Keywords
Leaf extract; Antibacterial activity; Wound isolates; Paullinia pinnata
Introduction
The medicinal importance of large number of plants has not been investigated. Globally, plants have served as the richest source of raw materials for traditional as well as modern medicine, particularly in Africa and Asia [1]. Many of today’s modern drugs have their origin in traditional plant medicine [2]. The therapeutic efficacies of many indigenous plants for several disorders have been described by practitioners of traditional herbal medicines [3].
Paullinia pinnata, a climbing shrub with compound leaves and winged rachis, inflorescences stand axillary on long stalks, and bearing paired collected tendrils with white flowers. P. pinnata grows in evergreen and mixed forests up to an altitude of 1200 m. The plant is a perennial climber with a height of 2.5 to 8.0 m. The fruit is up to 2.5 cm long [4]. In traditional medicine, various parts of P. pinnata are used for treating various diseases. In South West Nigeria, the leaf juice of P. pinnata is used to treat sore throat [5]. An infusion from P. pinnata is used for fever while the roots are used for the treatment of jaundice, leprosy, snake bites vomiting and nausea [6,7]. The whole plant is used to treat dysentery in Ghana. The roots, mashed with seeds of Piper guineense, are applied as a styptic to cut veins and to treat leprosy [8]. The roots are also chewed for coughs and pulmonary diseases, gonorrhoea, fractures or abscesses or used on open sores. It is also used for the treatment of Aphrodisiac [9].
Wounds, resulting from microbial infection, are the most common public health problems [10]. The common wound pathogens includes bacteria, fungi, protozoa and viruses among which the most common are beta haemolytic Streptococci [Streptococcus pyogens (S. pyogens)], Staphylococcus aureus (S. aureus), Pseudomonas aeuruginosa (P. aeuruginosa), Proteus spp, Escherichia coli (E. coli) and Enterococcus, Acinetobacter spp, Klebsiella spp and Coliforms [11-13]. Although wounds may heal through the body’s natural process of regenerating dermal and epidermal tissues, chronic ones cause apparent impact on human health and economic growth [14]. Current means of treating chronic wounds include irrigation, debridement, antibiotics, tissue grafts and proteolytic enzymes, which come with major drawbacks and unwanted side effects on human [15].
In this study, we investigate the antibacterial activity of P. pinnata against wound bacterial isolates with the possibility of formulating of antimicrobial agents of natural origin which could be used as cheaper alternative for the therapeutic management of wound infections.
Materials and Methods
Materials
Source of plant material and identification: Fresh mature leaf of Paullinia pinnata used for this research was collected from the Teaching and Research Farm of Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria on the 22nd of March 2017 with latitude (7 51’ North), longitude (3 56' East) and altitude 304 m. The leaf was green in colour with voucher specimen’s number: FPI 2211.
Gram positive bacteria include: Staphylococcus aureus, Staphylococcusspp (C1), Staphylococcus spp (C2), Streptococcus canis (LIO), Staphylococcus massiliensis (LIO), Streptococcus pyogen (LIO), Streptococcus spp (C3), Streptococcus spp (C4), Macrococcus spp (C5), Macrococcus spp (C6), Globicatella spp (C7) and Salinicoccus spp (C8). Gram negative bacteria include: Klebsiella pneumoniae (LIO), Klebsiella ozanae (LIO), Enterobacter asburiae (LIO), Aerococcus suis (LIO), Salmonella enterica (LIO), Liminorella spp (C9), Citrobacter spp (C10), Liminorella spp (C11), Citrobacter spp (C12), Proteus spp (C13), Yersinia spp (C14) and Proteus vulgarise (LIO).
Culture media used
All the culture media used in this study were manufactured by Lab M Ltd, UK. Nutrient broth and nutrient agar were used to subculture the Bacterial isolates, the wound isolates were stocked in the refrigerator on nutrient agar slant at 4°C. Media were prepared and sterilized at 121°C for 15 minutes.
Antimicrobial agents
Ampicillin (1 mg/mL each) was used as positive control in the antibacterial testing of the plant leaf extract and multiple antibiotic disc for Gram positive and Gram negative bacteria for antibiotic sensitivity testing of the test bacterial isolates.
Methods
Drying and extraction of the plant material
The leaf of Paullinia pinnata was collected and air-dried under the shade at room temperature (25°C ± 1°C) until constant weight was obtained and subsequently ground to powder at the Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife. The powdery form was weighed (1300 g). About 1300 g of the powdered leaf sample was soaked in 5 L of 70% ethanol for five days with regular agitation and then filtered. The extract was filtered out into a clean sterile conical flask using Whatman filter paper. The filtrate was concentrated in vacuum at low temperature (40°C) using a rotary evaporator. The yield (108 g) of crude extract was recovered. The yield obtained was dark in colour. The leaf extract was later stored into a glass petri dish.
Preparation of concentrations of crude ethanolic Paullinia pinnata leaf extract
The standard concentration 35 mg/mL was prepared by dissolving 175 mg of crude leaf extract of Paullinia pinnata in 5 mL of DMSO/H2O (2:3). The other concentrations (17.5 mg/mL 8.75 mg/mL, 4.38 mg/mL, 2.19 mg/mL) were prepared by double dilution of the standard solution.
Determination of the antibacterial activity of crude ethanolic Paullinia pinnata leaf extract
The susceptibility testing of the test bacteria to the plant leaf extract was determined using agar-well diffusion method with some modifications. The bacterial isolates were first grown in nutrient broth for 18 h before use. Then 0.2 mL of the standardized test isolates (108 cfu/mL of 0.5 McFarland standards) was then sub-cultured on molten Mueller-Hinton agar. The medium was allowed to set and wells were then bored into the agar medium using a sterile 6 mm cork borer. The wells were then filled up with 1 mL of the prepared extract concentrations (8.75 mg/mL, 17.5 mg/mL and 35 mg/mL). The remaining two wells were filled with 1 mg/mL of ampicillin and 1 mL dissolving solvent (DMSO/H2O) which serves as positive and negative controls, respectively. The plates were allowed to stand on the laboratory bench upright for 1 h to allow proper diffussion of the solution into the medium before incubating the plates in an incubator at 37°C for 24 h. The plates were later observed for the zones of inhibition and measured using transparent metre rule to the nearest millimetre (mm). The effects of the leaf extract on bacterial isolates were compared with standard antibiotic (ampicillin) at a concentration of 1 mg/mL which served as a positive control.
