Keywords
Trigonella foenum-graecum; Alloxan; Hypoglycemic effect; Dose dependency; Source dependency; Safety profile
Introduction
Diabetes mellitus (DM) is a multi-factorial, chronic endocrinological disorder and affects millions of people around the globe. It is characterized by hyperglycemia and glucose intolerance due to defective insulin secretion or defective insulin action or can be both [1]. According to an approximation of the World Health Organization (WHO), around 171 million people were affected by this disorder in 2000. It is predicted that the number will be doubled by the year 2030 [2-5]. Reasons behind this global prevalence attributed to [6] unhealthy diet, obesity, growth of aged population, and sedentary lifestyle [7]. The condition can be ameliorated by proper medical treatment and lifestyle changes. The currently available conventional classes of drugs for the treatment of hyperglycemia include insulin secretagogues sulfonylureas, meglitnides), insulin senstizers (biguanides, metformin, thiazolidinediones), and α- glucosidase inhibitor (miglitol, acarbose [8]. However, most glucose-lowering drugs induce side effects such as lactic acidosis, idiosyncratic liver cell injury, severe hypoglycemia, digestive discomfort, permanent neurological deficit, dizziness, headache, weight gain, edema, anemia, and swelling of legs or ankles [9-14]. The chronic nature, along with its relative complications making diabetes a costly disease [15] and impacting the whole socio-economic condition of a country. Furthermore, diabetes management without any side effects is still a challenge to the medical fraternity [16]. The Traditional medicinal plants with multifarious active principles and properties have been used long before prehistoric period to treat a great variety of human diseases, including diabetes [17]. Moreover, its lower cost and no or minimal side effects have attracted many healthcare professionals. Many medicinal plants have been identified as a fundamental source of potent anti-diabetic drugs in Bangladesh, including Achyranthesaspera, Trigonella foenum-graecum, Allium sativum, Asparagus racemosus, Centellaasiatica, Ecliptaalba, Mangiferaindica, Ocimum sanctum, Phyllantusemblica, Heliotropiumindicum, Teminaliabellirica [18]. Though the market for herbal medicines is hastily rising globally, the pieces of evidence for therapeutic efficacy is still sparse [19,20].
Trigonella foenum-graecum (Fenugreek), an annual plant belonging to the family Leguminosae, is widely distributed to the countries bordering on the Mediterranean's eastern shores and largely cultivated in India, China, Morocco, and Egypt [21]. Fenugreek commonly known as methi has been used as a useful source of antidiabetic medicinal compounds from its seeds, leaves, and extracts in various animal models [22-24]. Fenugreek seeds contain a rich resource of bioactive compounds like flavonoids (quercetin, vetexin, rutin), saponins (Graecunins, fenugrin B, Fenugreekine), amino acid (isoleucine, 4-hydroxyisoleucine, histidine, leucine, lysine), vitamins (A, B1, C), nicotinic acid and other bioactive constituents like volatile oils, mucilage, etc. [25,26]. T. foenum-graecum also imparts various pharmacological actions such as antidiabetic activity, antihyperlipidemic activity, stimulating/regenerating activity on β cells, antilithogenic activity, antioxidant activity, neuroprotective effects, anti-inflammatory, antipyretic activity, chemopreventive activity, aphrodisiac, wound healing, regulation of hyperthyroidism, anti-plasmodial activity, etc.[27-33]. Fenugreek extract plays an important role as a hypoglycemic agent by inhibiting α-amylase activity. Besides its hypoglycemic effect, the hypocholesterolemic effect of fenugreek seeds has also been recorded. Therefore, fenugreek seeds possess a dual function in the management of diabetes in alloxan-induced diabetic rats. Regardless of diabetes, it is also reported that T. foenum-graecum improved serum lipid profile with a significant fall in serum total cholesterol, LDL and VLDL cholesterol, and triglycerides [22] and also repaired the kidney and liver damage caused by alloxan-induced diabetic rats [34].
In view of the above consideration, the existing investigation strives to evaluate the pharmacological effect, therapeutic efficacy, related adverse effect, and safety profile of Trigonella foenum-graecum as antidiabetic, hypolipidemic medicine in a dose and source dependent manner in alloxan-induced diabetic rats.
