Keywords
Respiratory infections; SARS-CoV2; Ag rapid test
Introduction
Biological diagnosis of respiratory infections by SARS-CoV2 uses molecular tools targeting viral genes by RT-PCR analysis or isothermal gene amplification. These IVD assays need technical facilities and often take few hours. To be used in non-laboratory environment, some rapid test devices have been designed for SARS-CoV2 Ag detection by immunochromatographic assay.
Materials and Methods
Specimens were taken among those routinely analysed by RT-PCR for SARS-CoV2 diagnosis. They have been collected by mean of nasopharyngeal swab, and then swirled in 3 ml of viral transport medium (VTM). Such processing allowed using the same sample for each of the 5 assays (1 RT-PCR and 4 Ag test devices) with one routine nasopharyngeal collection.
Specimens were as follows:
• 29 PCR negative for SARS-CoV2 without any request for other diagnosis
• 18 PCR negative for SARS-CoV2 but positive for at least one respiratory virus by Panther Fusion®
• 86 PCR positive for SARS-CoV2.
The routine diagnosis has been conducted by RT-PCR (Thermo Fisher Scientific®/Applied Biosystems®TaqPath™ COVID-19 CE-IVD RT-PCR Kit catalog #A48067) targeting S, N and Orf 1ab genes.
For other respiratory infections diagnosis, if any requested, samples have been checked by PCR on Panther Fusion® (Hologic®) for Influenza A, Influenza B, Respiratory Syncitial Virus, Paramyxovirus (4 types), human Metapneumovirus, Rhino/ enterovirus and Adenovirus.
Analyses were processed day-by-day during February 2021 at the Virology Laboratory of "Dijon Bourgogne University Hospital" (France). Samples enrolled for Ag testing have been kept at 4-8°C and assayed within hours following RT-PCR analysis (Table 1).
| Ag test device |
Brand |
Lot |
SARS-CoV2 rapid antigen test
ref 9901- NCOV-01G |
RAPID TEST 1 |
QCO 3900531/sub: I-1 |
Panbio Covid-19 Ag rapid test
ref 41 FK 10 |
RAPID TEST 2 |
41 ADF 337 A |
SARS-CoV2 antigen rapid test
ref N/A |
All TestÒ, China |
ATNCP21010032 (cassettes) |
SARS-CoV2 antigen rapid test
ref N/A |
Acro BiotechÒ, USA |
NCP21010033ACO (cassettes) |
Table 1 COVID-19 Ag rapid test devices.
| |
Ag Test |
|
|
| SARS-CoV2 PCR |
RAPID TEST 1 |
RAPID TEST 2 |
AllTest |
AcroBiotech |
n |
|
| Negative |
Negative |
Negative |
Negative |
Negative |
29 |
|
| Negative |
Negative |
Negative |
Negative |
Negative |
18 |
Contains other respiratory viruses |
| Positive |
Positive |
Positive |
Positive |
Positive |
60 |
Comprises one UK and one ?69-70 variants |
| Positive |
Negative |
Negative |
Negative |
Negative |
16 |
Comprises 14 low burden (Ct>26) samples
and one ?69-70 variant among the two others |
| Positive |
Negative |
Negative |
Negative |
Positive |
1 |
Low burden sample |
| Positive |
Slight positive |
Negative |
Positive |
Positive |
2 |
High burden (Ct<26) samples |
| Positive |
Negative |
Negative |
Slight positive |
Slight positive |
3 |
Comprises one UK variant |
| Positive |
Slight positive |
Positive |
Positive |
Positive |
1 |
ZA variant |
| Positive |
Slight positive |
Slight positive |
Positive |
Positive |
1 |
UK variant |
| Positive |
Positive |
Slight positive |
Positive |
Positive |
1 |
UK variant |
| Positive |
Positive |
Positive |
Negative |
Slight positive |
1 |
?69-70 low burden variant |
Table 2 Summary onRapid antigen tests to detect SARS-CoV-2.
For each Ag test device, the VTM nasopharyngeal specimen was dispensed as 350 μl aliquot in the extraction buffers contained in each of the kits. So, for each of the four devices, specimens were processed of a same manner. Extracted sample was then added to the four test cassettes and migrated according to manufacturer’s instructions. Results were read and registered before the limit of timing. Assay with the four devices were processed simultaneously for each of the analyzed specimens [1,2].
Results and Discussions
PCR-negative samples
RT-PCR Thermo gave negative results on 29 specimens without any other demand and on 18 for which other viruses had been diagnosed (47 PCR-negative specimens).