Antibiotic disc sensitivity test for wound isolates used
The antibiotic disc sensitivity test was carried out on the test isolates to determine multiple antibiotic resistant microorganisms among them. Antibiotic susceptibility of the isolates was done using the Kirby-Bauer’s disc diffusion method and interpreted according to the guidelines of Clinical and Laboratory Standard Institute (CLSI, 2013).
Mueller-Hinton agar was prepared according to the manufacturer’s instruction. The medium was allowed to cool to 45-50°C and poured into sterile petri dishes. The sterile petri dishes were allowed to set on a level surface to a depth of approximately 4 mm. When the agar had solidified, plates were allowed to dry before use. Then 18-24 h old broth cultures of the isolates were standardized by diluting to 0.5 Mcfarland’s standard (108 cfu/mL). A sterile swab stick was inserted into the standardized isolate inocula, drained to remove excess inoculum load and inoculated by spreading on the surface of prepared Mueller-Hinton agar plate. After this, the inoculated Mueller-Hinton agar plate was allowed to dry for few minutes with the lid closed. Then antibiotic impregnated discs of known concentration were carefully applied on the inoculated Mueller-Hinton agar plates using sterile forceps.
The antibiotic discs used for Gram negative microorganisms were manufactured by ABTEK Biologicals Ltd. UK. These antibiotics include Gentamicin 10 μg (GEN), Cefuroxime 30 μg (CRX), Ceftazidime 30 μg (CAZ), Ciprofloxacin 5 μg (CPR), Nitrofurantoin 300 μg (NIT), Augmentin 30 μg (AUG), ofloxacin 5 μg (OFL) and cefixime 5 μg (CXM). The antibiotic discs used for Gram positive microoganisms were manufactured by MAXICARE MEDICAL LAB. These antibiotics include pefloxacin 10 μg (PEF), gentamycin 10 μg (CN), ampiclox 30 μg (APX), zinnacef 20 μg (Z), amoxacillin 30 μg (AM), rocephin 25 μg (R), ciprofloxacin 10 μg (CPX), streptomycin 30 μg (S), septrin 30 μg (SXT) and erythromycin 10 μg (E). The plates were then incubated at 37oC for 18-24 h. The diameters of the zones of inhibition were measured with a transparent calibrated ruler to the nearest millimeter and recorded. The results were recorded as Resistant (R) and Susceptible (S) according to the guideline of Clinical and Laboratory Standard Institute (CLSI, 2013).
Statistical Analysis
The experiments were carried out in triplicates. The results were presented as mean Standard Deviation (SD). Student’s ttest was used for comparison between the two means and a one-way analysis of variance (ANOVA) was used for comparison of more than two means.
Results and Discussions
Total yield of extract obtained from the leaf of Paullinia pinnata
The leaf extract collected was dark in colour and the yield was 108 g, which was 8.31% of the powdered leaf sample (1300 g) used. Fractions of 5.4 g of n-hexane, 4.5 g of ethyl acetate, 1.7 g of acetone and 0.9 g of ethanol were obtained.
Diameter of zone of inhibition exhibited by crude ethanolic leaf extract of P. pinnata and ampicillin against the wound bacterial isolates: Tables 1 and 2 shows the antibacterial effect of Crude ethanolic leaf extract of P. pinnata and standard antibiotic against test bacterial isolates. Out of the 24 test bacteria used, 16 was susceptible to the crude ethanolic leaf extract of P. pinnata at concentrations of 35 mg/mL, 17.5 mg/mL and 8.75 mg/mL, respectively. The zones of inhibition exhibited by the test bacteria at concentration of 35 mg/mL ranged between 5.00 mm and 20.00 mm, the lowest zone of inhibition of 5.00 mm was observed for Macrococcus bruencis, while the highest zone of inhibition 22.00 mm was observed for Staphylococcus muscae. Twenty out of the 24 test bacteria screened were susceptible to ampicillin with the zones of inhibition ranging between 15.00 mm to 30.00 mm, while Enterobacter asburiae was resistant to this antibiotic.
| Test Bacterial |
Zones of Inhibition (mm)** |
| Crude Extracts of ethanol |
Ampicillin |
| (8.75 mg/mL) |
(17.5 mg/mL) |
(35 mg/mL) |
(1 mg/mL) |
| Macrococcus spp (C6) |
0 |
0 |
5.00 ± 1.41 |
25.00 ± 1.22 |
| Aerococcus suis |
9.00 ± 1.41 |
10.00 ± 1.41 |
12.00 ± 1.41 |
24.00 ± 2.12 |
| Staphylococcus aureus |
8.00 ± 1.41 |
12.00 ± 1.41 |
15.00 ± 1.41 |
20.00 ± 1.50 |
| Citrobacter spp (C12) |
12.00 ± 0.71 |
11.00 ± 1.41 |
15.00 ± 0.71 |
21.00 ± 0.00 |
| Globicatella spp (C7) |
11.00 ± 1.41 |
17.00 ± 1.41 |
19.00 ± 0.85 |
22.00 ± 1.55 |
| Streptococcus canis |
5.00 ± 1.41 |
7.00 ± 1.41 |
11.00 ± 1.41 |
25.00 ± 1.88 |
| Staphlococcus spp (C2) |
10.00 ± 1.41 |
11.00 ± 1.41 |
16.00 ± 1.41 |
25.00 ± 1.05 |
| Citrobacter spp (C10) |
9.00 ± 1.41 |
11.00 ± 1.41 |
15.00 ± 1.27 |
25.00 ± 0.34 |
| Proteus vulgaris |
9.00 ± 0.71 |
12.00 ± 0.71 |
15.00 ± 1.84 |
26.00 ± 0.13 |
| Staphlococcus massiliensis |
0 |
13.00 ± 0.00 |
18.00 ± 1.41 |
19.00 ± 0.71 |
| Salmonela enterica |
0 |
0 |
0 |
25.00 ± 0.85 |
| Staphlococcus spp (C1) |
10.00 ± 0.14 |
14.00 ± 0.14 |
20.00 ± 1.41 |
24.00 ± 1.27 |
| Salinicoccus spp (C8) |
8.00 ± 0.71 |
11.00 ± 1.41 |
15.00 ± 1.41 |
22.00 ± 0.87 |
| Streptococcus pyogenes |
6.00 ± 1.41 |
9.00 ± 1.41 |
12.00 ± 1.41 |
19.00 ± 2.44 |
Table 1 The diameter of zone of inhibition of the crude ethanolic extract of P. pinnata leaf against the test wound bacterial isolates.
