Introduction
Candida spp. are able to form biofilm by adhering to surfaces of medical devices such as pacemakers, joint replacement, prosthetic heart valve, silicone voice prostheses, end tracheal tubes, catheters and cerebrospinal fluid shunts; this biofilm can lead to acute disseminated infection [1]. Candida spp. are the most common cause of fungal infection. The frequency of hospital acquired Candida infection especially blood stream infection is due to the increased use of immune suppressive therapy in cancer and transplant patients [2].
Biofilms are aggregates of microorganisms, which are formed due to the attachment of cells to host surface in aqueous environment [3]. The pathogenicity of Candida spp. is attributed to some factors such as the ability to hedge host defences, adherence biofilm formation on host tissue and on medical devices, and production of hydrolytic enzymes like proteases, phospholipases and haemolysin [4]. Biofilms formed by Candida spp. are very difficult to diagnose and treat because of their high antifungal resistance. The implant infections are difficult to treat therefore, treatment can require surgical removal and later replacement of the infected device [5-8].
Lactic acid bacteria (LAB) are well known to have a positive effect on maintenance of human health and potential interfering bacteria by producing various compounds such as organic acid ,hydrogen peroxide, diacetyl, bacteriocins and biosurfactants which inhibit the growth of pathogens [9].
LABs from different sources are documented to have antiadhesion activity. Zárate, Nader?Macias [10] reported that Lactobacillus acidophilus CRL 1259 and Lactobacillus paracasei CRL 1289 isolated from vaginal have inhibited the attachment of staphylococcus aureus and streptococci. Balcázar et al. [11] also found that Lactococcus lactis CLFP 101, Lactobacillus plantarum CLFP 238, and Lactobacillus fermentum CLFP 242 can inhibit adhesion of several fish pathogens (Aeromonas hydrophila, Aeromonas salmonicida, Yersinia ruckeri and Vibrio anguillarum) to host intestinal mucus under in-vitro condition. Furthermore, LAB are capable to interfere with the pathogens adhesion on epithelial cells of urogenital and intestinal tract [12]. Supernatants produced by LAB contain compounds which can reduce adhesion of pathogenic micro-organisms to glass [13], silicone rubber [14], surgical implants [15] and voice prosthesis [16]. Many LAB are known to inhibit the growth Candida species by different ways such as competition for adhesion sites or production of different antagonistic metabolites which inhibit the growth [17]. The aim of this study was to determine the anti-adhesion capability of cellfree supernatant produced by LAB isolates from honey against five pathogenic Candida spp.
Materials and Methods
Honey samples
Honey samples used in this study were Al-Sedar honey from Libya, Tualang honey from Malaysia, Al-Hanon honey from Libya, and Al-Maray honey from Yemen.
Isolation of lactic acid bacteria from honey samples
LAB strains were isolated from honey samples following the method described by Aween et al. [18]. Approximately, 10 g of honey samples were suspended in 90 mL peptone water (0.1 % w/v) in stomacher bags and the bags were manually agitated. Then 1 mL was added to 9 mL of MRS broth (Oxoid CM359) followed by incubation at 30°C for 24 to 48 h. Appropriate serial dilution with peptone water (0.1 % w/v) was carried out and 0.1 mL of appropriate dilution was spread plated on several modified media namely, MRS agar (Oxoid) [19], MRS agar with 0.8 % CaCO3 [20]. MRS agar with 1 % glucose, tomato juice agar with 0.8 % CaCO3 and tomato juice agar with 1 % glucose. All plates were incubated anaerobically for 48h at 37 °C. Isolated single colonies were tested for catalase activity with 4 % H2O2 and Gram stained. Catalase negative colonies were streaked on MRS agar containing 0.8 % CaCO3 incubated at 37 °C for 48 h to obtain pure colonies. All catalase negative and Gram positive LAB isolates were kept at -20 °C in MRS broth containing 15% (v/v) glycerol for further work.
Culturing of candida species
The Candida spp. used were obtained from the microbial collections at the Department of Medical Microbiology, University Putra Malaysia. All Candida spp. included strains of C. albcans ATCC14053, C. parapsilosis ATCC22019, C. tropicalis ATCC750, C. krusei ATCC 6258, C. glabrata ATCC 2001 were cultured on sabouraud dextrose agar (SDA, Oxoid ) and incubated at 35°C for 24 h and 48 h to check for viability and purity. The pure isolates of Candida spp. were maintained on SDA at 4°C.
The cell free supernatant preparation
Approximately 3 mL of overnight culture of LAB in MRS broth (Oxoid CM359) were inoculated into 600 ml of MRS broth and incubated at 37°C for 24 h in incubator shaker (Orbital shaker incubator, LM-530 RD) at 150 rpm. Then the cell free supernatant (CFS) was prepared by centrifuging the broth at 11500 rpm for 10 min at 4 °C (Mini Spin, Eppendorf, AG 22331, Hamburg). The supernatant of each isolates was filtered using sterile filter (0.45 μm-pore-size filter, Millipore) [21] and the CFS was used for analysis.
