Keywords
Anti-VEGF; Post-injection reflux; Post-injection IOP spike; Intravitreal injection
Introduction
There are many diseases which can cause blindness; some are preventable by using Anti-VEGF, among these diseases, the most common worldwide is the wet type of age-related macular degeneration (AMD), which causes neovascularization subsequently blindness if left untreated [1]. The second one is diabetic macular edema and retinal vascular diseases [2-4]. While anti-VEGF injected, it causes a sudden increase in the IOP. Still, no one had measured the immediate post-injection IOP to know how much eye volume increases with the standard doses to causes this spike; we measured within less than 15 seconds postinjection and with the standard Goldmann tonometry which is the most accurate one. We did not check these patients for long terms and the effects of IOP spike on the optic nerve because it’s not our aim.
This study tries to determine the impact of different types of IOPlowering, prophylactic, therapies in reducing the IOP spikes and the reflux post IVI of Bevacizumab and Ranibizumab. Immediately before and immediately after the Intravitreal injection in the operative theater on slit-lamp by Goldmann tonometer carried out.
Methodology and Material
A retrospective data recording performed on patients who received anti vasoproliferative endothelial growth factor (VEGF) intravitreal injections (IVI). The inclusion and exclusion criterion are the following:
Inclusion criteria
Any patient indicated for IVI anti-VEGF injection.
Exclusion criteria
Allergy to the used or related drug, renal problem, liver problem, pregnancy, baseline intraocular pressure (IOP) >21, glaucoma patients’ underwent Ahmet valve or filtration surgery and vitrectomized patients.
A total of 200 patients, (88 women and 112 men), each of them injected with one eye (200 eyes). Among them, 170 patients received Ranibizumab, and 30 received Aflibercept. The topical prophylactic IOP lowering therapy, which included Timolol maleate eye drop, Brimonidine tartrate eye drop and Dorzolamide hydrochloride eye drop, have been administrated three hours before the injection, according to the groups. While oral prophylactic IOP-lowering medication; Acetazolamide tablet (500 mg) used and given 2 hours before the injection according to the groups. The patients had been divided into four groups, and each group subdivided to include 50 eyes as following:
Group A: Neither drops (topical) nor oral hypotensive had been given,
Group B: Only acetazolamide tabs 500mg 2 hours before injection,
Group C: Timolol maleate eye drop, Brimonidine tartrate eye drop and Dorzolamide hydrochloride eye drop had given 3 hours before injections, they were given 5 minutes apart.
Group D: Received both topical and oral hypotensive prophylactic therapy (combined group B and C).
All the injections were performed by the same surgeon, in the operation theatre setting, under topical anesthesia using a sterile technique; every patient in all groups has been checked for IOP just before receiving the intravitreal injection, on the table in operation theater and immediately after the injection, (within less than 15 seconds), by Goldmann applanation tonometry.
The Ranibizumab and Bevacizumab were injected in the dose of 0.05 ml through 30 gauge needle according to Euretina Expert Consensus Recommendations IVI Guidelines Procedure 2018 [1], the bevel completely inserted trans-sclerally then directed into the center of the vitreous cavity and after injection, in a reversed manner the needle is withdrawn to retain the formed scleral tunnel, immediately a counter pressure performed with the help of a cotton-tip applicator. Then the injection area was monitored for the evidence of the of any Anti-VEGF reflux. Immediately after the injection, the visual acuity was assessed as seeing hand movement or not, those who did not saw hand movement underwent Paracentesis. Followed by topical antibiotics both after the injection and after the applanation. For every individual eye, a disposable applanation prism of Goldmann tonometry had been used.