| Test Bacterial |
Zone of Inhibition (mm)** |
| Crude Extract of ethanol |
Ampicillin |
| 8.75 mg/mL |
17.5 mg/mL |
35 mg/mL |
1 mg/mL |
| Streptococcus spp (C3) |
5.00 ± 1.41 |
9.00 ± 1.41 |
13.00 ± 1.41 |
0 |
| Liminorella spp (C9) |
6.00 ± 1.41 |
10.00 ± 1.41 |
12.00 ± 1.41 |
17.00 ± 2.44 |
| Klebsiella ozanae |
0 |
0 |
0 |
19.00 ± 0.00 |
| Streptococcus spp (C4) |
0 |
0 |
0 |
13.50 ± 1.20 |
| Klebsiella pneumonia |
0 |
0 |
0 |
18.00 ± 2.22 |
| Enterobacter asburia |
0 |
0 |
0 |
0 |
| Proteus spp (C13) |
0 |
0 |
0 |
0 |
| Yersinia spp (C14) |
0 |
0 |
0 |
0 |
| Liminorella spp (C11) |
0 |
0 |
0 |
0 |
| Macrococcus spp (C5) |
7.00 ± 1.88 |
15.00 ± 1.41 |
18.00 ± 1.41 |
30.00 ± 1.32 |
All organisms are clinical isolates, locally isolated from human infected wound. **: mean of three replicates; SD: Standard Deviation; C1-C14: Organisms code.
Table 2 The diameter of zone of inhibition of the crude ethanolic extract of P. pinnata leaf against the test wound bacteria continues.
Antimicrobial activities exhibited by the fractions obtained from the crude leaf extract of Paullinia pinnata against the wound bacteria isolates: Table 3 shows the results of antimicrobial activities of the extract fractions (partitioned crude) at a concentration of 10 mg/mL on wound isolates. Nhexane fraction inhibited the growth of 15 out of the 16 susceptible wound isolates with the zones of inhibition ranging between 6.00 mm and 22.00 mm. The highest zone of inhibition of 22.00 mm was observed against Aerococcus suis, while the lowest zone of inhibition of 6.00 mm was observed against Citrobacter spp (C5). Also, Streptococcus spp (C3) was resistant to n-hexane fraction. On the other hand, the ethyl acetate fraction inhibited the growth of 14 out of the 16 susceptible wound isolates while Streptococcus canis and S. pyogenes were resistant to this fraction. The zones of inhibition exhibited by the ethyl acetate fraction against susceptible test isolates ranged between 7.00 mm and 28.00 mm. The highest zone of inhibition of 28.00 mm was observed against Aerococcus suis lowest zone of inhibition 7 mm was observed against Macrococcus spp (C6). The organisms were resistance to acetone and ethanol fractions.
| Test Bacteria |
Zones of Inhibition (mm)** |
Ethanol
(10 mg/mL) |
Acetone
(10 mg/mL) |
Ethyl acetate (10 mg/mL) |
N-hexane
(10 mg/mL) |
| Proteus vulgaris |
0 |
0 |
20.00 ± 1.84 |
16.00 ± 0.00 |
| Staphlococcus massiliensiss |
0 |
0 |
24.00 ± 1.41 |
14.00 ± 1.41 |
| Staphylococcus aureus |
0 |
0 |
26.00 ± 1.27 |
16.00 ± 2.69 |
| Aerococcus suis |
0 |
0 |
28.00 ± 1.27 |
22.00 ± 1.41 |
| Macrococcus spp (C5) |
0 |
0 |
25.00 ± 1.4 |
17.00± 0.00 |
| Liminorella spp (C9) |
0 |
0 |
15.00± 1.41 |
8.00± 0.14 |
| Staphlococcus spp (C2) |
0 |
0 |
22.00 ± 0.85 |
17.00± 0.71 |
| Staphlococcus spp (C1) |
0 |
0 |
9.00 ± 1.41 |
18.00± 0.85 |
| Streptococcus canis |
0 |
0 |
0 |
8.00 ± 1.88 |
| Streptococcus pyogenes |
0 |
0 |
0 |
17.00 ± 1.41 |
| Citrobacter spp (C12) |
0 |
0 |
21.00 ± 1.88 |
15.00± 1.85 |
| Streptococcus spp (C3) |
0 |
0 |
9.00 ± 1.41 |
0 |
| Globicatella spp (C7) |
0 |
0 |
25.00± 1.85 |
18.00± 1.21 |
| Macrococcus spp (C6) |
0 |
0 |
7.00 ± 1.21 |
15.00 ± 1.41 |
| Citrobacter spp (C10) |
0 |
0 |
10.00± 1.22 |
6.00± 1.88 |
| Salinicoccus spp (C8) |
0 |
0 |
22.00 ± 1.41 |
15.00± 1.85 |
Table 3 Antimicrobial activities exhibited by the Paullinia pinnata leaf fractions against the wound bacterial isolates.
The synergetic antibacterial effect of ethyl acetate/Nhexane fractions P. pinnata leaf extract against the wound bacterial isolates: There was considerably increase in the antibacterial activity of the plant extract on the wound isolates resulting from the combined activities of two most active fractions (N-hexane/ethyl acetate). The zone of inhibition ranged from 12.00 mm to 26.00 mm. The Highest zone of inhibition of 26 mm was observed against Aeococcus suis and the lowest zone of inhibition of 12.00 mm was observed against Macrococcus spp (C6). The combined fractions have activities on all the sixteen susceptible wound bacteria with a higher zone of inhibition as compared to each fraction singly applied. Table 4 shows the sensitivity pattern of the effect of the combined fraction.
| |
Zones of Inhibition (mm)** |
| Wound Bacteria |
Ethyl acetate/n-hexane (10 mg/mL) |
| Proteus vulgaris |
20.00 ± 0.00 |
| Staphlococcus massiliensis |
23.00 ± 1.21 |
| Staphylococcus aureus |
24.00 ± 2.10 |
| Aerococcus suis |
26.5 ± 0.68 |
| Macrococcus spp (C5) |
22.00 ± 1.66 |
| Liminorella spp (C9) |
16.00 ± 1.41 |
| Staphlococcus spp (C2) |
14.00 ± 1.85 |
| Staphlococcus spp (C1) |
18.5.00 ± 1.21 |
| Streptococcus canis |
13.00 ± 1.14 |
| Streptococcus pyogenes |
24.00 ± 1.85 |
| Citrobacter spp (C12) |
14.00 ± 1.88 |
| Streptococcus spp (C3) |
18.00 ± 1.22 |
| Globicatella spp (C7) |
17.5.00 ± 1.41 |
| Macrococcus spp (C6) |
12.00 ± 2.44 |
| Citrobacter spp (C10) |
20.00 ± 1.22 |
| Salinicoccus spp (C8) |
22.00 ± 1.88 |
**=mean of three replicates; SD: Standard Deviation
Table 4 The synergetic antibacterial effect of ethyl acetate/n-hexane fractions of P. pinnata leaf extract against the wound bacterial isolates.