Determination of the anti-adhesion activity of LAB supernatants against biofilm candida species by microtiter plate method
The anti-adhesion activity of the LAB CFS against Candida spp. was performed in pre-coating and co-incubation experiments. The pre-coating experiments was carried out as described by Gudiña et al. [22] . A 96-wells microtiter plates were coated with different CFS. A 200 μL of CFS were pipetted into the wells and the microtiter plates were incubated at 37 °C for 24 h. Then, the CFS were removed and the plates washed twice with 100 μL of phosphate buffer saline (PBS) pH 7.2 to remove non- adhering supernatant. After that, 150 μL of each 24h culture Candida spp. suspension (1.5× 107 CFU/mL) cultured in sabouraud dextrose broth (SDB, Oxoid CM147) were added to each well then the microtiter plate was again incubated at 37 °C for 24 h.
Non–adhering cells were removed by gently washing twice the wells with PBS pH 7.2. Quantification was done using the crystal violet assay [23,24]. The biofilm was fixed for 15 min by adding 100 μL of 99% methanol to each well and the plate was air dried. After that, 100 μL of crystal violet 2% was added and held for 20 min then the excess crystal violet was removed by pipette and, residue in the wells was washed with tap water. The stain bound to the adherent fungi was solubilized with 100 μL of 33% glacial acetic acid per well and the optical density readings of each well were measured at 595 nm using a micro Elisa auto reader (Model 680, BioRad). Candida suspension without CFS was prepared as control. The percentage reduction in adherence was calculated using the following equation according to Gudiña et al. [22] as [ % microbial adhesion = 1- (ODc /OD0) × 100] where ODc represents the optical density of the well with CFS and Candida suspension; OD0 represents the optical density of the Candida suspension without CFS (control). The microtiter plate anti-adhesion assay estimates the percentage reduction of Candida adhesion in relation to the control wells which were at 0 % in the absence of LAB CFS. The analysis was carried out in triplicates and the mean of optical density was taken. In co-incubation experiment, suspension of Candida spp. in SDB (1.5 ×107 CFU / mL) were added to each well together with different LAB CFS (200 μL supernatant: 150 μL Candida culture), and incubated at 37 °C for 24 h. Determination of percent adhesion was carried following the method described above.
Effect of heating on LAB CFS anti-adhesion activity
The CFS of LAB isolates was heat treated at 60, 80 and 100 °C for 30 min and at 121 °C for 15 min, then the samples were cooled in ice water. Then the CFS were tested against Candida spp. biofilm by using pre-coating experiment following the method described by Gudiña et al. [22].
Effect of different pH adjustments on LAB CFS anti-adhesive activity
The pH of CFS of LAB were adjusted to different pH values 3, 5, 6,7 and 9 using 0.1 N HCL or 0.1 NaOH and read by pH meter (METTLER TOLEDO). Then the CFSs were tested against Candida spp. biofilm using pre-coating experiment as described above.
Identification of LAB isolates by API 50 CH and 16S rDNA
The four LAB isolates that showed anti-adhesion activity were identified using API 50 CHL kit assay following the method described by the manufacturer [25]. The identity of the LAB isolates were further confirmed by 16S rDNA, using two primers 16S forward (5-AGAGTTTGATCCTGGCTC-3) and 16S reverse: (5- CGGGAACGTATTCAC-CG-3) Magnusson et al. [26] which were synthesized at 1st Base, Malaysia. The chromosomal DNA of the four strains of LAB was extracted using the Wizard® Genomic gram positive DNA purification kit (USA). The purified DNA of per sample was processed to the polymerase chain reaction (PCR) using Fail SafeTM Pre Mix kit Epicentre® (an Illumina® company). A 5 μl of each amplification mixture were subjected to electrophoresis in 1.5% (1.5 g agarose powder with 100 mL in 1 x TEA buffer for 45 min and 90 volts. The partial 16S rDNA sequences (approximately 1400 bp) were determined by 1st Base, Malaysia and sequences were compared with databases (Gen- Bank).
Statistical analysis
All data were presented as mean ± standard deviation. Data were analysed by using two-way analysis of variance (ANOVA) using general linear model (GLM) procedure of SAS, and Tukey’s test at P<0.05 to evaluate the significant differences between groups.
Results
The identification of four LAB isolated from honey samples that showed anti-adhesion activity against five strains of pathogenic Candida spp. is presented in Table 1. The results from API 50 CHL kit identified the isolate HS from Al-Sedar honey as Lactobacillus plantarum2, and other three isolates HH from Al-Hanon honey, HC from Tualang honey and HM from Al-Maray as L. curvatus. However, the results from 16S rDNA sequence were slightly different: HS was identified as L. plantarum, HH as L. curvatus, HC as Pediococcus acidilactici and HM as P. pentosaceus.
