Review Article - (2022) Volume 13, Issue 3
A review of the risk factors, pathophysiology, diagnosis and
treatment of narcolepsy
Steven Jervis1*,
Anthony Payton2,
Arpana Verma3,
Marcus Lowe1,
Rachel Thomasson4 and
Kay Poulton1,3
1Transplantation Laboratory, Manchester Royal Infirmary, Manchester University NHS Foundation Trust, United Kingdom
2Department of Informatics, Imaging & Data Sciences, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, United Kingdom
3Department of Population Health, Health Services Research and Primary Care, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, United Kingdom
4Department of Neurology, Manchester Centre for Clinical Neurosciences, Salford Royal Hospital, United Kingdom
*Correspondence:
Steven Jervis, Transplantation Laboratory, Manchester Royal Infirmary, Manchester University NHS Foundation Trust,
United Kingdom,
Email:
Received: 08-Mar-2022, Manuscript No. ipjnn-22-12666;
Editor assigned: 10-Mar-2022, Pre QC No. P-12666;
Reviewed: 19-Mar-2022, QC No. Q-12666;
Revised: 24-Mar-2022, Manuscript No. R-12666;
Published:
31-Mar-2022
Abstract
Narcolepsy is hypothesized to be an autoimmune disease targeting
the hypocretin/orexin producing neurons within the hypothalamus
and is categorised into subsets based on the symptoms presented.
Epidemiological studies have proposed that there is an additional
requirement for an environmental trigger combined with potential
genetic predisposition to trigger the onset of this spectrum of disorders.
Despite epidemiological evidence which suggests the condition arises
due to a breach of immunological tolerance, the absence of a defined
autoantigen remains an element of debate in the aetiology of the
disorder. Currently there are no definitive diagnostically useful genetic
variants observed in narcolepsy suffers which could be exploited at
disease onset to support its diagnosis or clinical management
The most impactful genetic risk factor is the presence of human
leukocyte antigen (HLA) allele, DQB1*06:02, which increases the risk of
Narcolepsy over 20-fold. The discovery of the strong association of HLADQB1*
06:02 and disease onset was the original trigger to shift the focus
from its classification as a neurological disease to that of an immunemediated
condition. However, the presence of HLA-DQB1*06:02
alone cannot be the sole genetic risk factor to explain disease onset.
Primarily, not all narcolepsy patients express DQB1*06:02 (>95%)
and more importantly not all people who express DQB1*06:02 suffer
from narcolepsy – only 16% of the global population who express
DQB1*06:02 suffer from Narcolepsy. This has resulted in low usage of
this definition test in the clinical setting due to its lack of specificity and
because it is of most clinical utility if the result is negative, reducing the
likelihood of a diagnosis of narcolepsy.
The diagnosis of narcolepsy is often suspected from the clinical history
of the patient, supported by the results of a multiple sleep latency test
(MSLT). Combined with the determination of hypocretin/orexin in
the cerebral spinal fluid, these two tests are still considered the gold
standard tests for diagnosis. However, there are also documented
issues surrounding both tests in terms of level of sensitivity, specificity
and potential complications which limit their reliability as a diagnostic
resource. This review focuses on emerging evidence of genetic and
environmental risk factors linked with narcolepsy onset, their link to its
pathophysiology and we speculate how genetic traits could be better
utilised to effectively aid a diagnosis.
Keywords
Diagnosis; Narcolepsy; Pathophysiology; Polymorphism
Introduction
Narcolepsy is a rare, life-long disabling neurological
disorder that affects 1 in 3000 individuals. It is characterised
by excessive daytime sleepiness and presents as a result of
a loss of hypothalamic hypocretin producing neurons [1].
A cohort of sufferers also exhibit a sudden loss of muscle
tone while awake triggered by strong emotions, termed
cataplexy. Patients with narcolepsy can also experience
disturbed night time sleep, sleep paralysis, hypnagogic
hallucinations and/or hypnopompic hallucinations [2].
The term hypnagogic describes the period when an
individual falls asleep, whereas hypnopompic describes the
period when a person awakens. The onset of narcolepsy is
often seen during early adolescence or adulthood. The rate
of concordance in monozygotic twins for NT1 onset is 20-
30% [3]. Onset of the condition in a first-degree relative of
a NT1 sufferer is approximately 1-2% and the relative risk
for a first-degree family member of a NT1 sufferer is 10- to
40-fold higher than the general population [3].
Narcolepsy has a severe impact on the quality of life
for sufferers. Excessive daytime sleepiness consists of
periods of an irrepressible need to sleep during times which
would normally be characterized by wakefulness, leading
to sleep attacks that are promoted by sedentary activities
and physical inactivity [4]. Furthermore, symptoms of
narcolepsy may cause complications in relationships and
family life. Narcolepsy often goes undiagnosed for many
years and even following a diagnosis, it can still take time to
understand and manage symptoms. Female sufferers who
become pregnant may have to alter current medication as
some have been shown to pose risks to the unborn child.
In 2014, the international classification of sleep
disorders (ICSD3) categorised narcolepsy into two subtypes,
narcolepsy type 1 (NT1) and narcolepsy type 2 (NT2)
based on the presence or absence of hypocretin proteins
in the patient’s cerebrospinal fluid (CSF) [5]. Hypocretin
(also termed orexin) is the major biomarker associated
with narcolepsy [2]. Hypocretin is produced in the lateral
hypothalamus, derived from the protein precursor preprohypocretin,
which in turn is cleaved enzymatically into two
peptides-hypocretin 1 and 2 [5]. Hypocretin receptors 1 and 2 (HCRTR1 & HCRTR2) are the natural ligands for
hypocretin 1 and 2 respectively [6].
Patients diagnosed with NT1 have cataplexy with an
absence or low levels of hypocretin 1 in their CSF, whereas
NT2 patients do not have cataplexy and have normal levels
of hypocretin 1 in the CSF. Patients who show low levels of
hypocretin but do not exhibit the symptoms of cataplexy
are also included in the NT1 category. A select group of
narcolepsy patients who also suffer with cataplexy have
normal levels of hypocretin, and are also categorized as
NT1 by ICSD3 [7]. Previous studies have shown that the
onset of NT1 is likely due to a selective loss of hypocretin
producing neurons located in the hypothalamus. NT2
shares numerous clinical similarities with NT1 but
currently the pathogenesis remains unknown.
The global prevalence of narcolepsy comprises a mixture
of both the NT1 and NT2 subtypes. Research has shown
that males have a higher risk of developing narcolepsy
than females [8], but existing epidemiological studies have
included too few numbers for sex differences to be fully
conclusive.
Inherited Risk Factors
Human leucocyte antigens
The human leucocyte antigens (HLA) are cell-surface proteins responsible for regulation of the immune system. They are highly polymorphic including many allelic and phenotypic variants encoded by classical and non-classical loci. The classical loci are further divided into two classes, namely I and II. HLA class I loci include HLA-A, HLA-B and HLA-C, whereas class II include HLA-DRB1, -HLADQB1 and HLA-DPB1. The main function of HLA is to present foreign peptides to the immune system stimulating a targeted immune response. A further role of HLA is to maintain self-tolerance preventing the immune system attacking self-antigen. The HLA genes are encoded within the major histocompatibility complex (MHC) on the short arm of chromosome six – 6p21.3. The major genetic association with narcolepsy is HLA-DQB1*06:02 which is present in more than 95% of confirmed narcolepsy cases [9,10]. HLA-DQB1*06:02 is a high frequency allele, with as many as 27-32% individuals in English and European populations carrying this HLA type, and therefore a large percentage of the population could potentially be affected. HLA-DQB1*06:02 is in strong linkage disequilibrium (LD) with HLA- DQA1*01:02 and together these two genes encode separate chains which combine to form a complete HLA antigen. HLA-DQA1*01:02 is also seen in LD with other HLA-DQB1*06 alleles (including DQB1*06:03, 06:04 and 06:09) with no increase in frequency of narcolepsy onset. HLA-DQB1 is a HLA class II molecule, the primary function of which is to present antigen to CD4+ T cells, indicating that CD4+ T cells could play a central role in the pathogenesis of narcolepsy.