Antibiotic susceptibility testing of the test bacteria
The sensitivity test was carried out on the 16 microorganisms that were susceptible to the leaf extract of P. pinnata or its fractions to determine the multiple antibiotic resistant isolates among them. The result shows that Enterobacter asburiae and Klebsiella ozanae which exhibited multiple antibiotic resistances were susceptible to n-hexane fraction and n-hexane/ethyl acetate combined fractions of the plant leaf extract. Other wound isolates were not multiple antibiotics resistant. Aerococcus suis was resistant to Gentamicin, Cefuroxime, Augmentin and Nitrofurantoin but was susceptible to Ceftazidime, Cefixime, Ciprofloxacin, and Ofloxacin. Proteus vulgarise was resistant to Gentamicin, Ceftazidime, Ciprofloxacin, Augmentin and Cefixine but was susceptible to Cefuroxime, Nitrofuratoin and Ofloxacin (Table 5).
| Microorganism |
GEN |
CRX |
CAZ |
CPR |
NIT |
AUG |
OFL |
CXM |
| Diameter of Zone of Growth Inhibition (mm) |
| Enterobacter asburiae (LIO) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
| Klebsiella ozanae (LIO) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0(R) |
0 (R) |
0 (R) |
| Aerococcus suis (LIO) |
0 (R) |
0 (R) |
16 (S) |
18 (S) |
0 (R) |
0 (R) |
20 (S) |
0 (R) |
| Liminorella spp (C9) |
12 (S) |
10 (S) |
0 (R) |
20 (S) |
18 (S) |
0 (R) |
18 (S) |
0 (R) |
| Proteus vulgaris (LIO) |
18 (S) |
20 (S) |
0 (R) |
0 (R) |
20 (S) |
0 (R) |
20 (S) |
0 (R) |
| Citrobacter spp (C10) |
28 (S) |
14 (S) |
24 (S) |
0 (R) |
20 (S) |
30 (S) |
26 (S) |
20 (S) |
| Citrobacter spp (C12) |
20 (S) |
0 (R) |
0 (R) |
0 (R) |
18 (S) |
0 (R) |
0 (R) |
0 (R) |
GEN: Gentamicin (10 μg); CRX: Cefuroxime (30 μg); CAZ: Ceftazidime (30 μg); CPR: Ciprofloxacin (5 μg); NIT: Nitrofurantoin (300 μg); AUG: Augmentin (30 μg); OFL: Ofloxacin (5 μg) and CXM=Cefixime (5 μg).
Table 5 The antibiotic susceptibility profile of the test gram negative bacteria.
Table 6 shows the antibiotic susceptibility profile test Gram positive bacteria. Staphlococcus spp (C2) was resistant to all the antibiotics used except Ciprofloxacin and Septrin. Citrobacter spp (C12) was only susceptible to Pefloxacin, Ampiclox and Erythromycin. Streptococcus canis was resistant to all antibiotics except Ampiclox and Zinnacef. Streptococcus equi was resistant to all antibiotics except Ampiclox, Zinnacef and Erythromycin. Globicatella spp (C7) was only susceptible to Ampiclox and Zinnacef. Staphylococcus massiliensis was only resistant to Ampiclox but susceptible to all other antibiotics. Macrococcus spp (C5), Staphylococcus spp (C2), Staphylococcus spp (C1) and Staphylococcus aureus were resistant to Ampiclox and Amoxacillin but susceptible to other antibiotics. Macrococcus spp (C6) was susceptible to all the antibiotics except Ampiclox.
| Microorganism |
PEF |
CN |
APX |
Z |
AM |
R |
CPX |
S |
SXT |
E |
| Diameter of Zone of Growth Inhibition (mm) |
| Staphylococcus massiliensis (LIO) |
25 (S) |
22 (S) |
16 (S) |
24 (S) |
0 (R) |
20 (S) |
32 (S) |
24 (S) |
16 (S) |
28 (S) |
| Macrococcus lamae spp (C5) |
34 (S) |
24 (S) |
0 (R) |
12 (I) |
0 (R) |
22 (S) |
32 (S) |
30 (S) |
30 (S) |
26 (S) |
| Staphylococcus aureus (LIO) |
0 (S) |
0 (S) |
0 (R) |
20 (S) |
0 (R) |
20 (S) |
0 (S) |
20 (S) |
18 (S) |
0 (S) |
| Macrococcus spp (C6) |
20 (S) |
22 (S) |
0 (R) |
24 (S) |
16 (S) |
20 (S) |
26 (S) |
30 (S) |
16 (S) |
24 (S) |
| Salinicoccus spp (C8) |
30 (S) |
20 (S) |
0 (R) |
22 (S) |
0 (R) |
18 (S) |
32 (S) |
30 (S) |
16 (S) |
28 (S) |
| Staphylococcus spp (C1) |
30 (S) |
28 (S) |
0 (R) |
22 (S) |
0 (R) |
18 (S) |
30 (S) |
30 (S) |
16 (S) |
22 (S) |
| Staphylococcus spp (C2) |
0 (R) |
22 (S) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
30 (S) |
0 (R) |
0 (R) |
| Streptococcus canis (LIO) |
0 (R) |
0 (R) |
0 (R) |
25 (S) |
17 (S) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
| Streptococcus pyogene (LIO) |
0 (R) |
21 (S) |
16 (S) |
0 (R) |
0 (R) |
0 (R) |
22 (S) |
0 (R) |
14 (S) |
20 (S) |
| Streptococcus spp (C3) |
0 (R) |
0 (R) |
18 (S) |
10 (S) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
16 (S) |
| Globicatella spp (C7) |
0 (R) |
0 (R) |
0 (R) |
25 (S) |
17 (S) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
0 (R) |
PEF: Pefloxacin (10 g); CN: Gentamycin (10 g); APX: Ampiclox (30 g); Z: Zinnacef (20 g); AM: Amoxacillin (30 g); R: Rocephin (25 g); CPX: Ciprofloxacin (10 g); S: Streptomycin (30 g); SXT: Septrin (30 g) and E: Erythromycin (10 g)
Table 6 The antibiotic susceptibility profile of the test gram positive bacteria.