Table 1: Similarity index of LAB isolated from honey samples as determined by API 50CHL and 16S rDNA
| Sources |
Code |
API CHL 50 |
Similarity |
16S rDNA |
Similarity |
| Al-Sedar honey, Libya |
HS |
L. plantarum2 |
99.4% |
L. plantarum |
99.0 % |
| Al-Hanon honey, Libya |
HH |
L. curvatus |
99.4% |
L. curvatus |
96.0% |
| Tualang honey, Malaysia |
HC |
L. curvatus |
99.4% |
Pediococcusacidilactici |
99.0% |
| Al-Maray honey, Yemen |
HM |
L. curvatus |
97.4% |
Pediococcuspentosaceus |
99.0% |
It was observed that the Candida spp. had high ability to produce biofilm in 96-well microtiter plate. The different CFS showed variable anti-adhesion activity against the Candida spp. tested (Tables 2 and 3). Pre-coating of the polystyrene wells with CFS of L. curvatus HH showed significantly (P< 0.05) higher anti-adhesion activity against C. glabrata ATCC2001 and C. albicans ATCC14053 by 79.4% and 61.1%, respectively. However, the CFS produced by HS and HM showed significantly (P< 0.05) lower anti-adhesion activity against most Candida spp. especially C. tropicalis ATCC 750 and C. krusei ATCC6258 by 4.1% and 1.5%, respectively. The CFS of L. curvatus showed high anti-adhesion percentages for C. glabrata (79.4 %), C. albicans (61.1%) and C. parapsilosis (34.3 %). However, low anti-adhesion activity was obtained from CFS of L. plantarum against C. albicans (20 %), C.glabrata (15.8 %) and C. tropicalis (4.0 %).
Table 2: Percentage of anti-adhesion activity of LAB cell free supernatants against of Candida spp. as evaluated by pre-coating assay*
| Candida species |
LAB |
| HS |
HC |
HH |
HM |
| C. albicans |
20.4 ± 0.4f |
38.9 ± 0.5c |
61.1 ± 1.1b |
35.5 ± 0.3d |
| C. glabrata |
15.8 ± 0.4g |
35.7 ± 0.8d |
79.4 ± 0.4a |
26.0 ± 2.3e |
| C. parapsilosis |
10.6 ± 1.1h |
5.8 ± 0.1i |
34.3 ± 0.4d |
4.3 ± 0.8i |
| C. tropicalis |
4.0 ± 0.9ij |
24.7 ± 2.0e |
10.7 ± 0.8h |
18.2 ± 0.7fg |
| C. krusei |
12.0 ± 1.0h |
35.4 ± 03d |
26.9 ± 0.2e |
1.5 ± 0.9j |
*Titer plates were incubated at 37for C 48 h.The results are expressed as mean ± standard deviations of values obtained from triplicate experments
a-j Mean ± SD. Means with different superscripts are differ significantly (P < 0.05).
Table 3: Percentage of anti-adhesion activity of LAB cell free supernatants against of Candida spp. as evaluated by in co-incubation assay*
| Candida species |
LAB |
| HS |
HC |
HH |
HM |
| C. albicans |
50.0 ± 0.9de |
63.5 ± 0.3bc |
75.5 ± 2.1a |
14.3 ± 0.7j |
| C. glabrata |
63.0 ± 0.4bc |
42.0 ± 0.8ef |
58.4 ± 3.1cd |
36.3 ± 0.6fgh |
| C. parapsilosis |
27.4 ± 1.1hi |
16.8 ± 0.9ij |
57.5 ± 0.8cd |
30.4 ± 3.1h |
| C. tropicalis |
12.2 ± 0.9j |
14.4 ± 0.3j |
42.0 ± 0.8efg |
31.8 ± 0.4gh |
| C. krusei |
62 ± 0.02bc |
33.7 ± 0.4fgh |
70.0 ± 3.1b |
7.2 ± 0.9i |
*Titer plates were incubated at 37for C 48 h. The results are expressed as mean ± standard deviations of values obtained from triplicate experments.
a-j Mean ± SD. Means with different superscripts are differ significantly (P < 0.05).
Slightly different results were obtained when the CFS were evaluated by co-incubation experiment. The CFS of HH showed significantly (P< 0.05) higher anti-adhesion activity against most Candida spp. in which biofilm formation of C. albicans ATCC14053, C. krusei ATCC6258 and C. glabrata ATCC2001 was reduced by 75.5%, 70% and 58.4 %, respectively (Table 3). Similarly, biofilm formation was prevented by L. curvatus against C. albicans (75.5 %) and C. Krusei (70 %). In contrast CFS of P. pentosaceus HM and L. plantarum HS were not effective in reducing biofilm formation of C. krusei (7.2 %) and C. tropicalis (12.2 %). It was also observed that all the SFC of LAB was ineffective in preventing biofilm formation of C. tropicalis ATCC750.
The anti-adhesion activity of LAB isolates was stable after heating the CFS at 60, 80, 100 °C for 30 min and 121 °C for 15 min in precoating assay against most Candida spp. (Tables 4-7), especially, the supernatant produced by L. curvatus HH significantly (P<0.05) reduced the biofilm formation of C. albicans with percentages 57%, 55.3%, 55.9% and 58.6% at 60, 80, 100 °C and 121 °C, respectively. Additionally, the biofilm formation of C. glabrata was reduced by the heated CFS of L. curvatus HH with percentages 70%, 69.3%, 63.3% and 60.6%, respectively.