The 29 samples negative for SARS-CoV2 by RT-PCR were all negative by any of the four Ag tests.
The 18 samples with respiratory virus but negative for SARS-CoV2 were also negative by the four Ag tests.
Concordance for SARS-CoV2 negative results was then 100% for each of the Ag tests.
PCR-positive samples
RT-PCR Thermo gave positive results on 86 specimens.
• Ct ranged from 10.74 to 40.92 for S target (with 10 lacking of S response due to variations).
• Ct ranged from 12.59 to 32.97 for N target.
• Ct ranged from 10.41 to 33.68 for Orf1ab target.
• For 70 of 86 specimens, all the 3 Ct were <26.00.
Among the 86 specimens SARS-CoV2 positive by RT-PCR, 16 were negative by any of the rapid Ag tests. All their Ct ranged above 26.00, except for one wild type (S: Ct=25.16, N: Ct=23.06, Orf1ab: Ct=22.46) and for one variant del69-70 (S: negative by RT-PCR, N: Ct=23.58, Orf1ab: Ct=23.70).
For 60 of the RT-PCR positive samples, rapid Ag test positive results were obtained within 2 min for each of the device tested [3].
For 10 of the RT-PCR positive samples, rapid Ag testing failed (at least for one device) to quickly give a positive result, leading either to slight and late positive or to negative result. Among these 10, there was lacking 4 positive results for RAPID TEST 1, 6 for RAPID TEST 2, 2 for AllTest and none for AcroBiotech [4].
Taken together, quick and late positive results were as many as 66 by RAPID TEST 1, 64 by RAPID TEST 2, 68 by All Test and 70 by Acro Biotech, among the 86 specimens positive by RT-PCR.
Concordance for SARS-CoV2 positive results was then in our series:
• 77% for RAPID TEST 1
• 74% for RAPID TEST 2
• 79% for AllTest
• 81% for Acro Biotech.
PCR-positive – low and high burden
It is noteworthy that most of the discrepancies between RT-PCR and rapid Ag tests results were due to a low viral burden in some collected specimens. Indeed, 14 of the 16 samples negative by the 4 devices for rapid Ag testing were of Ct>26.00 whatever the target gene of SARS-CoV2. One sample, positive only by Acrobiotech, was of Ct>27.38 for any of the 3 target genes. Another sample was negative only by AllTest, with Ct>28.20.
Finally, unexpected negative results by Ag testing (although Ct<26.00 by RT-PCR) were 5 with RAPID TEST 1, 7 with RAPID TEST 2, 2 with AllTest and 2 with Acro Biotech. So, concordant results for RT-PCR positive specimens of Ct<26.00 were 65 for RAPID TEST 1, 63 for RAPID TEST 2, 68 for AllTest and 68 for Acro Biotech among the 70 samples of Ct<26.00 [5].
Concordance for Ct<26 positive results was then in our series:
• 92.9% for RAPID TEST 1
• 90.0% for RAPID TEST 2
• 97.1% for AllTest
• 97.1% for Acro Biotech.
Variants of SARS-CoV2
Among the 86 RT-PCR positive specimens, there was 10 variants of SARS-CoV2:
• Two of them (UK and del 69-70 variants) gave a strong and quick positive result by each of the Ag testing device.
• Three variant samples gave a negative result by each of the Ag testing device (low burden for two of them: UK and del 69-70 variants; high burden for the other: del 69-70 variant).
• Three variant samples gave positive results (even slight or late) for some of the device: RAPID TEST 1 with one ZA variant, RAPID TEST 2 with one UK variant, both RAPID TEST 1 and RAPID TEST 2 with another UK variant.
• Two variant samples gave a negative result by one of the 4 devices (AllTest with a del 69-70 variant, low burden) or by two (RAPID TEST 1 and RAPID TEST 2 with a UK variant, high burden).
Targeted Ag is N for AllTest and Acro Biotech but is not indicated for RAPID TEST 1 and RAPID TEST 2. As S variations were encountered with any kind of results, it seems not to be involved in assay performance, especially for AllTest and Acro Biotech, as S Ag is irrelevant for these devices.
4 kinds of COVID-19 Ag rapid test devices had been run with confirmed 47 negative specimens and 86 confirmed positive specimens of COVID-19 [6-8].
The accuracy of 4 brands’ products shows as data in Figure 1. Details please refer to the Table 2.