The Minimum Inhibitory Concentrations (MICs) and the Minimum Bactericidal Concentrations (MBCs) exhibited by antibiotic, crude and fractions of P. pinnata leaf extract against test bacteria.
The minimum inhibitory concentrations and the minimum bactericidal concentrations exhibited by the ampicillin, crude leaf extract, ethyl acetate and n-hexane fractions against the wound isolates are shown in Table 7.
| Bacterial Isolates |
Ampicillin
antibiotic |
Crude extract |
Ethylene fraction |
n-hexane fraction |
| MIC (µg/mL) |
MBC (µg/mL) |
MIC (mg/mL) |
MBC (mg/mL) |
MIC (mg/mL) |
MBC (mg/mL) |
MIC (mg/mL) |
MBC (mg/mL) |
| Proteus vulgaris |
500.0 |
1000 |
8.75 |
8.75 |
4.38 |
8.75 |
2.19 |
4.38 |
| Staphylococcus massiliensis |
62.50 |
125.0 |
4.38 |
8.75 |
4.38 |
8.75 |
2.19 |
4.38 |
| Staphylococcus aureus |
62.50 |
125.0 |
8.75 |
8.75 |
4.38 |
8.75 |
2.19 |
4.38 |
| Aeromonas suis |
500.0 |
1000 |
8.75 |
17.50 |
4.38 |
8.75 |
4.38 |
8.75 |
| Staphlococcus spp (C1) |
125.0 |
250.0 |
4.38 |
8.75 |
2.19 |
4.38 |
2.19 |
4.38 |
| Macrococcus spp (C5) |
500.0 |
1000 |
4.38 |
8.75 |
2.19 |
4.38 |
2.19 |
4.38 |
| Macrococcus spp (C6) |
500.0 |
1000 |
4.38 |
8.75 |
4.38 |
8.75 |
2.19 |
4.38 |
| Staphlococcus spp (C2) |
125.0 |
250.00 |
4.38 |
8.75 |
4.38 |
8.75 |
2.19 |
4.38 |
| Salinicoccus spp (C8) |
125.0 |
250.00 |
4.38 |
8.75 |
4.38 |
8.75 |
2.19 |
4.38 |
| Streptococcus canis |
62.50 |
125.00 |
4.38 |
8.75 |
4.38 |
8.75 |
2.19 |
4.38 |
| Streptococcus pyogenes |
62.50 |
125.00 |
2.19 |
4.38 |
4.38 |
8.75 |
2.19 |
4.38 |
| Citrobacter spp (C10) |
500.0 |
1000 |
17.50 |
35.0 |
8.75 |
17.5 |
8.75 |
17.5 |
| Citrobacter spp (C12) |
500.0 |
1000 |
8.75 |
17.50 |
4.38 |
8.75 |
2.19 |
4.38 |
| Streptococcus spp (C3) |
125 |
250 |
17.50 |
20.0 |
4.38 |
8.75 |
4.38 |
17.5 |
| Liminorella spp (C9) |
500.0 |
1000 |
8.75 |
17.50 |
4.38 |
8.75 |
4.38 |
17.5 |
| Globicatella spp (C7) |
250 |
500 |
17.50 |
35.0 |
4.38 |
8.75 |
2.19 |
4.38 |
Table 7 The minimum inhibitory concentrations and minimum bactericidal concentrations of the antibiotic, crude extract and fractions of P. pinnata leaf exhibited against the test bacteria.
The MICs exhibited by ampicillin against the test isolates ranged between 62.50 g/mL and 500.00 g/mL. The lowest MIC of 62.50 μg/mL was observed against S. massiliensis, S. aureus, S. canis, S. pyogens whereas the highest MIC of 500.00 μg/mL exhibited by ampicillin was observed P. vulgaris, A. suis, Macrococcus spp (C5), Macrococcus spp (C6), Citrobacter spp (C10), Citrobacter spp (C12), Liminorella spp (C9). The MBC exhibited by the ampicillin antibiotic against test isolates ranged between 125.00 μg/mL and 1000.00 μg/mL. The lowest MBC of 125.00 μg/mL was observed against S. massiliensis, S. aureus, S. canis, S. pyogene while the highest MBC of 1000.00 μg/mL was observed against P. vulgaris, A. suis, Macrococcus spp (C5), Macrococcus spp (C6), Citrobacter spp (C10), Citrobacter spp (C12), Liminorella spp (C9). The MICs exhibited by the crude extract against the test organisms ranged between 2.19 mg/mL and 17.50 mg/mL. The lowest MIC of 2.19 mg/mL was observed against S. pyogenes whereas the highest MIC of 17.50 mg/mL exhibited by crude extract was observed against Citrobacter spp (C10), Streptococcus spp (C3). The MBC exhibited by the crude extract against test isolates ranged between 4.38 mg/mL and 17.50 mg/mL. The lowest MBC of 4.38 mg/mL was observed against S. pyogenes while the highest MBC of 17.50 mg/mL was observed against Citrobacter spp (C10).
The MICs exhibited by n-hexane fraction against the test isolates used for this study ranged between 2.19 mg/mL and 8.75 mg/mL. The lowest MIC of 0.63 mg/mL was observed against Staphylococcus spp (C1), Macrococcus spp (C5), while the highest MIC of 2.19 mg/mL was observed against Citrobacter spp (C10). The MBC exhibited by the n-hexane fraction against the test isolates ranged between 4.38 mg/mL and 17.5 mg/mL. The lowest MBC of 4.38 mg/mL was observed against Staphylococcus spp (C1), Macrococcus spp (C5) and the highest MBC of 17.5 mg/mL was observed against Citrobacter spp (C10).
The MICs exhibited by ethyl acetate fraction against the test isolates ranged between 2.19 mg/mL and 8.75 mg/mL. The lowest MIC of 2.19 mg/mL was observed against P. vulgaris, S. aureus, S. massiliensis, Staphylococcus spp (C2), Staphylococcus spp (C1), Macrococcus spp (C5), Macrococcus spp (C6), Salinicoccus spp (C8), S. pyogene, S. canis, Citrobacter spp (C12) and the highest MIC of 8.75 was observed against Citrobacter spp (C10). The MBC exhibited by ethyl acetate fraction against test isolates ranged between 4.38 mg/mL and 17.5 mg/mL. The lowest MBC of 4.38 mg/mL was observed against P. vulgaris, S. aureus, S. massiliensis, Staphylococcus spp (C2), Staphylococcus spp (C1), Macrococcus spp (C5), Macrococcus spp (C6), Salinicoccus spp (C8), S. pyogene, S. canis, Citrobacter spp (C12), while the highest MBC of 17.5 mg/mL was observed against Citrobacter spp (C10) and Staphylococcus spp (C3).