Table 4: Percentage of anti-adhesion of Candida spp. with LAB supernatant after heat treatment at 60 °C at pre-coating assay after incubation for 48 h at 37°C.
| Candida species |
LAB |
| HS |
HC |
HH |
HM |
| C. albicans |
11.9 ± 0.4ijk |
40 ± 1.0bc |
57 ± 0.9c |
35 ± 0.3d |
| C. glabrata |
23.6 ± 1.2ef |
35.4 ± 0.9c |
70 ± 1.4a |
37 ± 0.5d |
| C. parapsilosis |
10.3 ± 0.2jkl |
61.5 ± 0.2b |
5.3 ± 0.4lm |
2.5 ± 0.3m |
| C. tropicalis |
8.3 ± 0.3kl |
15.3 ± 0.8hij |
16 ± 0.6hi |
10.2 ± 0.7ijk |
| C. krusei |
31.4 ± 1.3d |
17.2 ± 1.1gh |
22.8 ± 1.2fg |
45.7 ± 1.0b |
The results are expressed as mean ± standard deviations of values obtained from triplicate experments.
a-mMean ± SD. Means with different superscripts are differ significantly (P < 0.05).
Table 5: Percentage of anti-adhesion of Candida spp. With LAB supernatant after heat treatment at 80 °C at pre-coating assay after incubation for 48 h at 37°C.
| Candida species |
LAB |
| HS |
HC |
HH |
HM |
| C. albicans |
36.1 ± 0.5ghij |
15.3 ± 0.6no |
55.3 ± 1.2c |
30.8 ± 0.50 |
| C. glabrata |
33.3 ± 0.7hij |
29.3 ± 0.4ijk |
69.3 ± 0.8b |
27.3 ± 0.9jkl |
| C. parapsilosis |
44.2 ± 0.3efg |
37.1 ± 0.8efgh |
19.2 ± 1.0mno |
21.4 ± 0.4lmn |
| C. tropicalis |
48.1 ± 0.3cd |
25.4 ± 0.4klm |
43.6 ± 0.6cde |
14.2 ± 1.4no |
| C. krusei |
27.7 ± 1.1ijk |
42.5 ± 0.3def |
55.5 ± 0.2c |
78.7 ± 0.3a |
The results are expressed as mean ± standard deviations of values obtained from triplicate experments
a-o Mean ± SD. Means with different superscripts are differ significantly (P < 0.05).
Table 6: Percentage of anti-adhesion of Candida spp. With LAB supernatant after heat treatment at 100 °C at pre-coating assay after incubation for 48 h at 37°C.
| Candida species |
LAB |
| HS |
HC |
HH |
HM |
| C. albicans |
21.1 ± 1.1defg |
13.7 ± 0.9fgh |
55.9 ± 1.2b |
21.0 ± 0.8defg |
| C. glabrata |
21.4 ± 0.2def |
12.9 ± 0.5gh |
63.3 ± 0.4a |
14.6 ± 0.9fgh |
| C. parapsilosis |
9.60 ± 0.5h |
8.40 ± 0.4h |
22.8 ± 1.0cde |
10.8 ± 1.0h |
| C. tropicalis |
23.7 ± 1.2cde |
16.5 ± 0.9efgh |
21.6 ± 1.3def |
40.9 ± 1.1b |
| C. krusei |
30.7 ± 1.3c |
20.9 ± 1.0defg |
19.5 ± 1.4efg |
28.0 ± 1.0cd |
The results are expressed as mean ± standard deviations of values obtained from triplicate experments.
a-h Mean ± SD. Means with different superscripts are differ significantly (P < 0.05).
Table 7: Percentage of anti-adhesion of Candida spp. With LAB supernatant after heat treatment at 121 °C at pre-coating assay after incubation for 48 h at 37°C.
| Candida species |
LAB |
| HS |
HC |
HH |
HM |
| C. albicans |
11.8 ± 1.0ef |
25.0 ± 1.1d |
58.6 ± 0.8a |
33.2 ± 0.4b |
| C. glabrata |
12.4 ± 0.4ef |
34.0 ± 0.9b |
60.6 ± 1.2a |
10.8 ± 0.8f |
| C. parapsilosis |
14.6 ± 0.5ef |
14.0 ± 1.3ef |
12.4 ± 0.9ef |
15.4 ± 1.0cdef |
| C. tropicalis |
17.5 ± 0.7cde |
22.2 ± 1.0dc |
22.2 ± 0.6dc |
16.2 ± 0.3cdef |
| C. krusei |
11.9 ± 1.1ef |
11.0 ± 0.4f |
24.1 ± 1.0d |
10.7 ± 0.5f |
The results are expressed as mean ± standard deviations of values obtained from triplicate experments.
a-f Mean ± SD. Means with different superscripts are differ significantly (P < 0.05).
The anti-adhesion activity of supernatants was good at pH ranged from 3 to 5, but decreased rapidly at pH 6 and, the activity was lost when pH was adjusted to 7 against most Candida species. The supernatant of isolate L. curvatus HH observed a loss of anti-adhesion activity at acidic condition. However, CFS of L. curvatus HH was more effective at pH7 especially, against biofilm formation of C. glabrata ATCC 2001 and C. albicans ATCC 14053 with percentages 65.9 % and 58.6 % respectively (Figures 1-5). The results from this study indicate that different strains of LAB produce different types of anti-adhesion compounds against Candida spp.