HLA allele frequencies vary between populations. This also supports the association of HLA- DQB1*06:02 with the onset of narcolepsy, as populations in which there are fewer individuals who have this HLA type (for example, Greece and India) also see lower incidence of narcolepsy. Other HLA class I genes have been shown to be associated with the onset of narcolepsy including HLA-A*11:01, HLA-B*35:01 and HLA-C*04:01 [11]. In several populations, HLA-B*35:01 and C*04:01 are often inherited together, and these HLA Class I genes may also be inherited in combination with HLA- DQB1*06:02 as an extended haplotype. The role of HLA class I is to present antigens to CD8+ T cells suggesting a potential cytotoxic element to the pathogenesis of narcolepsy involving activated cytotoxic T cells. The associations of both HLA class I and II alleles may suggest that a full HLA haplotype of alleles inherited in combination may be linked with an increased risk of narcolepsy onset. However, DQB1*06:02 is a common allele in the general population, the presence of which alone is insufficient for narcolepsy onset. The λ value for HLA of narcoleptic patients was calculated to be between 2 and 4 which is below the relative risk in first degree family members [9]. This implies that additional genetic factors are involved in the genetic predisposition to narcolepsy onset.
T cell receptors
Polymorphisms detected in the genes encoding the T cell receptor alpha (TRA) on chromosome 14q11.2 have also been suggested to predispose patients to narcolepsy [12]. The T cell receptor (TcR) binds foreign or selfpeptides presented by HLA allowing the stimulation and regulation of immune responses. In 2009, a study of 1,614 confirmed narcolepsy cases compared against 2,148 controls from mixed European, United States and Canadian ancestry showed that three single nucleotide polymorphisms (SNPs) within the TRA gene-rs12587781 (p = 1.90 × 10-13), rs12636646 (p = 4.86 × 10- 12) & rs1154155 (p = 1.90 × 10-13) are associated with NT1 [12]. This genome wide association study (GWAS) demonstrated an increase in relative risk of narcolepsy onset if any or a combination of these three SNPs are present. All three TRA SNPs are located within the encoding region of the J segment which is central to the process of VDJ recombination. During thymic education, T cells undergo somatic recombination to increase the diversity of the TcR. Recombination of both TRA and T cell receptor beta (TRB) chains results in the generation of T cell clones with a unique complete TcR. TRA recombination occurs between the 5’ end located within one of the 46 functional variable (V) regions and the 3’ end located in one of the 49 functional joining (J) segments. Added to this, variation is also introduced by amino-acid junctional diversity generated by N and P inclusions in the V-J boarder region. Diversity in the TRB is able to produce an even more expansive repertoire of proteins as it is a result of the recombination of 48V, 2D and 13J segments [13]. Identification of these SNPs via GWAS suggests that the mutated forms of these markers may be involved in non-random VJa choices during the process of recombination, however this remains unproven [12]. These findings coupled with the HLA- DQB1*06:02 association reinforce an indicative autoimmune basis of narcolepsy pathophysiology.
In 2009, an ImmunoChip consortium was formed to create a SNP array to target fine-mapping of loci associated with various autoimmune conditions [14]. The ImmunoChip was designed to allow deep replication from large-scale meta-analyses in nine autoimmune conditions. Using this platform, the consortium conducted a collaborative GWAS to identify genetic risk factors associated with narcolepsy in addition to HLA-DQB1*06:02 [14]. The group investigated a total of 111,240 SNP markers with a minor allele frequency of ≥ 1% which were located outside the HLA region on chromosome 6. 1,886 confirmed narcolepsy cases were analysed for these markers and compared against 10,421 healthy controls of mixed European, United States and Canadian ancestry. The results showed that the strongest association with narcolepsy was a SNP located on chromosome 14 in the TRA region (rs1154155). Further fine-mapping using the ImmunoChip showed that this SNP was located close to the J10 segment of the locus confirming the results published earlier that year [12]. There are, however, weaknesses in the ImmunoChip approach. Primarily, the chip is designed for use in white European populations and is therefore less informative for other ethnicities. Another limitation of this study lies with the use of the ImmunoChip in that it does not cover the whole genome and utilises data from earlier GWAS studies for the selection of markers included in the assay [15].
Other immunoregulatory genes
A polymorphism within the P2Y purinoceptor 11 (P2RY11) gene on chromosome 19 has also been reproducibly associated with an increased risk of narcolepsy onset [16-18]. In 2010, a GWAS investigation conducted by a worldwide collaboration in 7122 patients showed that a SNP located downstream of P2RY11 (rs2305795) was associated with the condition [17]. P2RY11 is a member of a large family of more than 20 purinergic receptors. The purinergic signalling pathway plays a fundamental role in immune regulation, proliferation, apoptosis and chemotaxis in lymphocytes and monocytes [19,20]. P2RY11 is a low affinity receptor and detects high concentrations of extracellular adenosine triphosphate (ATP) [17]. ATP is traditionally localised intracellularly, however, during inflammation, ATP levels rise within the extracellular space and produce a cascade of concentrationdependent effects on the immune system [21]. In humans, P2RY11 expression is marked in brain tissue and leucocytes, in particular CD8+ T cells and natural killer cells [22]. It has been suggested that immune cell death in the presence of high ATP is controlled by a balance of multiple purinergic receptors including P2RY11 [17]. Narcoleptic patients who carried the mutated version of P2RY11 had a less efficient P2RY11 function because the presence of the minor allele (A) of rs2305795 results in decreased P2RY11 expression and therefore decreased sensitivity of CD8+ T cells to an ATP-induced cell death. The association between the minor allele of P2RY11 and onset was only seen in individuals of European ancestry despite Asian and African American populations also being studied. Furthermore, P2RY11 expression is predominantly seen in CD8+ T cells which account for approximately ~20% of peripheral blood mononuclear cells (PBMCs). Within whole blood, P2RY11 is expressed in granulocytes, which account for 65% of all leucocytes and may reduce the ability to identify differences in the expression of P2RY11 in the CD8+ T cells. Hence, attempts to confirm a direct role in narcolepsy onset for this mutation in isolation may prove inconsequential.
Holm and co-workers showed that a nonsynonymous mutation in exon 21 of the DNA methyltransferase 1 (DNMT1) gene, also encoded on chromosome 19, causes a rare hereditary form of narcolepsy-associated deafness and cerebellar ataxia [23]. The P2RY11 and DNMT1 genes are in linkage so it was suggested that both mutations could be related at the pathophysiological level. This was proven not to be the case, but rs3826784 located in the intronic region of the eukaryotic initiation factor (EIF3G) gene was the most significant within the region investigated. Eukaryotic initiation factors initiate eukaryotic translation by forming a complex with the 40S ribosomal subunit and recruiting 43S to the preinitiation complex [24]. This complex recognises and binds the 5’ cap structure of mRNA and regulation of the AUG initiation codon [25]. The polymorphic EIF3G gene is in high LD with P2RY11 but not with DNMT1. DNMT1 also plays a role in the differentiation of CD4+ T cells into regulatory T cells (Tregs) [26]. A mutation causing a lack of expression of DNMT1 could consequently lead to an absence of Treg cells and may result in a loss of immunological tolerance. The discovery of the EIF3G mutation is of particular interest as findings have implicated a role of the H1N1 pandemic virus of 2009 as a trigger for narcolepsy development [16]. The disruption of the EIF pathways seen in H1N1 influenza cases could indirectly alter the immune response to the infection and increase narcolepsy risk and the severity of symptoms in individuals who are homozygous for the mutated variant of rs3826784 (G:G).
Cathepsins are enzymes primarily located within the lysosomal/endosomal compartment of professional antigen presenting cells (monocytes, dendritic cells, B cells) [27]. They play an important role in the exogenous pathway of antigen processing which concludes with the loading of peptides onto the HLA class II protein [27]. HLA class II is then expressed on the surface of a professional antigen presenting cell initiating cellular interactions leading to elimination of the pathogen. Deficiencies in selected cathepsins have been shown to impair immune cell development resulting in defective immune effector cell function [27]. Cathepsin H (CTSH) is one of eleven cathepsins and is unique with instrumental importance in both exopeptidase and endopeptidase activities. It is expressed at high levels in immune cells which are also HLA class II positive, most notably B cells, monocytes and dendritic cells. A global collaborative study of immune gene markers led by Faraco and co-workers in 1886 patients with narcolepsy identified two SNPs (rs34593439 and rs3825932) located in the intronic region of the gene encoding Cathespin H located on chromosome 15 [14]. The SNP rs3825932 has a C substituted for T introducing the negatively charged amino acid alanine that could affect trafficking or the cleavage of peptides for presentation. It is speculated that changes in CTSH may alter the HLA class II peptide repertoire that is presented to CD4+ T cells. This altered repertoire of presented peptides may promote the recognition of a self-antigen, increasing the risk of developing narcolepsy.