The MBC exhibited by the n-hexane fraction against the test isolates ranged between 4.38 mg/mL and 17.5 mg/mL. The MICs exhibited by ethyl acetate fraction against the test isolates ranged between 2.19 mg/mL and 8.75 mg/mL.
The extent and rate of killing exhibited by the most active (ethyl acetate) fraction of P. pinnata leaf extract against Proteus vulgarise and Staphylococcus aureus: The extent and rate of killing of Proteus vulgarise exhibited by ethyl acetate fraction at 1 X MIC, 2 X MIC and 3 X MIC concentrations (8.75 mg/mL, 17.50 mg/mL and 35.00 mg/mL respectively) are shown in Figure 1. The percentage of the organisms killed by the extract at 1 X MIC in 0 minutes was 11% and at 15 minutes, it rose to 15%. After 30 minutes of contact time with this extract, the percentage of the organisms killed was 22%. When the contact time was 60 minutes, the percentage of the organisms killed was 24% and 26% at 90 minutes and this rose to 29% after 120 minutes of contact time. When the MIC was doubled, the percentage of organisms killed by the extract within 0 minutes contact time was 18% and at 15 minutes of contact time, the percentage of cells killed increased to 44% and with the increase of the contact time to 30 minutes, the percentage of organisms killed was 54%. When the contact time was 60 minutes, the percentage of the organisms killed was 100%, after 90 minutes of contact time, the percentage of the cells killed was 100% and at 120 minutes of the contact time interval, the percentage of the organisms killed has increased to 100%. The extent and rate of kill of the antibiotic at 3 X MIC after 0 minutes contact time was 28 and this increased to 62 after 15 minutes and also increased to 68% after 30 minutes of the contact time interval. Finally after 60, 90 and 120 minutes of exposure of the organisms to the extract, the percentage of cells killed has increased to 100%.
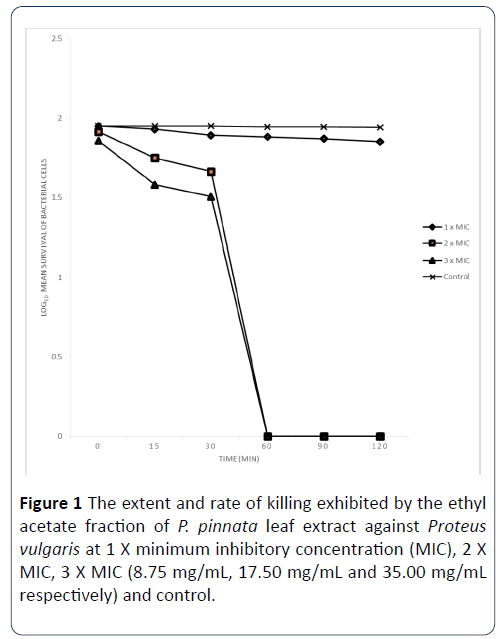
Figure 1: The extent and rate of killing exhibited by the ethyl acetate fraction of P. pinnata leaf extract against Proteus vulgaris at 1 X minimum inhibitory concentration (MIC), 2 X MIC, 3 X MIC (8.75 mg/mL, 17.50 mg/mL and 35.00 mg/mL respectively) and control.
Each designated point represents the mean log10 survival of bacterial cells at a particular time interval in the presence of the extract.
The extent and rate of killing of S. aureus cells by ethyl acetate fraction P. pinnata at 1 X MIC, 2 X MIC and 3 x MIC are shown in Figure 2. The percentage of the organisms killed by the extract at 1X MIC in 0 minutes was 52%, while the percentage of organisms killed at 15 minutes rose to 55%, and after 30 minutes of contact time with this extract, the percentage of the organisms killed was 57% and eventually rose to 65% after 60 minutes. When the contact time was increased to 90 minutes, the percentage of the cells killed was still 65% and this rose to 80% after 120 minutes of contact time. When the MIC was doubled, the percentage of organisms killed by the extract within 0 minutes contact time was 54%, at 15 minutes of contact time, the percentage of cells killed increased to 62%, and with the increase of the contact time to 30 minutes, the percentage of organisms killed was 64%. When the contact time was 60 minutes, the percentage of the organisms killed was 72, after 90 minutes of contact time interval, the percentage of the organisms killed has increased to 75%, and increased sequentially to 100% at 120 minutes of contact time. The extent and rate of kill of the extract at 3 X MIC after 0 minutes contact time was 55% and this increased to 75% after 15 minutes and increased to 80% after 30 minutes of the contact time interval. Finally, after 60, 90, 120 minutes of exposure of the organisms to the extract, the percentage of cells killed has increased to 100%.
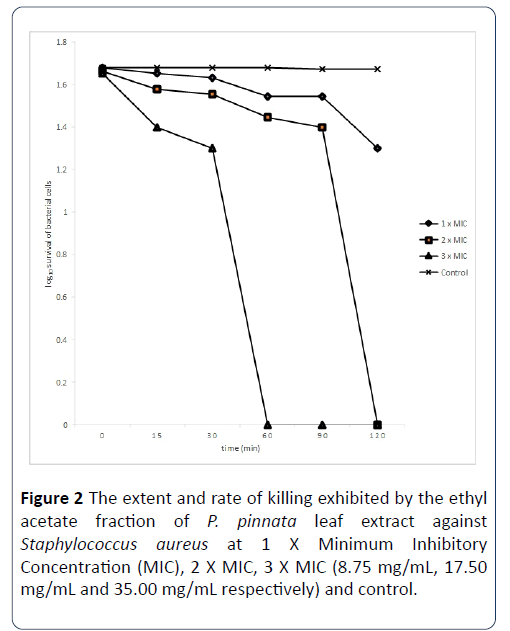
Figure 2: The extent and rate of killing exhibited by the ethyl acetate fraction of P. pinnata leaf extract against Staphylococcus aureus at 1 X Minimum Inhibitory Concentration (MIC), 2 X MIC, 3 X MIC (8.75 mg/mL, 17.50 mg/mL and 35.00 mg/mL respectively) and control.