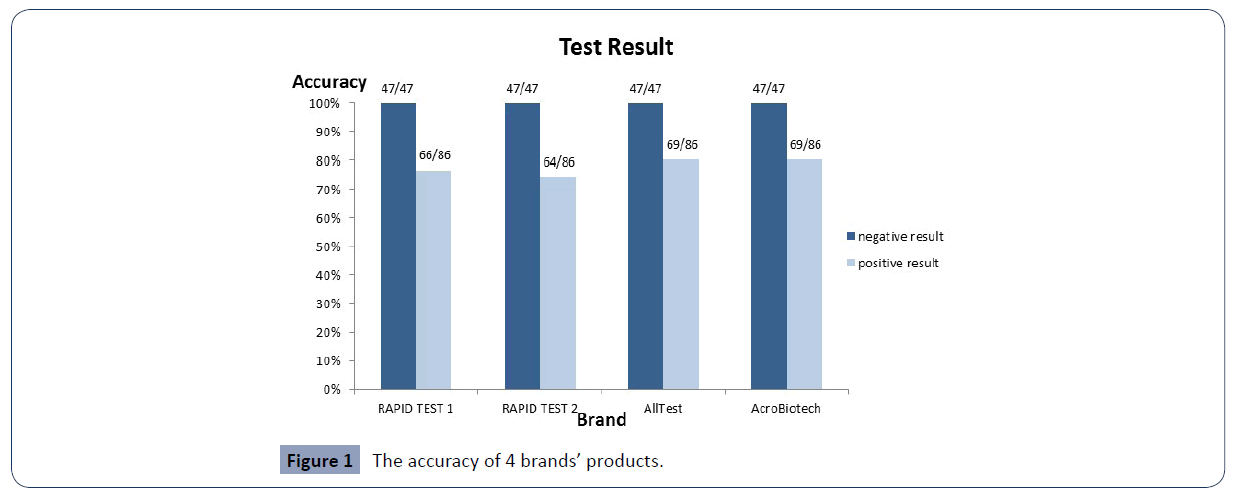
Figure 1 The accuracy of 4 brands’ products.
Discussion
As we analyzed VTM suspended nasopharyngeal swabbing, Ag concentration was slightly less than obtained by directly swirling the swab in extraction buffer. Instead of complete transfer of Ag by swab directly in extraction buffer, we took a 350 μl aliquot of the VTM suspension, which correspond to 1/12 of Ag burden collected by swabbing. Concordance given above for the 4 Ag test devices has been established with samples diluted in VTM. If specimen would be unloaded directly in extraction buffer, the Ag concentration would be 12 times higher than in VTM, which would correspond to RT-PCR reactivity at Ct=29.42 (26.00+3.42) [9-12].
Rapid Ag test devices are alternative for SARS-CoV2 diagnosis when lacking time or laboratory environment. Molecular diagnosis based upon viral RNA amplification is better because of its lower limit of detection. In our study, it appears that nasopharyngeal specimens should be positive by rapid Ag testing if viral burden correspond to Ct of around 30 or less by RT-PCR Thermo. This is very frequent with virus-producing patients. The highest the Ct, the highest the chance for an Ag rapid test to be negative, but also the lowest for the patient to be contagious. At least for devices targeting N Ag, molecular variations within S gene do not influence performance of the test, which is the case for AllTest and Acro Biotech. In our series, these 2 devices gave most of the expected positive results.
36516
References
- Santhanam M, Algov I, Alfonta L (2020) DNA/RNA Electrochemical Biosensing Devices a Future Replacement of PCR Methods for a Fast Epidemic Containment. Sensors 20:4648.
- Chinese Center for Disease Control and Prevention (2020) Public protection guidelines for Novel coronavirus pneumonia, People's Medical Publishing House (PMPH).
- European Centre for Disease Prevention and Control(2020) An overview of the rapid test situationfor COVID-19 diagnosis in the EU/EEA.Stockholm:EUCDC.
- Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR,et al. (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotech 28:595-599.
- Clinical and Laboratory Standards Institute (2017) Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures Approved Guideline (2nd Edn). Wayne, PA: Clinical and Laboratory Standards Institute. CLSI Document EP17-A2.
- Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, et al. (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotech 28:595-599.
- Su S, Wong G, Shi W, Liu J,Lai ACK,et al. (2016) Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol 24:490-502.
- Wang D,Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323: 1061-1069.
- WHO (2020)Laboratory testing for coronavirus disease(COVID-19) in suspected human cases.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K,et al. (2002) Molecular Biology of the Cell. 4th edition. New York: Garland Science. B Cells and Antibodies.