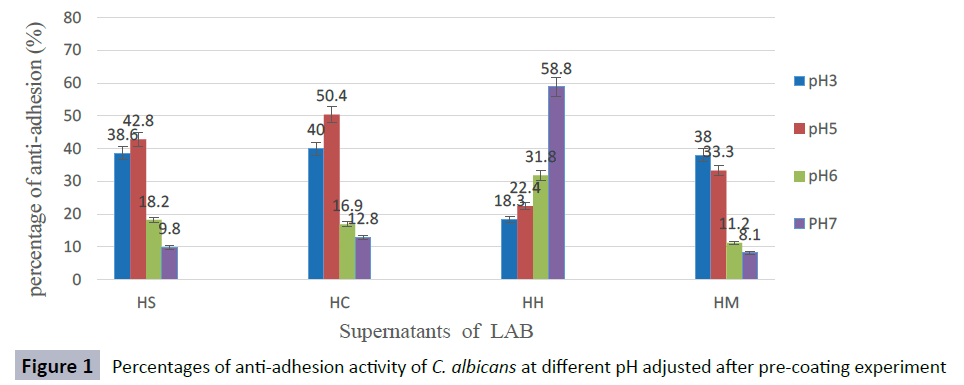
Figure 1: Percentages of anti-adhesion activity of C. albicans at different pH adjusted after pre-coating experiment
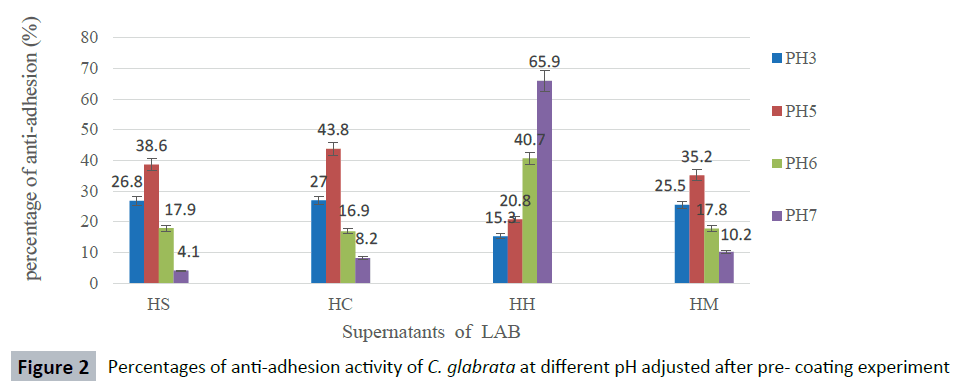
Figure 2: Percentages of anti-adhesion activity of C. glabrata at different pH adjusted after pre- coating experiment
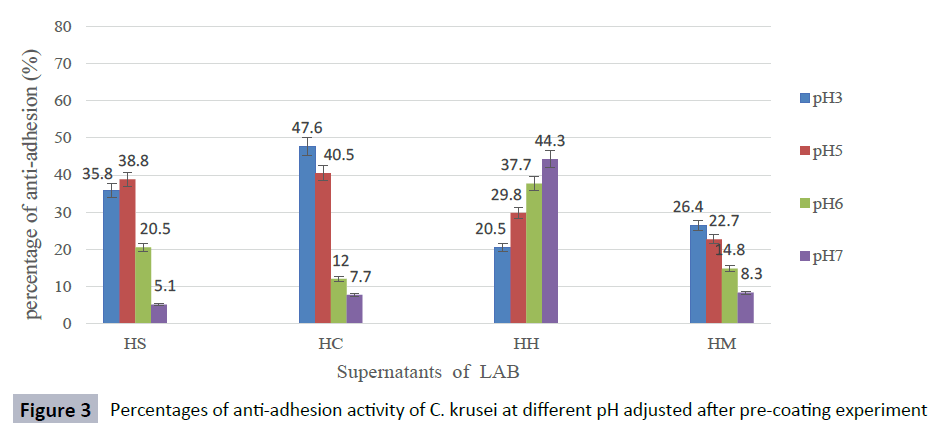
Figure 3: Percentages of anti-adhesion activity of C. krusei at different pH adjusted after pre-coating experiment
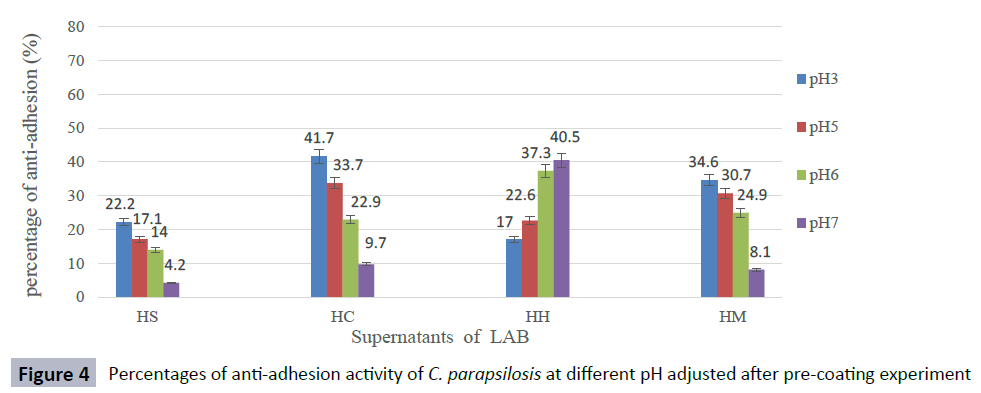
Figure 4: Percentages of anti-adhesion activity of C. parapsilosis at different pH adjusted after pre-coating experiment
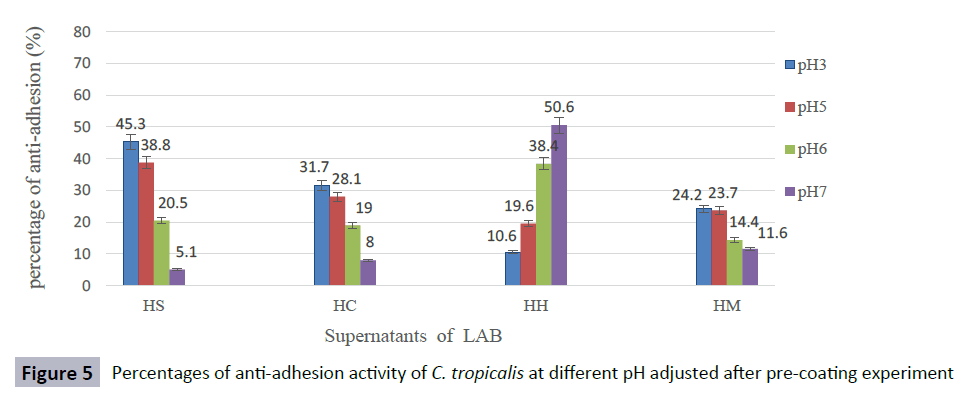
Figure 5: Percentages of anti-adhesion activity of C. tropicalis at different pH adjusted after pre-coating experiment
Discussion
Candida spp. have the ability to form biofilms that are responsible for survival of these species. This study showed that all Candida spp. formed biofilms on polystyrene surfaces similar to that reported by Silva et al. [27] and Parahitiyawa et al. [28]. LABs from different sources have been documented to have ability to prevent biofilm formation. The presence of LAB in honey was reported by several researchers [29-32]. Aween et al. [33] isolated LAB from honey and identified as strains of Lactobacillus acidophilus and demonstrated that they have antibacterial activities against Gram- positive bacteria. In this study LAB was detected in 10 from the 15 honey samples with variable antifungal activity against Candida spp. Four of the LAB were identified as L. plantarum HS, Pediococcus acidilactici HC, Lactobacillus curvatus HH and Pediococcus pentosaceus HM which showed good antifungal activity and anti-adhesion activity against Candida spp. Additionally, Atanassova et al. [34] reported that L. paracasei subsp. paracasei M3 isolated from Bulgarian yellow cheese had antifungal activity against strains of Candida spp. included C. albicans, C. pseudointermedia and C. blankii. Similarly, Ogunshe et al. [35] also observed that L. acidophilus and L. plantarum isolated from vaginal had antifungal activity against strains of pathogenic Candida spp.