SNPs within the Tumour Necrosis Factor ligand superfamily member 4 (TNFSF4) have also been linked with narcolepsy [14]. Located on chromosome 1, rs7553711, identified using the ImmunoChip assay, was more strongly associated with the onset of narcolepsy in Faraco’s study than any other SNP [14]. Point mutations of the TNFSF4 gene are also strongly linked with other autoimmune conditions including systemic lupus erythematosus (SLE) and systemic sclerosis [28,29]. Polymorphisms upstream of the TNFSF4 gene confer susceptibility to SLE, whereas the polymorphisms identified in narcoleptic patients are located further downstream of rs7553711. The association of narcolepsy with SNPs in the TNFSF4 gene is consistent with a primary role of antigen presentation to T cells in the pathogenesis of narcolepsy. TNFSF4 is primarily expressed on the surface of HLA class II positive professional antigen presenting cells and acts as a costimulatory ligand during the formation of an immune synapse. Changes in the structure of TNFSF4 may therefore affect the configuration of this synapse and the subsequent immune response to infection, increasing the risk of an autoimmune condition developing, including narcolepsy.
The genetic factors mentioned all encode molecules which have an essential role in the immune system (Fig. 1). The altered immunogenetic factors create an immune environment which, in the presence of an environmental agent (e.g., an infection) combine to cause selective damage to the hypocretin producing neurons. However, despite growing evidence from genetic studies in global populations of patients with narcolepsy, the specific genetic and environmental triggers of narcolepsy onset remain undetermined.
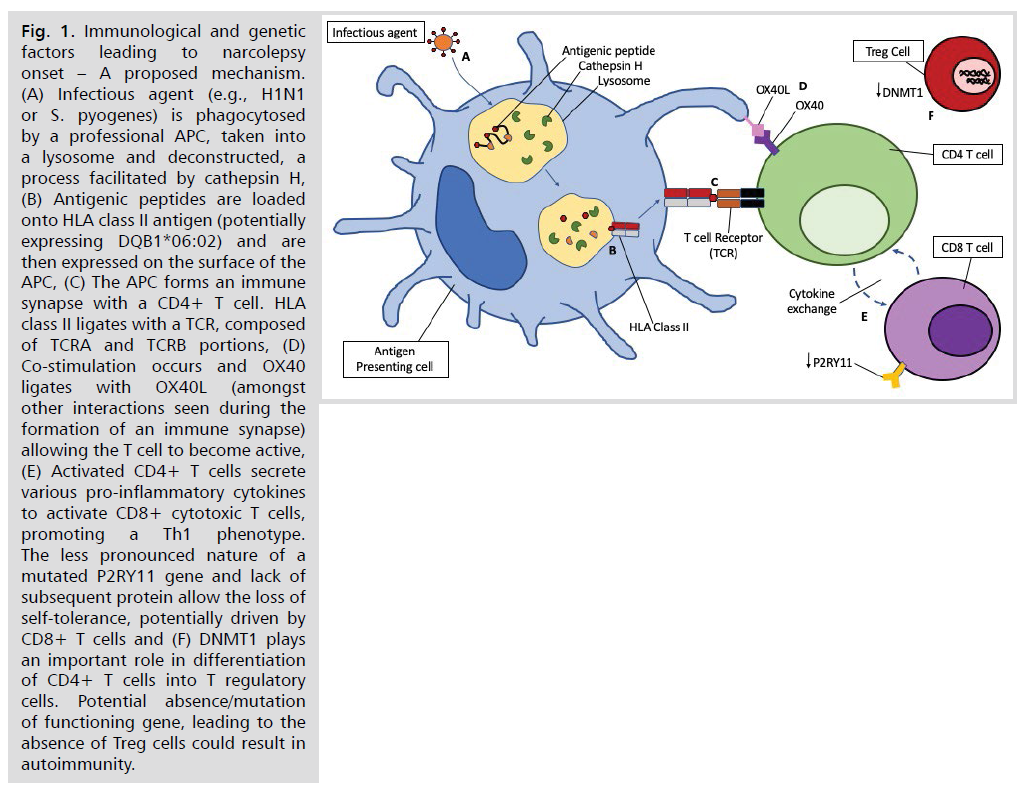
Fig 1: Immunological and genetic factors leading to narcolepsy onset – A proposed mechanism. (A) Infectious agent (e.g., H1N1 or S. pyogenes) is phagocytosed by a professional APC, taken into a lysosome and deconstructed, a process facilitated by cathepsin H, (B) Antigenic peptides are loaded onto HLA class II antigen (potentially expressing DQB1*06:02) and are then expressed on the surface of the APC, (C) The APC forms an immune synapse with a CD4+ T cell. HLA class II ligates with a TCR, composed of TCRA and TCRB portions, (D) Co-stimulation occurs and OX40 ligates with OX40L (amongst other interactions seen during the formation of an immune synapse) allowing the T cell to become active, (E) Activated CD4+ T cells secrete various pro-inflammatory cytokines to activate CD8+ cytotoxic T cells, promoting a Th1 phenotype.
The less pronounced nature of a mutated P2RY11 gene and lack of subsequent protein allow the loss of self-tolerance, potentially driven by CD8+ T cells and (F) DNMT1 plays an important role in differentiation of CD4+ T cells into T regulatory cells. Potential absence/mutation of functioning gene, leading to the absence of Treg cells could result in autoimmunity.
Environmental Risk Factors
Vaccines
The most widely documented potential environmental
trigger of narcolepsy is the activated monovalent vaccine
used between 2009 and 2010 to protect against the
H1N1 influenza virus. The H1N1 virus first emerged in
Mexico and California in April 2009 and quickly spread
globally and was declared a pandemic by the World Health Organisation in June 2009 [30]. A MF-59-adjuvanted
vaccine (Focteria) produced by Novartis and A (H1N1)
pdm09 termed Pandemrix produced by GlaxoSmithKline
were distributed to several European countries. Alternate
inactivated monovalent unadjuvanted vaccines were
administered in the United States of America, and the
AS03-adjuvanted Arepanrix was dispersed to Canada.
Arepanrix and Pandemrix had similar pH1N1 antigens and
used the same AS03 adjuvant, however the manufacturing
processes for the two vaccines differed. More than 1300
people who received the Pandemrix vaccine developed
narcolepsy [31]. GSK acknowledged the link and some
patients were awarded compensation. However, the
mechanism of action that led to narcolepsy onset following
vaccine administration remains unclear. Shortly after the
administration of Pandemrix (August 2010) the increased
incidence of NT1 was reported firstly in Finland [32],
then Sweden, followed by Norway, France, Ireland and
the United Kingdom [33-36]. The incidence reported was
seen mainly in children and young adolescents in certain
populations the relative risk for vaccinated versus nonvaccinated
was significantly higher [37].
A multi-country assessment of the incidence of
narcolepsy and the adjuvanted pandemic H1N1 vaccines
was published in 2018 [38]. Based on a case-control
analysis, it has been demonstrated that other than an
increased narcolepsy incidence rate after the vaccination
campaigns in Sweden, no other associations between AS03
or MF59 adjuvanted pH1N1 vaccines was seen [40].
Several limitations within this retrospective study include
weak statistical power due to low vaccine coverage in some
of the participating countries, diagnostic misclassification
due to differing procedures, and factors that may have
included selection bias.
The incidence of NT1 in China sharply increased 4
months after the 2009 H1N1 pandemic with rates returning
to normal immediately afterwards [16]. This led to an
observational study suggesting that genetic associations
with onset of a condition can change in response to a
new environmental trigger. The study strengthened the
association with SNPs located in the TRA and P2RY11-
DNMT1 complex and identified three new polymorphisms
associated with onset (rs9648789, rs10995245 &
rs2252931). A limitation with observational studies
like this one by Han and co-workers is that bias
cannot be completely excluded and no clear relationship
to the vaccine and narcolepsy onset is acknowledged.