Results
The mean IOP pre-injection in all groups was 14.44, while postinjection was 28.73 with a p-value of <0.001, statistically, it is highly significant, Table 1. In the Group D, which received both topical and systemic IOP-lowering therapy showed lowest IOP mean for both pre-injection (12 mmHg) and post-injection (17 mmHg) while the highest post-injection IOP mean had been recorded in the group A (38 mmHg) and the p-value showed a highly significant difference between them, Table 2. Only 12% patient got significant IOP rise of 60 mm Hg with a VA of NLP post-injection in the Group A and 2% in Group D who was hyperopic, and the patients underwent immediate anterior chamber paracentesis, till the was able to count the finger.
Table 1. Difference of IOP pre-injection & post-injection differences.
IOPMeanNStd. DeviationP valuePre injection14.442003.189<0.001Post injection28.732009.304
Table 2. Difference between Pre & post-injection IOP by groups.
GroupsPre injection IOPPost injection IOPP valueMeanStd. DeviationMeanStd. DeviationGroup A163388<0.001Group B163334<0.001Group C153274<0.001Group D122172<0.001
Regarding the reflux; the incidence of reflux among total all groups were 32% (64 cases), and the mean IOP among them were 38.16 mmHg with a very highly significant p-value <0.001, Table 3. The reflux was significantly seen in the Group A; retrospectively the incidence of non-reflux cases was 20%, and they had high myopia; neither reflux nor high spike happened, with a peak post-injection IOP reached 25 mmHg while the remaining 80% developed reflux. In reverse, in the Group D reflux happened to the hyperopic eyes with a mean IOP of 17 mmHg, and the incidence of 4% and 96% were non-refluxing Table 2 and Figure 1.
Table 3. Correlation between the reflux and the volume.
IOPRefluxNumber of casesMean IOPStd. DeviationP-valuePost injectionYes6438.168.289<0.001No13625.597.296
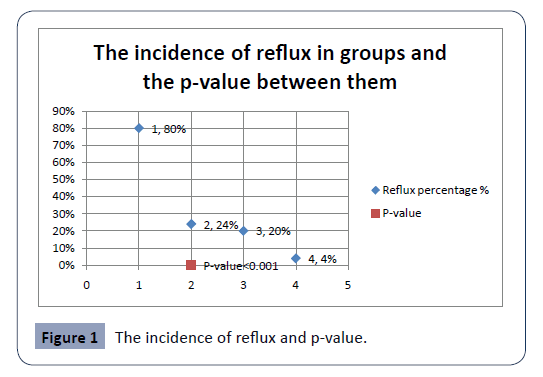
Figure 1: The incidence of reflux and p-value.
Therefore, decreasing the volume of the vitreous, with topical and systemic therapy, statistically significantly reduces the chance of reflux to happen (p<0.001), Table 3.
Discussion
This study shows the amount of IOP spike and the reflux, immediately after the intravitreal anti-VEGF injection, studies showed post-injection IOP raising, but it was not immediate post-injection, that had been conducted by Singer MA et al and Schmidt-Erfurth U, years ago [5,6], therefore a safety measure should be taken in consideration as the use of anti-VEGF is increasing. The current study determines the real increase of immediate post-injection with different types of anti-VEGF in different groups, to demonstrate which way is the safest method of choice to prevent the IOP spike and the reflux, to consider which one needs anterior chamber paracentesis. In this setting, the standard anti-VEGF dose and needle bore size used, the latter to demonstrate which way is effective in preventing the reflux. The logic behind acute post-injection IOP spike is the change in the vitreous volume which is nearly 4 ml, and the volume of anti- VEGF is 0.05 ml; therefore, the increase in volume nearly will be 1.25%, this change in the volume might cause structural changes in the aqueous outflow [7].