Methods and Materials
All the chemicals used in the current study were of the highest analytical grade. Dried seeds of Trigonella foenum-graecum purchased from Allahrdan Shop, Banasree, Dhaka, Bangladesh. Alloxan was brought from Sigma Aldrich, Germany. Humalyzer 3000 was used to assess the blood parameters of rodents. Glucometer of Alere GI of AlereInc, USA was taken from Shahbag, Dhaka, Bangladesh. All blood parameter analyzing kits were purchased from Plasmatic Laboratory Product Limited.
Extraction procedure
At first, seeds of T. foenum-graecum were properly washed and dried in sunlight for several days. Afterward, the dried seeds were crushed into powder using high capacity grinding machine, and then the powdered materials were soaked in methanol for 14 days with occasional vigorous shaking and stirring. Next, the extract was filtered using Whatman No.1 Filter paper. The filtrate liquid was taken for the next step to reduce the volume using a rotary evaporator at low temperature and pressure.
Experimental design and animal handling
Thirty healthy adult Wister albino male rats were obtained from the animal unit, Department of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh, and individually housed in stainless steel cages at 12 ± 1 h light/dark cycle and controlled temperature (25°C) in the Institute of Nutrition & Food Science, University of Dhaka. The rodents were fed with a standard pellet diet and water ad libitum. Before inaugurating the study, the rats were stored there for acclimatization. Afterward, the bodyweight of each rat has weighed. The rats were distributed into 6 groups where an even division of rodents as per their body weight has been taken place, and each group contained 5 rats.
Group 1: Normal Control
Group 2: Diabetic Control
Group 3: Low Dose (100 mg/kg body weight)
Group 4: Medium Dose (400 mg/kg body weight)
Group 5: High Dose (750 mg/kg body weight)
Group 6: Commercial Preparation (400 mg/kg body weight)
In the first two weeks, the rats were given normal food and water twice daily without inducing diabetes. On the 14th day, a chemical agent, alloxan (150 mg/kg body weight), was injected into all groups via intraperitoneal route for diabetic induction except group 1. After 72 hours, the blood glucose level was checked. It has been found that diabetes was induced in all rats belonged to groups 2-5 .and treatment was initiated on the 18th day, which was continued for twenty-eight days. The blood glucose level was measured once every week. The doses were administered orally.
Statistical analysis
The findings of all study parameters belong to several groups were represented as mean ± SD. “One Way Anova Test” of SPSS 16” software was used to investigate the inter-group variations in results to find the statistical significance. Here, the statistical significance level was set at a ‘p’ value of p<0.05. And high statistical significance was set at 'p' value of p<0.01 In terms of results, the inter-group diversity was considered statistically significant and highly significant when the p-value was found less than 0.05 and 0.01 respectively.
Results
Change in body weights
The pre-treatment & post treatment body weight(gram) of rats belonged to different groups are shown in Figure 1
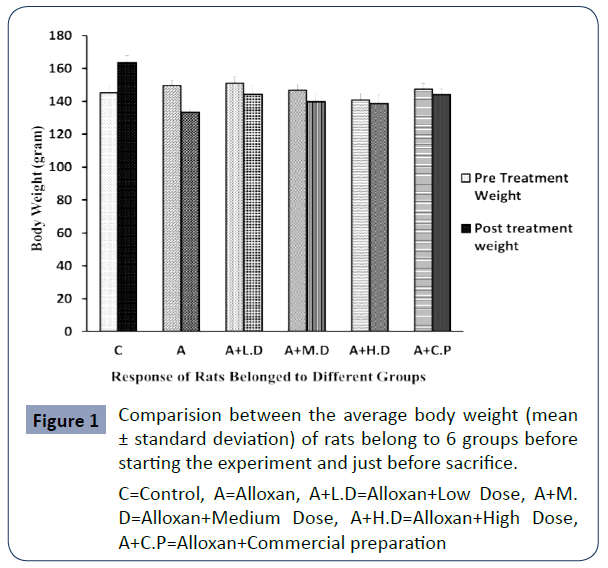
C=Control, A=Alloxan, A+L.D=Alloxan+Low Dose, A+M. D=Alloxan+Medium Dose, A+H.D=Alloxan+High Dose, A+C.P=Alloxan+Commercial preparation
Figure 1 Comparision between the average body weight (mean ± standard deviation) of rats belong to 6 groups before starting the experiment and just before sacrifice.