Theoretically this may imply that the H1N1 virus alone
could be the triggering environmental factor in an Asian
population. This theory has been suggested by others
who demonstrated that the influenza virus is capable of
damaging hypocretin neurons in immunodepleted mice
resulting in a narcolepsy- resembling phenotype [39].
An international study conducted in 2015 set out to
investigate the effect of Pandemrix and determine potential
links to the onset of narcolepsy [40]. They concluded that
differences in the viral proteins used to construct the vaccine
may explain the association. The discovery of antibodies directed against a protein fragment contained within the
influenza virus used in the synthesis of the Pandemrix
vaccine supported this theory. The fragment, which is
part of the influenza nucleoprotein, cross reacted with
the hypocretin receptor 2 (HCRTR2), which has shown
to be associated with narcolepsy [41]. Levels of HCRTR2
antibodies were much higher in the sera of Finnish patients
who had been vaccinated with Pandemrix compared to sera
from normal Italian individuals who had been administered
Focteria to combat the H1N1 infection. Antibodies to
the HCRTR2 cross-reacted with influenza nucleoprotein
peptides in 17 of the 20 samples tested from people who
had since been diagnosed with narcolepsy following the
Pandemrix vaccine in 2009. Further studies revealed that
the influenza antigen content in the Pandemrix vaccine had
significantly higher levels of the influenza nucleoprotein
compared to Focteria, which had 72% less nucleoprotein
than Pandemrix. However, the production of antibodies
and subsequent destruction of the HCRTR2 was not
suggested as a cause of the condition. One hypothesis is
that the antibodies produced could occupy the HCRTR2
sites within the brain, preventing the binding of hypocretin
to its natural ligand [42]. It was also reported that five of the
twenty cases investigated had antibodies directed toward
the nucleoprotein but did not develop narcolepsy, posing
questions to the clinical relevance of these findings. Also,
despite discovering an antibody against HCRTR2 post
vaccine, it does not explain the selective loss of neurons
seen in narcolepsy.
An investigation into the differences between Pandemrix
and Arepanrix found a higher amount of structurally altered
viral nucleoprotein in Pandemrix than Arepanrix resulting
in an increased response to the nucleoprotein in narcoleptic
children [42]. A fundamental flaw of this study was that
the viral component of each vaccine was manufactured in
different countries so a direct comparison of the proteomic
differences cannot be performed. The purification methods
employed by the manufacturers differed which may explain
the variation in the structure. An additional study showed
significant associations between post-Pandemrix narcolepsy
cases and HLA class II genes as well as a SNP marker
(rs12587781) in the gene coding for the TRA protein
[37]. The genetic associations observed indicated a strong
gene-environment interaction in which either antigenic
constituents or the adjuvant within the vaccine used may
be involved. A comprehensive meta-analysis published in
2018 showed a 5- to 14-fold increase in the incidence of
narcolepsy in children and adolescents and a 3- to 7-fold
increase in adults in the countries where Pandemrix was
administered [43]. Based on the cases reported it was
calculated that the vaccine attributable risk of narcolepsy
was significant in children and adolescents (1 per 18,400).
The presence of HLA-DQB1*06:02 would increase this
risk to 1 case per 4500 doses administered.
Ultimately, the exact mechanism of vaccine trigged
narcolepsy remains unknown. The adjuvant AS03 suggested
by some research groups is an attractive model but other
vaccines containing the AS03 adjuvant are not associated with increased risk of narcolepsy [43]. The discovery
of antibodies directed toward the hypocretin receptor 2
in a group of Finnish patients who were administered
the Pandemrix vaccine implicates a molecular mimicry
hypothesis. These antibodies may react with the influenza
nucleoprotein but their role in the pathophysiology of
narcolepsy remains controversial. Another caveat to the
potential impact of the Pandemrix vaccine on the increased
risk of narcolepsy is that despite the clear relationship
between the two, narcolepsy is still considered a rare disease
and the benefits of the vaccine outweighs the associated risks
of narcolepsy. Of the approximately 40 million vaccinated
since the outbreak, 30 million received Pandemrix [44]
and of these, only 1,333 confirmed cases of narcolepsy were
reported [31] generating an incidence rate of 0.0044%.
Streptococcal infections
Streptococcal infections are usually benign and selflimiting,
but potentially invasive diseases with associated,
well documented post-streptococcal disorders [45]. Poststreptococcal
disease has been linked to neurological
autoimmune conditions including Sydenham chorea
[46] and encephalitis lethargica [47]. Taking this into
consideration it has been suggested that the onset of
streptococcal infections may initiate an autoimmune
response against hypocretin producing neurons leading to
the development of narcolepsy. It has been postulated that
the streptococcal infections may lead to the destruction
of neurons via molecular mimicry or via superantigen
interactions with the HLA-TCR complex [45]. Another
suggested hypothesis is that the streptococcal infections
increase the permeability of the blood brain barrier,
exposing the hypocretin-secreting neurons to the immune
system, allowing more specific factors to trigger narcolepsy
[45]. Streptococcal infections have been shown to increase
the production of IL-17, a cytokine mainly produced by
Th17 cells [48], which in turn has been shown to increase
the permeability of the blood brain barrier [49].
A population-based case-control study detected antistreptococcal
antibodies in 65% of patients within one year
of narcolepsy onset which were still elevated 1-3 years
later [45]. For comparison, in uncomplicated streptococcal
infections the antibody titres are reported to increase after
2 weeks, peak at 2-4 months and then decease [50]. The
persistence of these antibodies in narcoleptic patients may
be due to an inability to clear the infection, possibly as
a consequence of their genetic background which is able
to promote and sustain a narcolepsy inducing immune
reaction. This may also further qualify the role of the strongly
associated HLA-DQB1*06:02. It has been shown that the
presence of this HLA allele shows a degree of protection
against septic shock following streptococcal infections,
suggesting a superior immune response to this bacterium
[51]. These findings also suggest structural similarities
between Streptococcus pyogenes and hypocretin [5].
The cellular arm of the immune response has also been
shown to play a role in narcolepsy onset post- streptococcal
infection. Ambati and co-workers published a study in 2015 demonstrating evidence of an increase in the amount of
IFNγ in the peripheral circulation of confirmed narcolepsy
cases in response to stimulation with streptodornase B
(a streptococcal protein) and streptococcus M proteins
[52]. This suggests a pro-inflammatory Th1 phenotype
is involved in streptococcal induced narcolepsy cases. In
contradiction, no inflammatory processes, including
lymphocytic infiltration have been observed in the
hypothalamus during post-mortem brain analysis of longterm
disease sufferers putting this hypothesis into question
[53,54].
Pathophysiology
The underlying pathological mechanism of NT1 is
the loss of hypocretin signalling. The hypocretin system is
made up of ~70,000 neurons producing hypocretin located
in the hypothalamus. All narcoleptic patients are born with
these neurons and the hypocretin deficiency develops later
in life. The exact mechanism that leads to the destruction
of the hypocretin neurons in narcoleptic patients is still
unknown. The only potential proposed hypothesis for the
development of narcolepsy is due to a loss of immunological
tolerance-the autoimmune hypothesis. An autoimmune
basis for narcolepsy has long been suspected and data
from genetic, epidemiologic and immunologic studies
strongly suggest this to be the case. The development of
an autoimmune condition is multifactorial and includes
both underlying genetic predispositions combined with
an environmental trigger(s) (Fig. 2.). In narcolepsy, both
have been demonstrated with strong genetic associations
with HLA-DQB1*06:02 and other immune based
polymorphisms, as well as suggested environmental triggers
including streptococcal infections, H1N1 infections and
H1N1 vaccination.