The current study showed that the prophylactic IOP lowering therapy before injection, significantly associated with a decrease in the post-injection spikes with the best prophylactic group when both topical and the systemic therapy used together in the same group giving a very highly significant p-value <0.001, Table 2, immediately at the post-injection period. While Frenkel et al recorded higher IOP after 20 minutes from IVI was <30 mmHg, therefore, he claims that the prophylactic therapy is ineffective in preventing the spikes [8], however, Hariprasad et al showed 13% had > 30 mmHg or more after 30 minutes from the injection [9], but ours was 25% immediately post-injection the mean IOP was 38 mmHg in the worse Group A and 4% of them the peak IOP was 60 mmHg, while the best result was in the Group D, with 25% of them revealed mean IOP of 17 mmHg. Theoulakis et al. and Kim et al. showed in their study that prophylactic IOP lowering therapy is effective in lowering the post-injection IOP [10,11], which supports our study findings. Regarding studies showed higher post-injection IOPs could be explained in terms of timing with the pre-injection treatments; Frenkel’s had given the therapy 5 minutes pre-injection which is a quite short interval to be effective for any topical drugs to reach its peak of action. While in Kim’s study a wider bore sized needle (27 gauge) used which might allow reflux to happen much more easily than 30 gauge needle which is used in the current study, and caused underestimation to the IOP, therefore, Kim’s IOP lowering was mechanical, (escaping “refluxing” the injected IVI through a wide scleral opening with 27 gauge needle), rather than the efficacy of his pre-operative IOP lowering agents and this idea shown by Carnota-Méndez et al [12,13].
Benz had used 0.1 ml of triamcinolone and 27 gauge needle and the reflux when happened they have a less significant rise in IOP [14], but in this study, the recommended dose, which is smaller in amount and a smaller bore needle size was used. Also, this method showed leakage, and the amount of reflux cannot be quantified. The smaller gauge associated with a higher rate in IOP immediately after injection, shown by Kim [12], which supports findings in the current study, here, this research provides a new method to prevent significantly both an IOP spike immediately after injection and the reflux. Although both the doses and the bore were smaller in our study, the leakage was seen in all groups, the Group A showed the highest incidence of 80%, even when tunnel carried out may be due to physics that increasing the volume in a limited space causing this reflux at the same time reflux did not happen in the same group in high myopic patients and the incidence was 6%, ( the axial length were more than 26.5 mm retrospectively done), this can be explained by that the volume of the eye directly affect the refluxing rather than the scleral rigidity, but Kim believes scleral rigidity has its effect [12]. The current study showed that the hyperopic eye which accounted 4%, (retrospectively the axial length were less than 21 mm), associated with an increased IOP in Group D, this finding supported by the same result in a study by Kotliar [15] and the same Group D, showed reflux in the hyperopic, therefore, these outcomes confidently prove that the scleral thickness not related to the reflux, also this study revealed that the high myopic patients in the Group A did not have reflux while hyperopic in the Group D had reflux, it’s the effect of volume of the eye that directly related to the spike in IOP and reflux rather than the volume of injection which is reverse to the Kim’s study that stated the spike might happen if the volume was more than 0.05 ml [12].
Here, even with the small dose paracentesis carried out in patients did not see hand movement in reverse to the study by Kotliar et al [15] they considered the paracentesis for 0.1 ml doses to avoid a spike in IOP. But still, post-injection paracentesis remains controversial, Saxena S et al [16].
In this study, the post-injection IOP measured immediately, patients were asked to sit on the operation bed, with a sterile disposable Goldmann tonometry the IOP checked and recorded for every single patient, the same thing done by Kim [12], the most accurate measure is Goldmann tonometry, but Kim used TonoPen; which can underestimate the IOP in comparision to the Goldmann tonometry, van der Jagt LH, Jansonius NM and Lim KS et al [17,18].
Therefore, the volume of the eye could have a direct correlation with both IOP spikes and reflux. More data collection and analysis on high myopia’s and hyperopia’s needed to prove that.
Whenever the recommended amount of IVI escaped, reflux, the actual IOP is challenging to be measured, and on the other hand, it might an impact on the improving efficacy on the retinal diseases.
Conclusion
This study concludes that combined topical and systemic ocular hypotensive therapy medications are gold standard pre-injection guidelines to prevent both post-injection IOP spikes and reflux.