LABs from different sources have been documented to have antiadhesion activity against Candida spp. Gudiña et al. [36] reported that L. acidophilus and L. paracasei ssp. Paracasei A20 had lower anti-adhesion activity against C. albicans strains. Fracchia et al. [24], also found that Lactobacillus CV8LAC isolated from cabbage have anti-adhesion activity against two C. albicans pathogenic CA- 2894 and DSMZ 11225. To date, the anti-adhesion of LAB isolated from honey has not been reported.
This study observed that the supernatants CFS of four LAB isolated from honey samples had good anti-adhesion activity against Candida spp. as evaluated by pre-coating and coincubation experiments. The highest anti-adhesion activity was obtained with CFS of L. curvatus HH that showed significantly (P< 0.05) anti-adhesion activity against C. glabrata ATCC2001 and C. albicans ATCC14053. The anti-adhesion activity of CFS was stable after heating at 60, 80, 100 °C for 30 min and after autoclaving at 121oC for 15 min. The CFS of L. curvatus HH significantly reduced the biofilms formation of C. albicans and C. glabrata.
The anti-adhesion activity of CFS of isolates HS, HC and HM diminished when pH of CFS was adjusted to pH 3 and 5 indicating that the anti-adhesion compounds produced by these isolates were acidic in nature, except for CFS from L. curvatus HH. CFS of HH lost the anti-adhesion activity at acidic condition but showed high anti-adhesion activity at pH 7 especially against biofilm formation of C. glabrata ATCC 2001 and C. albicans ATCC 14053 with percentages 65.9 % and 58.6 %, respectively. This may suggest that the compound was responsible for anti-adhesion activity in HH which has biosurfactant property.