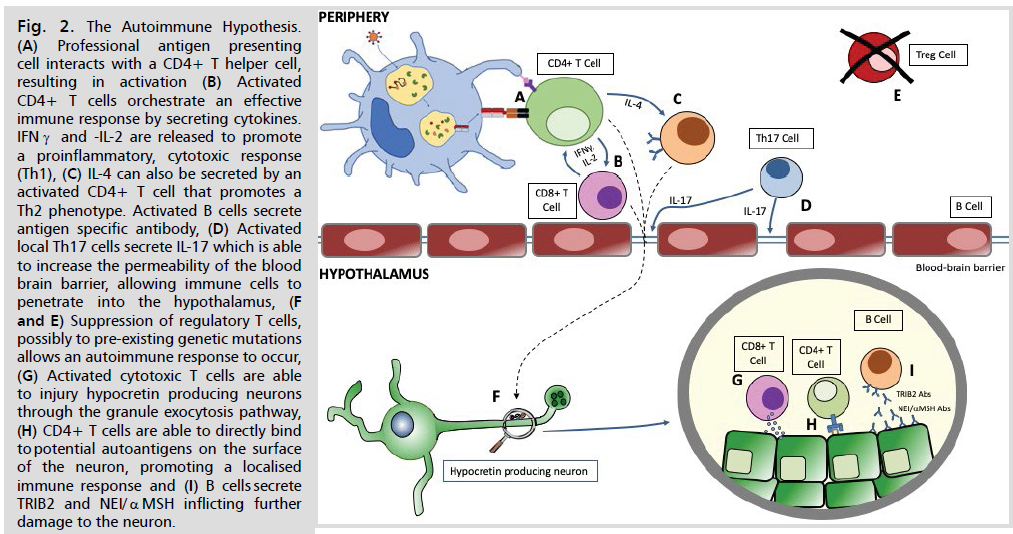
Fig 2: The Autoimmune Hypothesis. (A) Professional antigen presenting cell interacts with a CD4+ T helper cell, resulting in activation (B) Activated CD4+ T cells orchestrate an effective immune response by secreting cytokines. IFNγ and -IL-2 are released to promote a proinflammatory, cytotoxic response (Th1), (C) IL-4 can also be secreted by an activated CD4+ T cell that promotes a Th2 phenotype. Activated B cells secrete antigen specific antibody, (D) Activated local Th17 cells secrete IL-17 which is able to increase the permeability of the blood brain barrier, allowing immune cells to penetrate into the hypothalamus, (F and E) Suppression of regulatory T cells, possibly to pre-existing genetic mutations allows an autoimmune response to occur, (G) Activated cytotoxic T cells are able to injury hypocretin producing neurons through the granule exocytosis pathway, (H) CD4+ T cells are able to directly bind to potential autoantigens on the surface of the neuron, promoting a localised immune response and (I) B cells secrete TRIB2 and NEI/αMSH inflicting further
damage to the neuron.
In order to prove that a disease or condition is the
result of a loss of immunological tolerance, the presence
of autoreactive immune cells or autoantibodies must be
demonstrated and an autoantigen identified. Despite
many years of research, it remains unclear how the genetic
differences and environmental triggers actually contribute
to the onset of this condition. As a result, very few studies in
narcolepsy have produced confounding evidence to establish
the autoimmune pathogenesis. In 2010, two independent
studies showed the presence of antibodies against tribbles
homologue 2 (TRIB2)-a protein produced by many cells
including hypocretin neurons in narcoleptic patients [55].
These autoantibodies were originally described in uveitis
and were found to have higher titres in a small group of
narcoleptic patients when compared to healthy controls
[56]. This was the first study to report the impact of the
humoral immune response in narcolepsy. The impact of
TRIB2 autoantibodies is debatable and other studies have
shown that the levels of these antibodies are not increased
in confirmed narcolepsy cases [57]. Also, as TRIB2 is
expressed on other cell populations both in the CNS and
periphery, these antibodies are unlikely to have a direct role
in the onset of narcolepsy. TRIB2 antibodies have also been detected in individuals who do not suffer from narcolepsy
adding further question to the relationship between TRIB2
antibodies and disease onset. It has been postulated that
TRIB2 antibodies may be produced as a downstream
effect of neuron loss. Passive transfer of antibodies from
narcoleptic patients to murine models leads to sleep
behavioral disturbances or neurological changes [58] but
the specific mechanism by which these antibodies are able
to induce changes in the brain remains unknown.
Autoantibodies directed towards the C-terminal epitope
of neuropeptide glutamic acid-isoleucine/α-melanocytestimulating
hormone (NEI/αMSH) have also been
identified in narcoleptic patients following vaccination
with Pandemrix [59]. Investigation of narcolepsy patients’
sera or cerebral spinal fluid using immunohistochemistry
found a significant number to have NEI/αMSH antibodies
which were passively transferred into rats and their
electroencephalography (EEG) and behaviour analysed.
Results showed an increase in sleep fragmentation when
purified IgG antibodies were transferred from narcoleptic
patients that was not observed for the control group.
Apart from sleep fragmentation, no other symptoms
of narcolepsy were seen [59]. Despite the presence of
potential autoantibodies in narcoleptic cases, none of
the corresponding antigens (TRIB2, NEI/αMSH, anti
HCRT receptor 2) are exclusively expressed in hypocretin
producing neurons. This, plus the observation that these
antibodies have been detected in some healthy controls,
creates debate as to their role in the pathogenesis of
narcolepsy.
In 2013, an article by De la Herran-Arita and coworkers
was published suggesting that hypocretin was an
autoantigen. The group demonstrated functional analysis
of CD4+ T cells from narcoleptic patients that were able to
recognise hypocretin peptides presented by dendritic cells
homozygous for the heterodimer HLA-DQB1*06:02/
DQA1*01:02. These cells could also recognise peptides derived from H1N1 suggesting a potential link due to
molecular mimicry between hypocretin peptides and
similar peptides from H1N1. Unfortunately, the authors
were unable to replicate the findings resulting in the
retraction of the article [60].
Different studies of the autoimmune origins of
narcolepsy have yielded alternative hypotheses to
determine the etiology of the condition. Based on the
facts that there are both genetic and environmental links
with the disease there is a strong evidence base to support
narcolepsy being an autoimmune condition. The majority
of the evidence that contributes to this theory suggests
that it is mainly driven by the humoral response. This is
further supported by the absence of autoreactive CD4+ T
cells in confirmed narcoleptic cases [61] and a lack of T
cell infiltrations in affected hypothalamic areas seen in the
majority of post-mortems [62]. In the absence of evidence,
a defined cellular response cannot be completely ruled out.
The lack of cellular infiltrations seen during post mortems
could simply be explained if the neurological damage was
historic, completed many years previously and recent
studies which have shown a global increase of activated T
cells in NT1 patients retain the potential for a role of the
cellular immune response [63,64]. Nevertheless, crucial
pieces of information are missing to fulfil the autoimmune
hypothesis, including the lack of an autoantigen, a selfderived
structure for the immune system to target. Further
work into the autoimmune pathogenesis is required to
determine the autoantigen in a condition that shows all the
traditional aspects of an autoimmune condition without
the essential trigger.
Diagnosis
The current diagnostic classification for narcolepsy
is outlined in the third edition of the International
Classification of Sleep Disorders (ICSD3) published in 2014
[7]. Often patients attend sleep clinics long after symptom onset and often following several incorrect diagnoses.
The diagnosis of narcolepsy may be suspected from the
clinical history of the patient but is confirmed with an
overnight polysomnography, followed by a multiple sleep
latency test (MSLT). The overnight sleep study helps to
rule out other potential causes of daytime sleepiness and
in people with narcolepsy it may reveal fragmented, light
sleep and an early (within the first 15 minutes) transition
into rapid eye movement (REM) sleep. During the MSLT
the patient is encouraged to fall asleep every 2 hours for
20 minutes over an 8 to 10-hour period. Patients who
suffer with narcolepsy will tend to fall asleep in less than
8 minutes compared to healthy individuals who take on
average a minimum of 15 minutes [65]. Combined with
the shorter time to fall asleep, patients with narcolepsy
often have REM sleep during at least two of the daytime
naps during the MSLT, whereas healthy individuals rarely
have any daytime REM sleep [66]. The presence of sleeponset
REM periods (SOREMPs)-the occurrence of
REM sleep within 15 minutes is key in the diagnosis of
narcolepsy. The MSLT is the gold standard in the diagnosis
of narcolepsy and as a result must be performed under the
strictly defined conditions. Medications that suppress REM
sleep should be discontinued well in advance of the MSLT
and the patient should try to attain a sufficient amount
of sleep the night before the test. A significant group of
patients tested do not fully meet the criteria for a diagnosis
of narcolepsy. This group of patients show and suffer the
same symptoms as narcoleptic patients (excessive daytime
sleepiness, hypnagogic hallucinations, hypnopomphic
hallucinations) but fail the MSLT (lack of two incidences
of REM sleep). These patients are often diagnosed with
idiopathic hypersomnia and the symptoms are managed
with a working diagnosis of NT2.