Declarations
Funding: None
Conflict of interest: None declared
30950
References
- Grzybowski A, Told R, Sacu S, Bandello F, Moisseiev E, et al. (2018) 2018 Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. Ophthalmologica 239: 181-193.
- Ferris FL, Fine SL, Hyman L (1984) Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 102: 1640-1642.
- Jiang Y, Mieler WF (2017) Update on the Use of Anti-VEGF Intravitreal Therapies for Retinal Vein Occlusions. Asia Pac J Ophthalmol (Phila) 6: 546-553.
- Browning DJ, Stewart MW, Lee C (2018) Diabetic macular edema: Evidence-based management. Indian J Ophthalmol 66: 1736-1750.
- Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, et al. (2012) HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology 119: 1175-1183.
- Schmidt-Erfurth U (2010) Clinical safety of ranibizumab in age-related macular degeneration. Expert opinion on drug safety 9: 149-165.
- Tseng JJ, Vance SK, Torre KED, Mendonca LS, Cooney MJ, et al. (2012) Sustained increased intraocular pressure related to intravitreal antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. J Glaucoma 21: 241-247.
- Frenkel MP, Haji SA, Frenkel RE (2010) Effect of prophylactic intraocular pressure-lowering medication on intraocular pressure spikes after intravitreal injections. Arch Ophthalmol 128: 1523-1527.
- Hariprasad SM, Shah GK, Blinder KJ (2006) Short-term intraocular pressure trends following intravitreal pegaptanib (Macugen) injection. Am J Ophthalmol 141: 200-201.
- Theoulakis PE, Lepidas J, Petropoulos IK, Livieratou A, Brinkmann CK, et al. (2010) Effect of brimonidine/timolol fixed combination on preventing the short-term intraocular pressure increase after intravitreal injection of ranibizumab. Klin Monbl Augenheilkd 227: 280-284.
- Kim GN, Han YS, Chung IY, Seo SW, Park JM, et al. (2013) Effect of Dorzolamide/Timolol or Brinzolamide/Timolol prophylaxis on intravitreal anti-VEGF injection-induced intraocular hypertension. Semin Ophthalmol 28: 61-67.
- Kim JE, Mantravadi AV, Hur EY, Covert DJ (2008) Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol 146: 930-4.e1.
- Carnota-Méndez P, Méndez-Vázquez C, Otero-Villar J, Saavedra-Pazos JA (2014) Effect of prophylactic medication and influence of vitreous reflux in pressure rise after intravitreal injections of anti-VEGF drugs. European journal of ophthalmology 24: 771-777.
- Benz MS, Albini TA, Holz ER, Lakhanpal RR, Westfall AC, et al. (2006) Short-term course of intraocular pressure after intravitreal injection of triamcinolone acetonide. Ophthalmology 113: 1174-1178.
- Kotliar K, Maier M, Bauer S, Feucht N, Lohmann C, et al. (2007) Effect of intravitreal injections and volume changes on intraocular pressure: clinical results and biomechanical model. Acta Ophthalmol Scand 85: 777-781.
- Saxena S, Lai TY, Koizumi H, Farah ME, Ferrara D, et al. (2019) Anterior chamber paracentesis during intravitreal injections in observational trials: effectiveness and safety and effects. International Journal of Retina and Vitreous 5: 8.
- van der Jagt LH, Jansonius NM (2005) Three portable tonometers, the TGDc-01, the ICARE and the Tonopen XL, compared with each other and with Goldmann applanation tonometry*. Ophthalmic Physiol Opt 25: 429-435.
- Lim KS, Wickremasinghe SS, Cordeiro MF, Bunce C, Khaw PT (2005) Accuracy of intraocular pressure measurements in new zealand white rabbits. Invest Ophthalmol Vis Sci 46: 2419-2423.