Similarly, Gudiña et al. [22] reported that anti-adhesion activity of biosurfactant produced by a L. paracasei strain isolated from Portuguese dairy was stable at different pH values, being more effective at pH 7. The results from this study are in agreement with previous studies of Fracchia et al. [24] and Zakaria Gomaa [37], who reported that biosurfactants produced by LAB strains have high anti-adhesion activity against pathogenic C. albicans. These findings are consistent with Fracchia et al. [24], who reported that Lactobacillus CV8LAC isolated from cabbage showed anti-adhesion activity against two C. albicans pathogenic CA- 2894 (82%) and DSMZ 11225 (70%) in pre-coating and coincubation experiments. Recently, Zakaria Gomaa [37] reported that L. fermentum showed the highest anti-adhesion activity against C. albicans ATCC 70014 which the percentage of 84.69 %. The anti-adhesion activity of LAB has been attributed to the presence of biosurfactant in the CFS. The ability of biosurfactant to decrease pathogenic microorganisms attachment was observed by many researchers [24,36-40]. The effect of CFS as anti-adhesion depends on the properties of the supernatant, microorganism tested and surface properties. When the surface is conditioned with supernatant containing biosurfactant it becomes more hydrophilic and consequently, decrease microbial attachment [41]. LAB strains that produce biosurfactants can reduce microbial adhesion and combating colonization by pathogenic microorganisms not only in the biomedical field, but also in food industry [42-44]. The general mechanism in inhibition of adherence of Candida spp. by LAB are competitive with the adhesion sites, and also as a result of the effects of substances present in the supernatant of LAB. The current results indicated that L. curvatus HH had a significant anti-adhesion activity against Candida spp. The result is in agreement with studies of Rodrigues et al. [45] and Falagas, Makris [46] , who reported that biosurfactant isolated from Lactobacillus play an important role in care equipment such as catheters and other medical devices in hospitals.
Conclusion
This study shows that supernatant produced by LAB isolated from honey has anti-adhesion activity against Candida species. This indicates that the supernatant contains compounds which can be used as anti-adhesion on medical devices such as catheters, prosthesis and stents to prevent Candida species infections.
Acknowledgments
The authors would like to thank laboratory the staff at faculty of Science and Technology, Universiti Sains Islam Malaysia (USIM) for their assistance during the course of the study.
7326
References
- Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J (2009) Our current understanding of fungal biofilms. Crit Rev Microbiol 35: 340-355.
- Nucci M, Anaissie E (2001) Revisiting the source of candidemia: skin or gut? Clin Infect Dis 33: 1959-1967.
- Lynch JF, Lappin-Scott HM, Costerton JW (2003) Microbial Biofilms. Cambridge University Press,Cambridge. UK.
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J (2012) Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. Fems Microbiology Reviews 36: 288-305.
- Ozkan S, Kaynak F, Kalkanci A, Abbasoglu U, Kustimur S (2005) Slime production and proteinase activity of Candida species isolated from blood samples and the comparison of these activities with minimum inhibitory concentration values of antifungal agents. Memórias do InstitutoOswaldo Cruz 100: 319-324.
- Ramage G, Martínez JP, López-Ribot JL (2006) Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res 6: 979-986.
- Klotz SA, Gaur NK, De Armond R, Sheppard D, Khardori N, et al. (2007) Candida albicansAls proteins mediate aggregation with bacteria and yeasts. Med Mycol 45: 363-370.
- Harriott MM, Lilly EA, Rodriguez TE, Fidel PL Jr, Noverr MC (2010) Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156: 3635-3644.
- Pascual LM, Daniele MB, Ruiz F, Giordano W, Pájaro C, et al. (2008) Lactobacillus rhamnosus L60, a potential probiotic isolated from the human vagina. J Gen ApplMicrobiol 54: 141-148.
- Zárate G, Nader-Macias ME (2006) Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. LettApplMicrobiol 43: 174-180.
- Balcázar JL, Vendrell D, de Blas I, Ruiz-Zarzuela I, Muzquiz JL, et, al. (2008) Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 278: 188-191.
- Otero C, Nader-Macías ME (2007) Lactobacillus adhesion to epithelial cells from bovine vagina. J Gen Intern Med 2: 749-757.
- Velraeds MM, van der Mei HC, Reid G, Busscher HJ (1996) Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl Environ Microbiol 62: 1958-1963.
- Busscher HJ, van Hoogmoed CG, Geertsema-Doornbusch GI, van der Kuijl-Booij M, van der Mei HC (1997) Streptococcus thermophilus and its biosurfactants inhibit adhesion by Candida spp. on silicone rubber. Appl Environ Microbiol 63: 3810-3817.
- Tamang JP, Tamang B, Schillinger U, Franz CM, Gores M, et al. (2005) Identification of predominant lactic acid bacteria isolated from traditionally fermented vegetable products of the Eastern Himalayas. Int J Food Microbiol105: 347-356.
- Rodrigues L, van der Mei HC, Teixeira J, Oliveira R (2004) Influence of biosurfactants from probiotic bacteria on formation of biofilms on voice prostheses. Appl Environ Microbiol 70: 4408-4410.
- Rönnqvist D, Forsgren-Brusk U, Husmark U, Grahn-Håkansson E (2007) Lactobacillus fermentum Ess-1 with unique growth inhibition of vulvo-vaginal candidiasis pathogens. J. Med. Microbiol56: 1500-1504.
- Aween MM, Hassan Z, Muhialdin BJ, Noor HM, Eljamel YA (2012) Evaluation on antibacterial activity of Lactobacillus acidophilus strains isolated from honey. AJAS 9: 807- 817.
- De Man J, Rogosa d, Sharpe ME (1960) A medium for the cultivation of Lactobacilli. J ApplBacteriol23: 130-135.