Despite the clear and strong link between HLADQB1*
06:02 and narcolepsy, the presence of this allele
is of limited diagnostic value due to a high background
population frequency, it is more beneficial as a negative
predictor. Measuring hypocretin 1 levels in the CSF is a
highly specific and sensitive technique for the diagnosis of
narcolepsy (NT1 only). When compared to a recognised
reference standard, hypocretin 1 levels of ≤110pg per ml
is highly specific for a diagnosis of NT1 [7]. Care must
be taken when interpreting these results as low hypocretin
1 values in the CSF are also seen in patients suffering
other non-sleep related conditions [67]. CSF levels must,
therefore, be used to help build a diagnosis when combined
with the MLST and overnight polysomnography.
The Brighton Collaboration in 2013 published the
criteria for the diagnostic accuracy of narcolepsy for patients
who showed symptoms of the condition post vaccination
with Pandemrix [68]. The case definition and levels of
severity are broken down into three levels and covers criteria
including the presence of excessive daytime sleepiness, CSF
hypocretin deficiency, results of the MSLT and SOREMP.
The recently developed Narcolepsy Severity Scale (NSS), a self-administered scale that evaluates the severity of the
condition is a welcomed and accurate approach to assess
NT1 symptom severity, however the NSS is only useful
following the confirmation of disease diagnosis [69].
Treatment
The management of narcoleptic patients can be achieved
using both pharmacological and non- pharmacological
agents. Often a combination of both are employed in order
to manage the condition. Non-pharmacological agents
largely consist of developing set behavioural strategies.
Each strategy is individualised to each patient and consists
of avoiding sedentary activities, maintaining a regular night
time routine and planning daytime naps [70]. Caffeine
and energy drinks are also often consumed to fight bouts
of excessive daytime sleepiness but are not sustainable and
have been shown to have side effects which are detrimental
to subsequent sleep [71].
Antidepressants are widely used for the treatment of
NT1, particularly for the treatment of cataplexy. However,
despite their popularity, antidepressants have never been
proven for indication in narcolepsy in controlled clinical
trials. Antidepressants act by blocking norepinephrine and
serotonin reuptake resulting in a reduction in REM sleep
and cataplexy [72,73]. Both serotonin and norepinephrine
increase alertness, promote vigilance and focus attention.
Antidepressants are well tolerated and very few suffer side
effects although some experience issues including weight
gain, diarrhoea and anorexia [73]. There is however a severe
risk of cataplexy rebound if antidepressants are suddenly
removed from a patient's treatment plan [74].
Various pharmacological agents are approved by the
Food and Drug Administration (FDA) and European
Medicines Agency (EMA) that are available for NT1
sufferers. Modafinil is one of the first lines of treatment of
narcolepsy and helps promote wakefulness; however, the
mechanism of action remains undetermined. The drug is
long lasting with a half-life of 13.8 hours and the maximum
concentration in the periphery is achieved in 2-4 hours
[73]. Overall, Modafinil is well tolerated in both adults and
children and side effects are rare and mild. Modafinil can
be combined with other agents such as sodium oxybate
to improve narcolepsy management. Sodium oxybate
is the sodium salt of gamma hydroxybutyrate and is seen
as another front-line therapy. Sodium oxybate activates
the GABA type B receptor, but the precise mechanism of
sodium oxybate remains to be elucidated [75]. Sodium
oxybate has been shown to effectively improve sleep
quality, increase slow-wave sleep and promote wakefulness
[70]. Additionally, sodium oxybate is the only medication
approved by the FDA and EMA for the treatment of
cataplexy. However, sodium oxybate is not approved in the
UK, and NICE approval is required. Randomised control
trials and meta-analysis have shown that sodium oxybate
is currently the only available drug that can improve all of the core narcolepsy symptoms, and reduce the frequency of
cataplectic episodes [76]. The half-life of sodium oxybate
is only 40-60 minutes but the effects of the drug persist
much longer [73]. The administration of the drug is a
staged process, with low amounts (4.5g/night) taken
initially and then increased over a number of subsequent
weeks (increase of further 1.5g/night) to achieve the desired
dosage (max 9g/night). The drug is well tolerated if titrated
correctly, but adverse side effects have been observed (if
incorrectly titrated) including dizziness, headache, nausea,
weight loss and depression. Due to the power of sedation of
this drug, patients are advised to administer at the bedside.
When both modafinil and sodium oxybate are
ineffective, second line therapies like methylphenidate can
be administered. Methylphenidate (also known as Ritalin)
is a stimulant that blocks the re-uptake of monoamines,
primarily dopamine, allowing it to remain in circulation
for longer. Despite its wide utilization only one evidencebased
study has shown the significance of methylphenidate
in the treatment of narcolepsy [70]. Again, like modafinil
and sodium oxybate, the incidence of side effects is low
but some users can suffer tachycardia, hypertension,
anxiety and weight loss [77]. Another pharmacological
agent recently approved by the EMA but not the FDA is
Pitolisant. Pitolisant is a histamine H3 receptor agonist
able to activate histamine neurons and has been shown
to improve wakefulness in healthily animals and decrease
daytime sleepiness in orexin knockout mice [78]. Like
methylphenidate, Pitolisant is seen as an alternative therapy
if modafinil and sodium oxybate are ineffective. The drug
is generally well tolerated by both adults and children.
Amphetamines are considered a third line therapy for
narcolepsy. They are able to increase the concentration
of dopamine and norepinephrine and have been shown
to reduce excessive daytime sleepiness. However,
dextroamphetamine is the only amphetamine approved for
treatment of narcolepsy [73,79].
The treatment options for NT2 include modafinil,
sodium oxybate and methylphenidate as the main symptom
is excessive daytime sleepiness, but often less severe than
NT1. The reduced severity of symptoms is often reflected
in the lower dosages administered.
Future Directions
The past several years has seen great advances in
understanding the pathogenesis, underlying genetics,
and development of new therapeutic agents. However,
many questions remain unanswered. Future work should
be directed toward the clarification of the pathological
process that destroys the hypocretin producing neurons.
All the current data suggests that NT1 is an autoimmune
condition. All the factors are present for this to be the case; the genetic association, an environmental trigger and an
increase in peripheral immune cells have been classified.
Despite this, the lack of the elusive autoantigen remains
a constant frustration of researchers. Discovery of an
autoantigen would aid in the production of new effective
therapeutic drugs that target the condition rather than
treating the symptoms and may potentially result in the
development of a cure.
Interestingly, despite the increasing evidence promoting
an autoimmune hypothesis little success has been made
with treatments that are able to manipulate the immune
system. Therapies including corticoids, intravenous
immunoglobulin, plasmapheresis, immunoadsorption
have all been tested with variable efficacy [80-82]. These
therapies target the humoral arm of the immune response
potentially suggesting that antibodies are weakly associated
with the development of narcolepsy. However, a single
patient case study was published showing that intravenous
immunoglobulin treatment administered 15 days after
disease onset fully reversed the symptoms of the narcolepsy
and cataplexy [83]. This highlights the importance of early
diagnosis and if treated early the symptoms may be reversed
and enough hypocretin neurons may be saved to prevent
progression to disease. Future work should also focus on
the adaptive side of the immune system to determine how
significant a role it plays in development of narcolepsy and
immunomodulatory agents may be synthesised as a result.
For NT2, very little is known and the condition is
poorly defined. Further work in this area should focus
on the pathogenesis and the causative agents. The main
issue in narrowing the gap in knowledge of NT2 is the
poor classification of the condition in a group of highly
heterogenous patients. Genetic profiling of patients
suffering from NT2 or idiopathic hypersomnia may
provide further clarification on the condition and should
be transferred into the clinical setting. The severity of
the condition may be linked to the number of genetic
mutations an individual possesses or the strength of the
initial environmental trigger or a combination of both.
A clearer understanding pre-diagnosis combined with
the NSS may allow for a more specific and less invasive
diagnosis as well as giving the clinical team scope to tailor
the treatment options.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to acknowledge VH Bio Limited who
provided the educational grant.