Change in blood glucose level
The blood glucose level (mmol/dl) of all test group from day 1 to day 42 are expressing below in the mentioned graph in Figure 2.
C=Control, A=Alloxan, A+L.D=Alloxan+Low Dose, A+M. D=Alloxan+Medium Dose, A+H.D=Alloxan+High Dose, A+C.P=Alloxan+Commercial preparation
*Expresses the significant change,
** Expressing the high significant change.
Figure 2 Blood glucose level of six groups from day zero to day forty-two. The data were expressed as mean± standard deviation.
Safety profile study (Liver function test)
The SGOT level of all rats belonged to 6 groups are denoting the condition of liver is shown in below graph Figure 3.
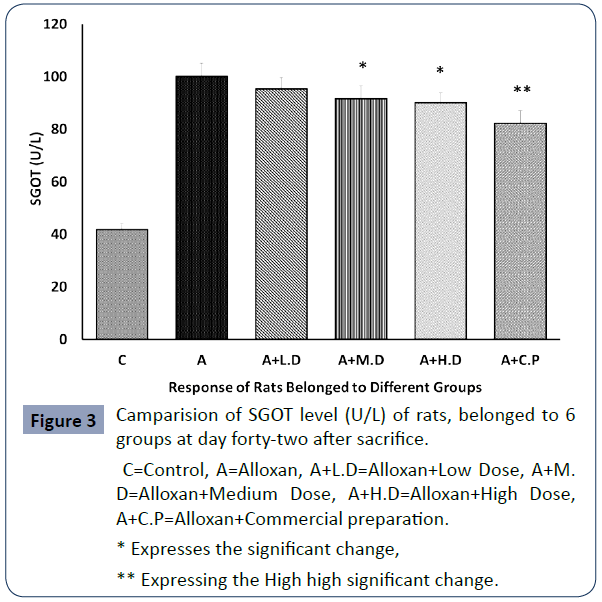
C=Control, A=Alloxan, A+L.D=Alloxan+Low Dose, A+M. D=Alloxan+Medium Dose, A+H.D=Alloxan+High Dose, A+C.P=Alloxan+Commercial preparation.
* Expresses the significant change,
** Expressing the High high significant change.
Figure 3 Camparision of SGOT level (U/L) of rats, belonged to 6 groups at day forty-two after sacrifice.
The level of SGPT of all rats that belonged to 6 groups is expressing the condition of liver are expressing via the blow shown graph Figure 4.
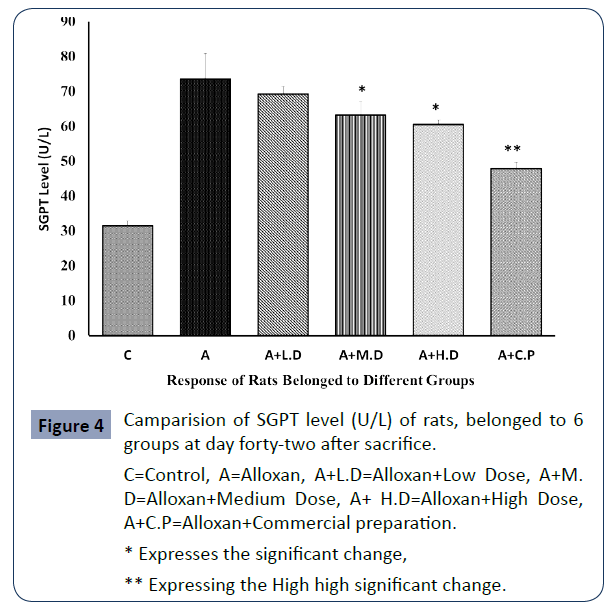
C=Control, A=Alloxan, A+L.D=Alloxan+Low Dose, A+M. D=Alloxan+Medium Dose, A+ H.D=Alloxan+High Dose, A+C.P=Alloxan+Commercial preparation.
* Expresses the significant change,
** Expressing the High high significant change.
Figure 4 Camparision of SGPT level (U/L) of rats, belonged to 6 groups at day forty-two after sacrifice.