- Panthavee W, Pramuan S, Nasakom W (2007) Identification and evaluation of lactic acid bacteria for Pla-som (fermented fish) starter. The 2nd International Conference on Fermentation Technology for Value Added Agricultural Products, Thailand.
- Ogunbanwo ST (2005) Functional properties of lactic acid bacteria isolated from ogi and fufu, two Nigerian fermented foods. Adv Food Sci 27: 14-21.
- Gudiña EJ, Teixeira JA, Rodrigues LR (2010) Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf B Biointerfaces 76: 298-304.
- Peeters E, Nelis HJ, Coenye T (2008) Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 72: 157-165.
- Fracchia L, Cavallo M, Allegrone G, Martinotti M (2010) A Lactobacillus-derived biosurfactant inhibits biofilm formation of human pathogenic Candida albicans biofilm producers. Current research. Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Formatex, Spain: 827-837.
- Tamminen M, Joutsjoki T, Sjöblom M, Joutsen M, Palva A, et al. (2004) Screening of lactic acid bacteria from fermented vegetables by carbohydrate profiling and PCR-ELISA. LettApplMicrobiol 39: 439-444.
- Magnusson J, Ström K, Roos S, Sjögren J, Schnürer J (2003) Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS MicrobiolLett 219: 129-135.
- Silva S, Henriques M, Martins A, Oliveira R, Williams D, et al. (2009) Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Med Mycol 47: 681-689.
- Parahitiyawa NB, Samaranayake YH, Samaranayake LP, Ye J, Tsang PW, et al. (2006) Interspecies variation in Candida biofilm formation studied using the Calgary biofilm device. APMIS 114: 298-306.
- Ruiz-Argueso T, Rodriguez-Navarro A (1975) Microbiology of ripening honey. ApplMicrobiol 30: 893-896.
- Bahiru B, Mehari T, Ashenafi M (2006) Yeast and lactic acid flora of tej, an indigenous Ethiopian honey wine: variations within and between production units. Food Microbiol 23: 277-282.
- Hosny I, El-Ghani SA, Nadir A. (2009) Nutrient composition and microbiological quality of three unifloral honeys with emphasis on processing of honey probiotic youghurt. Global Veterinaria 3: 107-112.
- Forsgren E, Olofsson TC, Vásquez A, Fries I (2010) Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie 41: 99-108.
- Aween MM, Hassan Z, Muhialdin BJ, Eljamel YA, Al-Mabrok ASW, et al. (2012) Antibacterial Activity of Lactobacillus acidophilus Strains Isolated from Honey Marketed in Malaysia against Selected Multiple Antibiotic Resistant (MAR) Gram-Positive Bacteria. J Food Sci 77: 364-371.
- Atanassova M, Choiset Y, Dalgalarrondo M, Chobertb JM, Doussetc X, et al. (2003) Isolation and partial biochemical characterization of a proteinaceous anti-bacteria and anti-yeast compound produced by Lactobacillus paracasei subsp. paracasei strain M3. Int J Food Microbiol87: 63-73
- Ogunshe AA, Omotoso MA, Bello VB (2011) The in vitro antimicrobial activities of metabolites from lactobacillus strains on Candida species implicated in Candida vaginitis. Malays J Med Sci 18: 13-25.
- Gudiña EJ, Rocha V, Teixeira JA, Rodrigues LR (2010) Antimicrobial and antiadhesive properties of a biosurfactant isolated from Lactobacillus paracasei ssp. paracasei A20. LettApplMicrobiol 50: 419-424.
- ZakariaGomaa E (2013) Antimicrobial and anti-adhesive properties of biosurfactant produced by lactobacilli isolates, biofilm formation and aggregation ability. J Gen ApplMicrobiol 59: 425-436.
- Gudiña EJ, Teixeira JA, Rodrigues LR (2010) Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf B Biointerfaces 76: 298-304.
- Ali O. (2012) Prevention of Proteus mirabilis biofilm by surfactant solution. Egypt Acad J of BiolSci 4: 1-8.
- Abedi D, Feizizadeh S, Akbari V, Jafarian-Dehkordi A (2013) In vitro anti-bacterial and anti-adherence effects of Lactobacillus delbrueckiisubspbulgaricus on Escherichia coli. Res Pharm Sci 8: 260-268.
- Zeraik AE, Nitschke M (2010) Biosurfactants as agents to reduce adhesion of pathogenic bacteria to polystyrene surfaces: effect of temperature and hydrophobicity. CurrMicrobiol 61: 554-559.
- Nitschke M, Costa S (2007) Biosurfactants in food industry. Trends Food Sci Tech 18: 252-259.
- Singh A, Van Hamme JD, Ward OP (2007) Surfactants in microbiology and biotechnology: Part 2. Application aspects. BiotechnolAdv 25: 99-121.
- Rodrigues L, Moldes A, Teixeira J, Oliveira R (2006) Kinetic study of fermentative biosurfactant production by Lactobacillus strains. BiochemEngJ 28: 109-116.
- Rodrigues L, Banat IM, Teixeira J, Oliveira R (2006) Biosurfactants: potential applications in medicine. J AntimicrobChemother 57: 609-618.
- Falagas ME, Makris GC (2009) Probiotic bacteria and biosurfactants for nosocomial infection control: a hypothesis. J Hosp Infect 71: 301-306.