REFERENCES
- Partinen M, Kornum BR, Plazzi G, et al. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol. 2014;13(6):600-613.
Google Scholar, Crossref, Indexed at
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369(9560):499- 511.
Google Scholar, Crossref, Indexed at
- Mignot E. Genetic and familial aspects of narcolepsy. Neurology. 1998;50(2 Suppl 1):S16-S22.
Google Scholar, Crossref
- Schiappa C, Scarpelli S, Atri AD, et al. Narcolepsy and emotional experience: a review of the literature. Behav Brain Funct. 2018;14(1):19.
Google Scholar, Crossref, Indexed at
- Seong MJ, Hong SB. Autoimmunity and Immunotherapy in Narcolepsy. Sleep Med Res. 2017;8(1):1-7.
Google Scholar, Crossref, Indexed at
- Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5): 573-585.
Google Scholar, Crossref
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387-1394.
Google Scholar, Crossref, Indexed at
- Silber MH, Krahn LE, Olson EJ, et al. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population- based study. Sleep. 2002;25(2):197-202.
Google Scholar, Crossref, Indexed at
- Mignot E, Lin L, Rogers W, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68(3): 686-699.
Google Scholar, Crossref
- Rogers AE, Meehan J, Guilleminault C, et al. HLA DR15 (DR2) and DQB1*0602 typing studies in 188 narcoleptic patients with cataplexy. Neurology. 1997;48(6):1550-1556.
Google Scholar, Crossref, Indexed at
- Tafti M, Lammers GJ, Dauvilliers Y, et al. Narcolepsy-Associated HLA Class I Alleles Implicate Cell-Mediated Cytotoxicity. Sleep. 2016;39(3):581-587.
Google Scholar, Crossref, Indexed at
- Hallmayer J, Faraco J, Lin L, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41(6):708-711.
Google Scholar, Crossref
- Lefranc MP, Giudicelli V, Ginestoux C, et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37:D1006-D1012.
Google Scholar, Crossref, Indexed at
- Faraco J, Lin L, Kornum BR, et al. ImmunoChip study implicates antigen presentation to T cells in narcolepsy. PLoS genetics. 2013; 14;9(2):e1003270.
Google Scholar, Crossref, Indexed at
- Cortes A, Brown MA. Promise and pitfalls of the Immunochip. Arthritis Res. Ther. 2011;13(1):1-3.
Google Scholar, Crossref, Indexed at
- Han F, Faraco J, Dong XS, et al. Genome wide analysis of narcolepsy in China implicates novel immune loci and reveals changes in association prior to versus after the 2009 H1N1 influenza pandemic. PLoS genetics. 2013;9(10):e1003880.
Google Scholar, Crossref, Indexed at
- Kornum BR, Kawashima M, Faraco J, et al. Common variants in P2RY11 are associated with narcolepsy. Nat. Genet. 2011;43(1):66-71.
Google Scholar, Crossref, Indexed at
- Degn M, Dauvilliers Y, Dreisig K, et al. Rare missense mutations in P2RY11 in narcolepsy with cataplexy. Brain. 2017;140(6):1657-1668.
Google Scholar Crossref, Indexed at
- Di Virgilio F, Boeynaems JM, Robson SC. Extracellular nucleotides as negative modulators of immunity. Curr Opin Pharmacol. 2009;9(4):507-13.
Google Scholar, Crossref Indexed at
- Bours MJ, Swennen EL, Di Virgilio F, et al. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358-404.
Google Scholar Crossref, Indexed at
- Bodin P, Burnstock G. Increased release of ATP from endothelial cells during acute inflammation. Inflamm Res. 1998;47(8):351-354.
Google Scholar, Crossref, Indexed at
- Moore DJ, Chambers JK, Wahlin JP, et al. Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription–polymerase chain reaction study. Biochim Biophys Acta. 2001;1521(1-3):107-119.
Google Scholar, Crossref, Indexed at
- Holm A, Lin L, Faraco J, et al. EIF3G is associated with narcolepsy across ethnicities. Eur J Hum Genet. 2015;23(11):1573-1580.
Google Scholar, Crossref, Indexed at
- Zhou M, Sandercock AM, Fraser CS, et al. Mass spectrometry reveals modularity and a complete subunit interaction map of the eukaryotic translation factor eIF3. Proc Nat Acad Sci. 2008;105(47):18139-18144.
Google Scholar, Crossref
- Hinnebusch AG. eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem Sci. 2006;31(10):553-562.
Google Scholar, Crossref, Indexed at
- Josefowicz SZ, Wilson CB, Rudensky AY. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J Immunol. 2009;182(11):6648-6652.
Google Scholar, Crossref, Indexed at
- Colbert JD, Matthews SP, Miller G, et al. Diverse regulatory roles for lysosomal proteases in the immune response. Eur J Immunol. 2009;39(11):2955-2965.
Google Scholar, Crossref, Indexed at
- Graham DS, Graham RR, Manku H,et al. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nat Genet. 2008;40(1):83-89.
Google Scholar, Crossref, Indexed at
- Gourh P, Arnett FC, Tan FK,et al. Association of TNFSF4 (OX40L) polymorphisms with susceptibility to systemic sclerosis. ARD. 2010;69(3):550-555.
Google Scholar, Crossref, Indexed at
- Broadbent AJ, Subbarao K. Influenza virus vaccines: lessons from the 2009 H1N1 pandemic. Curr Opin Virol. 2011;1(4):254-262.
Google Scholar, Crossref, Indexed at
- Gadroen K, Straus SMJM, Pacurariu A, et al. Patterns of spontaneous reports on narcolepsy following administration of pandemic influenza vaccine; a case series of individual case safety reports in Eudravigilance. Vaccine. 2016;34(41):4892-4897.
Google Scholar, Crossref
- Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7(3):e33536.
Google Scholar, Crossref, Indexed at
- Bardage C, Persson I, Örtqvist A, et al. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ. 2011;343:d5956.
Google Scholar, Crossref
- Heier MS, Gautvik KM, Wannag E, et al. Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Med. 2013;14(9):867-871.
Google Scholar, Crossref
- Dauvilliers Y, Arnulf I, Lecendreux M, et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain. 2013;136(Pt 8):2486-2496.
Google Scholar, Crossref, Indexed at
- O'Flanagan D, Barret AS, FoleyM, et al. Investigation of an association between onset of narcolepsy and vaccination with pandemic influenza vaccine, Ireland April 2009-December 2010. Euro Surveill. 2014;19(17):15-25.
Google Scholar, Indexed at
- Bomfim IL, Lamb F, Fink K, et al. The immunogenetics of narcolepsy associated with A(H1N1)pdm09 vaccination (Pandemrix) supports a potent gene-environment interaction. Genes Immun. 2017;18(2):75-81.
Google Scholar, Crossref, Indexed at
- Weibel D, Sturkenboom M, Black S, et al. Narcolepsy and adjuvanted pandemic influenza A (H1N1) 2009 vaccines - Multi- country assessment. Vaccine. 2018;36(41):6202-6211.
Google Scholar, Crossref, Indexed at
- Tesoriero C, Codita A, Zhang MD, et al. H1N1 influenza virus induces narcolepsy-like sleep disruption and targets sleep- wake regulatory neurons in mice. Proc Natl Acad Sci. 2016;113(3):E368-E377.
Google Scholar, Crossref
- Ahmed SS, Volkmuth W, Duca J, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med. 2015;7(294):294ra105.
Google Scholar, Crossref
- Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365-376.
Google Scholar, Crossref, Indexed at
- Vaarala O, Vuorela A, Partinen M, et al. Antigenic differences between AS03 adjuvanted influenza A (H1N1) pandemic vaccines: implications for pandemrix-associated narcolepsy risk. PLoS One. 2014;9(12):e114361.
Google Scholar, Crossref, Indexed at
- Sarkanen TO, Alakuijala APE, Dauvilliers YA, et al. Incidence of narcolepsy after H1N1 influenza and vaccinations: Systematic review and meta-analysis. Sleep Med Rev. 2018;38:177-186.
Google Scholar, Crossref, Indexed at
- Verstraeten T, Cohet C, Santos GD, et al. Pandemrix and narcolepsy: A critical appraisal of the observational studies. Hum Vaccin Immunother. 2016;12(1):187-193.