Safety profile study (Kidney functioning test)
The below mentioned values concerning the level of Creatinine (md/dl) of rats belonged to 6 group as a requirement of measuring the kidney functioning test are presenting in Figure 5.
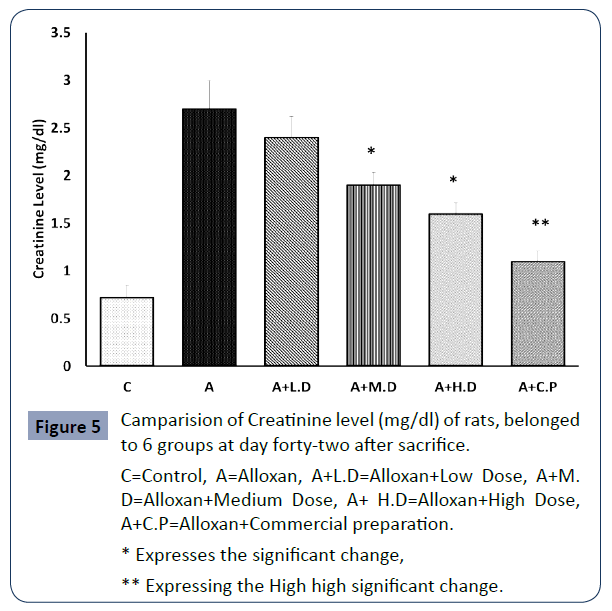
C=Control, A=Alloxan, A+L.D=Alloxan+Low Dose, A+M. D=Alloxan+Medium Dose, A+ H.D=Alloxan+High Dose, A+C.P=Alloxan+Commercial preparation.
* Expresses the significant change,
** Expressing the High high significant change.
Figure 5 Camparision of Creatinine level (mg/dl) of rats, belonged to 6 groups at day forty-two after sacrifice.
Safety profile study (Lipid profile)
The level of Total Cholesterol Level of all rats belonged to 6 groups are expressing in below Figure 6.
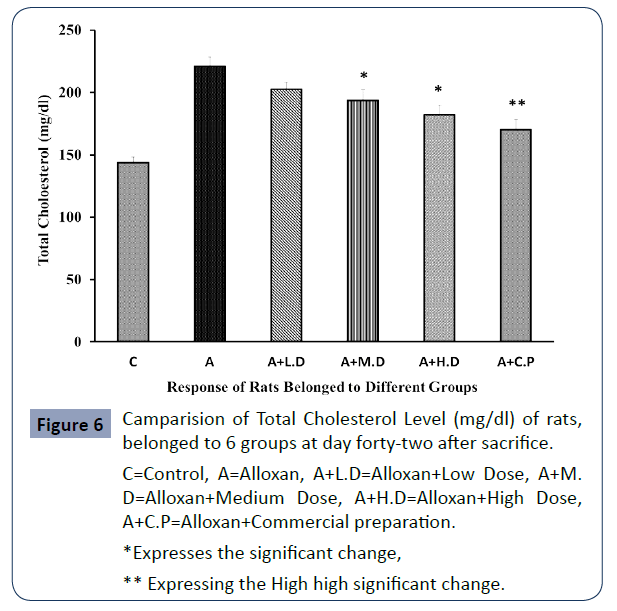
C=Control, A=Alloxan, A+L.D=Alloxan+Low Dose, A+M. D=Alloxan+Medium Dose, A+H.D=Alloxan+High Dose, A+C.P=Alloxan+Commercial preparation.
*Expresses the significant change,
** Expressing the High high significant change.
Figure 6 Camparision of Total Cholesterol Level (mg/dl) of rats, belonged to 6 groups at day forty-two after sacrifice.
Safety profile study (Lipid profile)
The level of HDL level (mg/dl) of all rats belonged to 6 groups are presenting in below drawn graph, Figure 7.
