited the synthesis remarkably as compared to control cells (50 and 100 μM at 24-h: P<0.01, 50 μM: P<0.01; 100 μM: P<0.001 at 48-h, 50 and 100 μM: P<0.001 at 72 h) at 24 and 72 h time interval. |
|
Determination of SOD activity
|
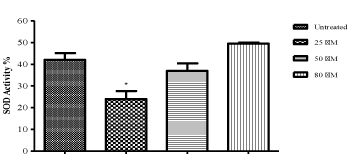
| As seen in Figure 3, the lower concentrations of ZNS decreased SOD activity and also this decrease is significant at 25 μM concentration as compared to control cells (P<0.05). However, no statistically significant differences were observed in the 50 and 80 M of ZNS treated groups (Figure 3). |
|
Measurement of intracellular glutathione
|
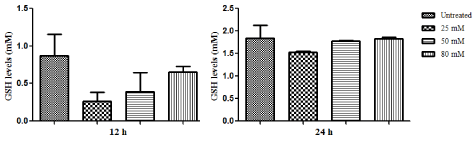
| As compared to untreated cells, lower doses of ZNS decreased intracellular GSH levels relatively (Figure 4). As it can be seen the effect of ZNS was more obvious at 12 h treated C6 cells. |
Discussion
|
| In patients with brain tumor, seizures are the onset symptom in 20–40% of the patients, while a further 20–45% of the patients will present them during the course of the disease. In this patient population, new generation drugs such as zonisamide are preferred because they have fewer drug interactions and cause fewer side effects [11,12]. The antioxidant activity of zonisamide suggests that it may be effective on tumors during use to avoid seizures in patient with glioma. Antioxidants are effective to provide the protection of normal cell cycle, inhibition of proliferation or induction of apoptosis, invasion of tumors and the prevention of angiogenesis, the suppression of inflammation [13]. Decreased antioxidant levels in the cells cause to increase in production of free radicals and damage DNA, proteins and lipids [14]. Anticancer activity, the levels of SOD and GSH antioxidant enzymes were evaluated after treatment of C6 glioma cells with various concentrations of zonisamide. Zonisamide (50 μM) demonstrated antiproliferative effects on C6 glioma cells (25.99% inhibition) and inhibited C6 glioma cell DNA synthesis. |
| These activities are valuable for management of cancers in case zonisamide may be a potential agent for glioma treatment. The other finding is a decline in SOD and GSH activates provided by lower concentration of zonisamide at the dose of 25 M. Unlike the decrease in GSH levels by zonisamide, the decrease in SOD levels was found to be a statistically significant. Especially lower dose of zonisamide showed pro-oxidant effects on C6 glioma cells. In this wise, anticancer activity of zonisamide might be patented by the inhibition of SOD levels. GSH levels were also decreased by treatment of zonisamide at 12 h, but this inhibition was not statistically significant. However, there is increasing evidence demonstrating that decreasing of GSH levels is an active phenomenon regulating the redox signaling events modulating cell death activation and progression by apoptosis through a variety of mechanisms [15,16]. GSH loss initiates the proapoptotic function of the released cytochrome c1 (cytc1), the formation of apoptosome disc and activation of caspases [15]. The apoptotic effects of zonisamide still need to be examined. |
| Anticancer drugs trigger the production of reactive oxygen species to kill the tumor cells and the studies have shown that the tumor cells with high levels of antioxidant enzymes are resistant to these anticancer drugs [17]. Decreasing of antioxidant enzyme levels by lower concentration of zonisamide in C6 glioma cells might be useful to overcome drug-resistance and using zonisamide with anticancer drugs during the glioma tumor treatment might enhance the effects of chemotherapy also shows the antiproliferative effect and prevents the tumor-induced seizures. |
Conclusion
|
| These results indicate that it seems to be useful to combine zonisamide with anticancer drugs for fighting against glioma cells because zonisamide showed the antiproliferative effects and reducing effects on antioxidant enzyme levels which may increase anticancer drugs efficiency to treat glioma cells. Understanding the relationship between zonisamide and glioma cells might provide useful information for improve the quality of life in cancer patients. |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |
| |