Google Scholar, Crossref, Indexed at
- Aran A, Lin L, Nevsimalova S, et al. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32(8):979-983.
Google Scholar, Crossref, Indexed at
- Zomorrodi A, Wald ER. Sydenham's chorea in western Pennsylvania. Pediatrics. 2006;117(4):e675-e679.
Google Scholar, Crossref, Indexed at
- Vincent A. Encephalitis lethargica: part of a spectrum of post-streptococcal autoimmune diseases? Brain. 2004;127(Pt 1):2-3.
Google Scholar, Crossref
- Lundgren A, Bhuiyan T, Novak D, et al. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine. 2012;30(26):3897-3907.
Google Scholar, Crossref, Indexed at
- Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173-1175.
Google Scholar, Crossref, Indexed at
- Kaplan EL, Ferrieri P, Wannamaker LW. Comparison of the antibody response to streptococcal cellular and extracellular antigens in acute pharyngitis. J Pediatr. 1974;84(1):21-28.
Google Scholar, Crossref, Indexed at
- Bondinas GP, Moustakas AK, Papadopoulos GK. The spectrum of HLA-DQ and HLA-DR alleles, 2006: a listing correlating sequence and structure with function. Immunogenetics. 2007;59(7):539-553.
Google Scholar, Crossref, Indexed at
- Ambati A, Poiret T, Svahn BM, et al. Increased beta-haemolytic group A streptococcal M6 serotype and streptodornase B-specific cellular immune responses in Swedish narcolepsy cases. J Intern Med. 2015;278(3):264-276.
Google Scholar, Crossref, Indexed at
- Hong SC, Hayduk R, Lim J, et al. Clinical and polysomnographic features in DQB1*0602 positive and negative narcolepsy patients: results from the modafinil clinical trial. Sleep Med. 2000;1(1):33-39.
Google Scholar, Crossref
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991-997.
Google Scholar, Crossref, Indexed at
- Cvetkovic-Lopes V, Bayer L, Dorsaz S, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120(3):713-719.
Google Scholar, Crossref, Indexed at
- Kawashima M, Lin L, Tanaka S, et al. Anti-Tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy are associated with recent onset of cataplexy. Sleep. 2010;33(7):869-874.
Google Scholar, Crossref, Indexed at
- Lind A, Ramelius A, Olsson T, et al. A/H1N1 antibodies and TRIB2 autoantibodies in narcolepsy patients diagnosed in conjunction with the Pandemrix vaccination campaign in Sweden 2009-2010. J Autoimmun. 2014;50:99-106.
Google Scholar, Crossref, Indexed at
- Katzav A, Arango MT, Kivity S, et al. Passive transfer of narcolepsy: anti-TRIB2 autoantibody positive patient IgG causes hypothalamic orexin neuron loss and sleep attacks in mice. J Autoimmun. 2013;45:24-30.
Google Scholar, Crossref, Indexed at
- Bergman P, Adori C, Vas S, et al. Narcolepsy patients have antibodies that stain distinct cell populations in rat brain and influence sleep patterns. Proc Natl Acad Sci. 2014;111(35):E3735-E3744.
Google Scholar, Crossref, Indexed at
- De la Herran-Arita AK, Kornum BR, Mahlios J, et al. Retraction of the research article: "CD4(+) T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy". Sci Transl Med. 2014;6(247):247rt1.
Google Scholar, Crossref
- Kornum BR, Burgdorf SK, Holm A, et al. Absence of autoreactive CD4(+) T-cells targeting HLA-DQA1*01:02/DQB1*06:02 restricted hypocretin/orexin epitopes in narcolepsy type 1 when detected by EliSpot. J Neuroimmunol. 2017;309:7-11.
Google Scholar, Crossref, Indexed at
- Ramberger M, Högl B, Stefani A, et al. CD4+ T-cell reactivity to orexin/hypocretin in patients with narcolepsy type 1. Sleep. 2017;40(3):zsw070.
Google Scholar, Crossref, Indexed at
- Lecendreux M, Churlaud G, Pitoiset F, et al. Narcolepsy type 1 is associated with a systemic increase and activation of regulatory t cells and with a systemic activation of global t cells. PLoS One. 2017;12(1):e0169836.
Google Scholar, Crossref, Indexed at
- Hartmann FJ, Bernard-Valnet R, Quériault C, et al. High-dimensional single-cell analysis reveals the immune signature of narcolepsy. J Exp Med. 2016;213(12):2621-2633.
Google Scholar, Crossref, Indexed at
- Dement W, Rechtschaffen A, Gulevich G. The nature of the narcoleptic sleep attack. Neurol. 1966;16(1):18-33.
Google Scholar, Crossref, Indexed at
- Vogel G. Studies in psychophysiology of dreams. III. The dream of narcolepsy. Arch Gen Psychiatry. 1960;3:421-428.
Google Scholar, Crossref, Indexed at
- Kornum BR. Narcolepsy. Nat Rev Dis Primers. 2017;3:16100.
- Poli F, Overeem S, Lammers GJ, et al. Narcolepsy as an adverse event following immunization: case definition and guidelines for data collection, analysis and presentation. Vaccine. 2013;31(6):994-1007.
Google Scholar, Crossref, Indexed at
- Dauvilliers Y, Barateau L, Lopez R, et al. Narcolepsy Severity Scale: a reliable tool assessing symptom severity and consequences. Sleep. 2020;43(6):zsaa009.
Google Scholar, Crossref, Indexed at
- Barateau L, Lopez R, Dauvilliers Y. Treatment Options for Narcolepsy. CNS Drugs. 2016;30(5):369-379.
Google Scholar, Crossref, Indexed at
- Snel J, Lorist MM. Effects of caffeine on sleep and cognition. Prog Brain Res. 2011;190:105-117.
Google Scholar, Crossref
- Lopez R, Dauvilliers Y. Pharmacotherapy options for cataplexy. Expert Opin Pharmacother. 2013;14(7):895-903.
Google Scholar, Crossref, Indexed at
- Billiard M, Bassetti C, Dauvilliers Y, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13(10):1035-1048.
Google Scholar, Crossref, Indexed at
- Poryazova R, Siccoli M, Werth E, et al. Unusually prolonged rebound cataplexy after withdrawal of fluoxetine. Neurology. 2005;65(6):967-968.
Google Scholar, Crossref, Indexed at
- Huang YS, Guilleminault C. Narcolepsy: action of two gamma-aminobutyric acid type B agonists, baclofen and sodium oxybate. Pediatr Neurol. 2009;41(1):9-16.
Google Scholar, Crossref
- Boscolo-Berto R, Viel G, Montagnese S, et al. Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2012;16(5):431-443.
Google Scholar, Crossref, Indexed at
- Leonard BE, Denise MC, John W, et al. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum Psychopharmacol. 2004;19(3):151-180.
Google Scholar, Crossref
- Lin JS, Dauvilliers Y, Arnulf I, et al. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin-/- mice and patients. Neurobiol Dis. 2008;30(1):74-83.
Google Scholar, Crossref, Indexed at
- Thorpy MJ, Dauvilliers Y. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med. 2015;16(1):9-18.
Google Scholar, Crossref, Indexed at
- Fronczek R, Verschuuren J, Lammers GJ. Response to intravenous immunoglobulins and placebo in a patient with narcolepsy with cataplexy. J Neurol. 2007;254(11):1607-1608.
Google Scholar, Crossref, Indexed at
- Valko PO, Khatami R, Baumann CR, et al. No persistent effect of intravenous immunoglobulins in patients with narcolepsy with cataplexy. J Neurol. 2008;255(12):1900-1903.
Google Scholar, Crossref, Indexed at
- Plazzi G, Poli F, Franceschini C, et al. Intravenous high-dose immunoglobulin treatment in recent onset childhood narcolepsy with cataplexy. J Neurol. 2008;255(10):1549-1554.
Google Scholar, Crossref, Indexed at
- Dauvilliers Y, Abril B, Mas E, et al. Normalization of hypocretin-1 in narcolepsy after intravenous immunoglobulin treatment. Neurology. 2009;73(16):1333-1334.
Google Scholar, Crossref, Indexed at