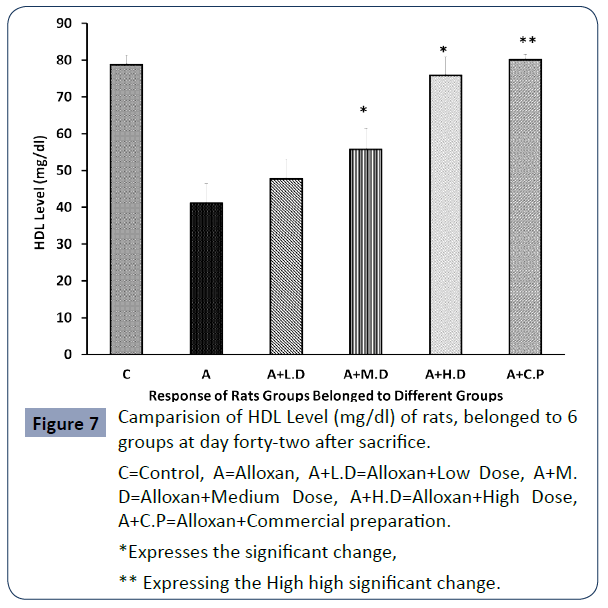
C=Control, A=Alloxan, A+L.D=Alloxan+Low Dose, A+M. D=Alloxan+Medium Dose, A+H.D=Alloxan+High Dose, A+C.P=Alloxan+Commercial preparation.
*Expresses the significant change,
** Expressing the High high significant change.
Figure 7 Camparision of HDL Level (mg/dl) of rats, belonged to 6 groups at day forty-two after sacrifice.
Safety profile Study (Lipid profile)
The below mention values regarding the level of LDL level (mg/ dl) of rats belonged to 6 groups are presenting in below, Figure 8.
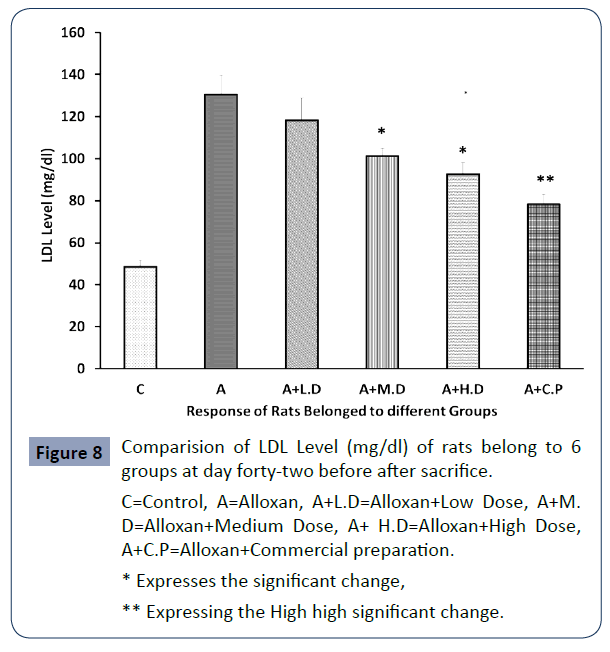
C=Control, A=Alloxan, A+L.D=Alloxan+Low Dose, A+M. D=Alloxan+Medium Dose, A+ H.D=Alloxan+High Dose, A+C.P=Alloxan+Commercial preparation.
* Expresses the significant change,
** Expressing the High high significant change.
Figure 8 Comparision of LDL Level (mg/dl) of rats belong to 6 groups at day forty-two before after sacrifice.
Safety profile study (Lipid profile)
The below mention values regarding the level of Triglyceride level (mg/dl) of rats belonged to 6 groups are given below Figure 9.
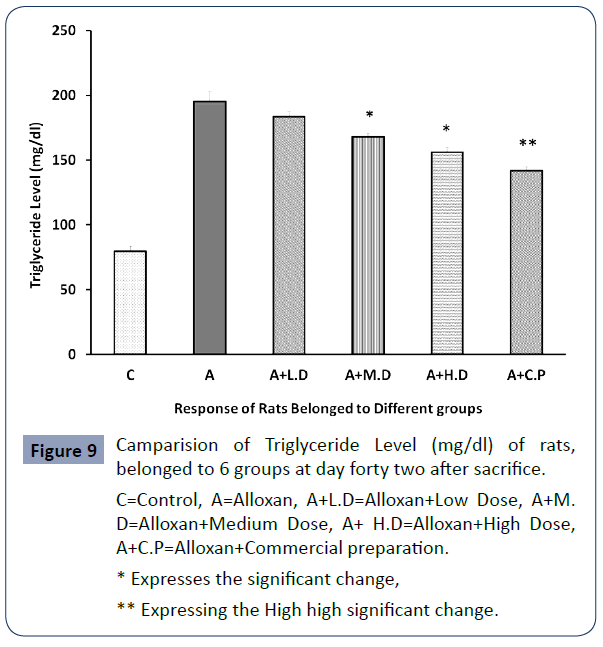
C=Control, A=Alloxan, A+L.D=Alloxan+Low Dose, A+M. D=Alloxan+Medium Dose, A+ H.D=Alloxan+High Dose, A+C.P=Alloxan+Commercial preparation.