Each designated point represents the mean log10 survival of bacterial cells at a particular time interval in the presence of the extract.
The extent and rate of killing exhibited by ampicillin antibiotic against Proteus vulgarise and Staphylococcus aureus: The extent and rate of killing of Proteus vulgarise exhibited by Ampicillin antibiotic at 1 X MIC, 2 X MIC and 3 X MIC concentrations (8.75 mg/mL, 17.50 mg/mL and 35.00 mg/mL respectively) are shown in Figure 3. The percentage of the organisms killed by the antibiotic at 1 X MIC in 0 minutes was 8.33%, while the percentage of organisms killed at 15 minutes rose to 46.28%, after 30 minutes of contact time with this antibiotic, the percentage of the organisms killed was 50.41%. At contact time of 60 minutes, the percentage of the organisms killed was 79.34% meanwhile at contact time of 90 minutes, the percentage of the cells killed was 91.74% and this rose to 100% after 120 minutes of contact time. When the MIC was doubled, the percentage of organisms killed by the antibiotic within 0 minutes contact time was 16.67%, at 15 minutes of contact time, the percentage of cells killed increased to 50.41%, and with the increase of the contact time to 30 minutes, the percentage of organisms killed was 54.55%. When the contact time was 60 minutes, the percentage of the organisms killed was 95.87%. After 90 and 120 minutes of the contact time interval, the percentage of the organisms killed has increased to 100%. The extent and rate of kill of the antibiotic at 3 X MIC after 0 minutes contact time was 20.83 and this increased to 58.68 after 15 minutes and also increased to 62.81 after 30 minutes of the contact time interval. After 60, 90 and 120 minutes of exposure of the organisms to the antibiotic, the percentage of cells killed increased to 100%.
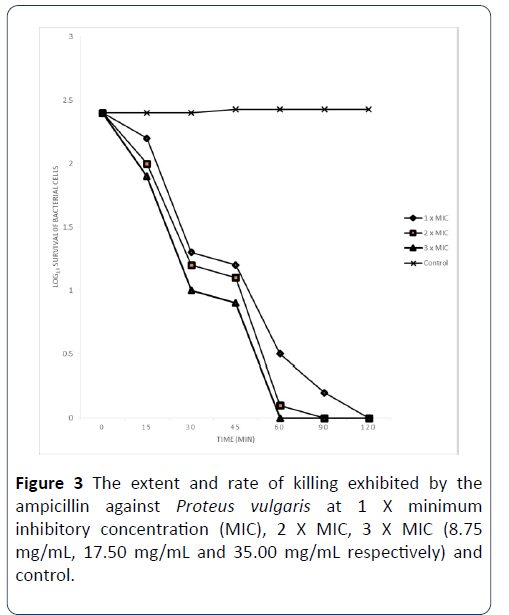
Figure 3: The extent and rate of killing exhibited by the ampicillin against Proteus vulgaris at 1 X minimum inhibitory concentration (MIC), 2 X MIC, 3 X MIC (8.75 mg/mL, 17.50 mg/mL and 35.00 mg/mL respectively) and control.
The extent and rate of killing of S. aureus by Ampicillin antibiotic at 1 X MIC, 2 X MIC and 3 X MIC is shown in Figure 4. The percentage of the organisms killed by the antibiotic at 1 X MIC in 0 minutes was 8.70% while the percentage killed at 15 minutes rose to 43.48%, after 30 minutes of contact time with this antibiotic, the percentage of the organisms killed was 52.17%. At contact time of 60 minutes, the percentage of the organisms killed was 61.21% and at 90 minutes, it was 78.45% and eventually rose to 82.76% after 120 minutes of contact time. When the MIC was doubled, the percentage of organisms killed by the antibiotic within 0 minutes contact time was 17.39%, at 15 minutes of contact time, and increased to 52.17%; and with the increase of the contact time to 30 minutes, the percentage of organisms killed was 65.22%, which increased to 78.45% at 60 minutes. The percentage of the organisms killed increased to 91.38% and 100% after 90 and 120 minutes of the contact time interval, respectively. The extent and rate of kill of the antibiotic at 3 X MIC after 0 minutes contact time was 26.09% and this increased to 69.57% after 15 minutes and it rose to 82.61% after 30 minutes of the contact time interval. After 60 minutes of contact time interval, the percentage of the organisms killed was 91.38%. Finally, at 90 and 120 minutes of exposure of the organisms to the antibiotic, the percentage of cells killed increased to 100%.
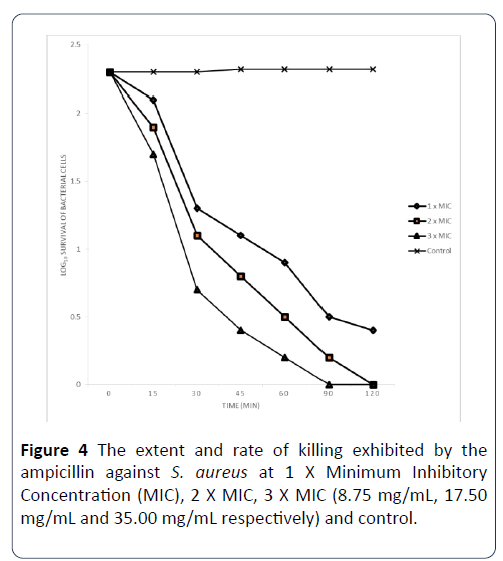
Figure 4: The extent and rate of killing exhibited by the ampicillin against S. aureus at 1 X Minimum Inhibitory Concentration (MIC), 2 X MIC, 3 X MIC (8.75 mg/mL, 17.50 mg/mL and 35.00 mg/mL respectively) and control.
Each designated point represents the mean log10 survival of bacterial cells at a particular time interval in the presence of the extract.
Leaf extract of Paullinia pinnata was investigated to know its’ antimicrobial potency against some bacterial isolates comprising both Gram-positive and Gram-negative isolates. The crude extract at a concentration of 8.75 mg/mL, 17.5 mg/mL and 35 mg/mL and its fractions (10 mg/mL) inhibited the growth of 16 bacterial isolates out of the 24 isolates used for this study. It was therefore, observed that this plant extract compared favourably with the standard antibiotic ampicillin. Thus, the leaf extract of P. pinnata appears to be a potential source of antibacterial compounds that could be relevant in the treatment of infections caused by these microorganisms. The result shows that Enterobacter asburie and Klebsiella ozanae are multiple antibiotic resistant microorganisms but were susceptible to n-hexane fraction, ethyl acetate/n-hexane fraction of the plant extract. Thus, n-hexane and ethyl acetate may serve as the best solvents to extract the bioactive components of P. pinnata. The combined fraction of P. pinnata showed appreciable level of activity against most of the test isolates at a concentration of 10 mg/mL. Thus, these combined fractions (synergy) can also serve as base for the formulation of novel natural antimicrobial agents.