* Expresses the significant change,
** Expressing the High high significant change.
Figure 9 Camparision of Triglyceride Level (mg/dl) of rats, belonged to 6 groups at day forty two after sacrifice.
Discussion
It has been observed that body weight was decreased in every single group than that of negative control. Here the depletion of body weight was highest in diabetic control group. And in treatment groups, with the enhancement of dose, depletion was reduced. Lowest reduction was observed in Commercial preparation treated groups.
Diabetic induced group2 rats experiencedan elevated blood glucose level than all other groups. This may be due to destruction of beta cells and untreated condition. There was no significant decline in the blood sugar level at low dose but the medium and high dose yield significantly fall (p<0.05) in blood glucose level. A high statistically significant decrease (p<0.01) in blood glucose level was noticed in commercial preparation. However, all the groups have the capacity to lower the increased blood sugar level compared to diabetic group.On the other hand, the Blood glucose level of rats was detected to be normal belonged to group 1 (Normal Control group).
The diabetic induced group rats exhibited highest level of SGOT, SGPT and Creatinine than all other groups due to destructive effect of alloxan. On the contrary, it was seen that the SGOT, SGPT and Creatinine levels were significantly lowered (p<0.05) at medium and high dose. In contrast at low dose level null significance was found when compared with group 2 (p>0.05).
Additionally, SGOT, SGPT and Creatinine levels were meticulously (p<0.01) decreased in commercial preparation compared to diabetic group.
Ethanolic extract of Trigonella foenum-graecum was effective in lowering triglycerides, LDL-C and total cholesterol in the serum at all dose levels were the reduction came statistically significant in case of medium and high dose and high statistical significance was observed in case of commercial preparation.
HDL-C level increased compared to the diabetic induced group at all dose levels. There was no significant increase in the HDL-C level at low dose. However, a significant increase was noticed at medium, high dose and high statistical significance was observed inn case of commercial preparation.
So it can be conferred that the Commercial preparation can decline the blood sugar level more efficiently than that of lab-based preparations. Different reasons may come responsible for the enhanced potentiality of commercial preparation for e.g: fewer errors in preparations, the season of seed collection, or geological areal of cultivation. Apart from that response of rat’s belonged to medium and high doses seem to be relatively similar from visual and statistical inspection. It may occur due to the saturation of receptors.
Conclusion
Ethanolic extract of T. foenum-graecum exhibit dose and source dependent activity against diabetes. The commercial preparation imparted best effects than that of all other groups. Most likely Furthermore, it was also investigated that this seed extract of can improved the altered pathological condition of alloxan induced diabetic rats. From the view point of safety study, it can be terminated that the doses of ethanolic extract of T. foenum-graecum were not toxic to the rats and did not significantly alter the pathological state in healthy individual. Hence, It might, therefore be presumed that ethanolic extract of T. foenum-graecum could be effectively used as an alternative therapy in the prevention and treatment of diabetes
35549
References
- Kharroubi AT, Darwish HM (2015) Diabetes mellitus: The epidemic of the century. World J Diabetes 6:850-867.
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053.
- King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995-2025: prevalence, numerical estimates and projections. Diabetic care 21:1414-1431.
- Mohler ML, He Y, Wu Z, Hwang DJ, Miller DD (2009) Recent and emerging anti‐diabetes targets. Medicinal research reviews 29:125-95.
- Bagust A, Hopkinson PK, Maier W, Currie CJ (2001) An economic model of the long-term health care burden of Type II diabetes. Diabetologia 44:2140-2155.
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, et al. (1998) Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care 21:518-524.
- Modi P (2007) Diabetes beyond insulin: review of new drugs for treatment of diabetes mellitus. Current drug discovery technologies 4:39-47.
- Prabhakar PK, Doble M (2011) Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin J Integr Med 17:563-574.
- Harsch IA, Kaestner RH, Konturek PC (2018) Hypoglycemic side effects of sulfonylureas and repaglinide in ageing patients-knowledge and self-management. J Physiol Pharmacol 69.
- Zimmerman BR (1997) Sulfonylureas. Endocrinology and metabolism clinics of North America 26:511-522.
- Consoli A, Formoso G (2013) Do thiazolidinediones still have a role in treatment of type 2 diabetes mellitus? Diabetes Obes Metab 15:967-977.
- Bastaki A (2005) Diabetes mellitus and its treatment. Int J Diabetes and Metabolism 13:111.
- Afroz A, Alramadan MJ, Hossain MN, Romero L, Alam K, et al. (2018) Cost-of-illness of type 2 diabetes mellitus in low and lower-middle income countries: a systematic review. BMC Health Services Research 18:972.
- Kameswararao B, Kesavulu MM, Apparao C (2003) Evaluation of antidiabetic effect of Momordicacymbalaria fruit in alloxan-diabetic rats. Fitoterapia 74:7-13.
- Middleton E, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:623-751.
- Ocvirk S, Kistler M, Khan S, Talukder SH, Hauner H (2013) Traditional medicinal plants used for the treatment of diabetes in rural and urban areas of Dhaka, Bangladesh–an ethno botanical survey. J Ethnobiol Ethnomed 9:43.
- Ernst E, Pittler MH, Stevinson C, White A (2001) The desktop guide to complementary and alternative medicine: an evidence-based approach. Mosby International Ltd.
- Bailey CJ, Day C (1989) Traditional plant medicines as treatments for diabetes. Diabetes Care 12: 553–564.
- Snehlata HS, Payal DR (2011) Fenugreek (Trigonella foenum-graecum L.): an overview. International Journal of Current Pharmaceutical Review and Research 2:169-187.
- Raju J, Gupta D, Rao AR, Yadava PK, Baquer NZ (2001 )Trigonella foenum-graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol Cell Biochem 224:45-51.
- Srinivasan K (2006) Fenugreek (Trigonella foenum-graecum) A review of health beneficial physiological effects. Food reviews international 22:203-224.
- Khalki L, M’hamed SB, Bennis M, Chait A, Sokar Z (2010) Evaluation of the developmental toxicity of the aqueous extract from Trigonella foenum-graecum (L.) in mice. J Ethnopharmacol 131:321-325.
- Ghosh B, Chandra I, Chatterjee S (2015) Fenugreek (Trigonella foenum-graecum L.) and its necessity. Fire J Engineering and Technology 1:66-67.
- Yadav UC, Baquer NZ (2014) Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm Biol 52:243-254.
- Rizvi SI, Mishra N (2013) Traditional Indian medicines used for the management of diabetes mellitus. J Diabetes Res 2013: 712092.
- Khan V, Najmi AK, Akhtar M, Aqil M, Mujeeb M, et al. (2012) A pharmacological appraisal of medicinal plants with antidiabetic potential. J Pharm Bioallied Sci 4:27-42.
- Gauttam VK, Kalia AN (2013) Development of polyherbalantidiabetic formulation encapsulated in the phospholipids vesicle system. J Adv Pharm Technol Res 4:108-117.
- Uemura T, Goto T, Kang MS, Mizoguchi N, Hirai S, et al. (2011) Diosgenin, the main aglycon of fenugreek, inhibits LXRα activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J Nutr 141:17-23.
- Saxena A, Vikram NK (2004) Role of selected Indian plants in management of type 2 diabetes: a review. J Altern Complement Med 10:369-378.
- Kumar P, Kale RK, Baquer NZ (2012)Antihyperglycemic and protective effects of Trigonella foenum-graecum seed powder on biochemical alterations in alloxan diabetic rats. Eur Rev Med Pharmacol Sci 16:18-27.
- Srinivasan K (2006) Fenugreek (Trigonella foenum-graecum): A review of health beneficial physiological effects. Food reviews international 22:203-224.
- Thakran S, Siddiqui MR, Baquer NZ (2004) Trigonella foenum-graecum seed powder protects against histopathological abnormalities in tissues of diabetic rats. Mol Cell Biochem 266:151-159.