Time-kill assays are expressed as the rate of killing by a fixed concentration of an antimicrobial agent (in vitro) and it’s one of the most reliable methods for determining tolerance of microorganisms to drugs. The killing rate of the most active fraction and antibiotic ampicillin was determined by using Staphylococcus aureus as Gram-positive representative and Proteus vulgarise as Gram-negative representative. It was observed that the rate at which the antibiotic and ethyl acetate fraction eliminated the cells increased with increase in the concentrations of the antibiotic, fraction and contact time intervals. The results show a monophasic effects as reported by Akinyele et al. [12]. These fractions also kill the wound isolates at a shortest contact time. Pankey et al. [13] reported that the ability of plant leaf extract to eliminate or kill organisms at the shortest period of time is generally accepted definition of bactericidal activity in antibiotics and which is in supports of our findings.
The antibacterial activity of plant leaf extract is considered significant if the MIC of the extract is less than or equal to 200 mg/mL [14]. The MICs exhibited by the crude extract, the active fractions (n-hexane and ethyl acetate) and the combined fraction were below 200 mg/mL as observed in this study. This is an indication that the leaf extract of P. pinnata exhibited significant antibacterial activity against the test isolates and suggests it to be a veritable source of bioactive compounds that can serve as a base for the formulation of potent antibacterial especially against wound infection [15-18].
Conclusion
The study concluded that the leaf extract of P. pinnata were found to possess antibacterial activity which compared positively with ampicillin. The mode of antibacterial action exhibited by the extract could be concluded to be by cytoplasmic membrane disruption which leads to the death of the test bacteria. The presence of secondary metabolites, bioactive compounds and membrane disruption properties of this plant may be responsible for its antibacterial potency.
The antibacterial potential of the crude leaf extract of P. pinnata against both Gram positive and Gram negative bacterial isolates at low concentration and minimal contact time established the effectiveness of the Paullinia pinnata leaf as a potential source of broad spectrum antimicrobial drugs in the treatment of multiple drug resistant pathogens. Antimicrobial drug formulated from this plant will assist in treating infections caused by test microorganisms and most importantly will aid in wound healing. Paullinia pinnata formulations in to antimicrobial agent could be more effective and cheaper compared to the expensive synthetic drugs.
These findings could be of help to Pharmaceutical Industries and Research Institutes in the formulation of new antimicrobial agents of natural origin for wound healing and to cater for the problem of multi-drug resistant bacteria. Paullinia pinnata if used to synthesize drug will reduce the rate of morbidity recorded from patients with high risk of wound infection.
Recommendation
Paullinia pinnata is commonly sourced in most local environs. Its antibacterial potential against both Gram positive and Gram negative bacterial isolates at low concentration and minimal contact time established the effectiveness of the Paullinia pinnata leaf as a potential source of broad spectrum antimicrobial drugs in the treatment of multiple drug resistant pathogens. Antimicrobial drug formulated from this plant will assist in treating infections caused by test microorganisms. The study has provided useful information on antibacterial properties of P. pinnata which could be harnessed for the development of cheaper alternative antibacterial agent capable of combating microorganisms implicated in human wound infections.
Authors Contribution
All contributing authors have agreed to the submission of this manuscript for publication. AOO, FO conceived and designed the study, performed the experiments, analyzed data and interpreted the results JOA involved in analyzing the data, interpreted the results and wrote the paper. OOO monitored leaf extract production experiment, interpreted the results and edited the manuscripts.
24175
References
- Tsakala O, Pen RS, Miemanang K, Krohn H, Dongo E, et al. (2006) Paullinoside A and Paullinomide A: A New Cerebroside and a New Ceramide from the leaves of Paullinia pinnata. J Natural Sc 61b: 1123-1127.
- Blanks T, Brown S, Cosgrave B, Woody J, Bentley VO, et al. (1998) The Body shop Book of Wellbeing mind, body, and soul. Ebury Press London, pp: 173-192.
- Natarajan V, Venugopal PV, Menon T (2003) Effect of Azadarichta indica (neem) on the growth pattern of dermatophtyes. Ind J Med Microbiol 21: 98-101.
- Watson L, Dallwitz MJ (2007) The families of flowering plants: Descriptions, illustrations, identification and information retrieval. WorldCat 5: 1-10.
- Fred-Jaiyesimi AA, Anthony O (2011) Larvicidal activities of the extract and fractions of Paullinia pinnata Linn leaf. Pharmacognosy Communications 1: 37-40.
- Gill LS (1992) Ethnomedicinal uses of plants in Nigeria. Am J Plant Sci 5: 82-83.
- Chabra SC, Makuna RLA, Mshiu EN (1991) Plants used in traditional medicine in Eastern Tanzania. J Ethnopharmacol 33: 147-57.
- Dokosi OB (1998) Herbs of Ghana, Ghana Universities Press, pp: 615-623.
- Abbiw D (1990) Useful plants of Ghana, Intermediate technology publication Ltd and the royal. J Trop Ecol 7: 286-287.
- Odimegwu DC, Ibezim EC, Esimone CO, Nworu CS, Okoye FBC (2008) Wound healing and antibacterial activities of the extract of Dissotis theifoila (Melastomataceae) stem formulated in a simple ointment base. J Med Plant Rev 2: 11-16.
- Mulu A, Moges F, Tessema B, Kassu A (2006) Pattern and multiple drug resistance of bacterial pathogens isolated from wound infection at University of Gondar Teaching Hospital, Northwest Ethiopia. Ethiopia Med J 44: 125-131.
- Mehta M, Dutta P, Gupta V (2010) Bacterial isolates from burn wound infections and their antibiograms: a eight-year study. Ind J Plastic Surgery 40: 25-28.
- Ozumba UC (2007) Bacteriology of wound infections in the surgical wards of a teaching hospital in Enugu, Nigeria. Afr J Med Science 36: 341-344.
- Ousey K, McIntosh C (2009) Topical antimicrobial agents for the treatment of chronic wounds. British J Community Nurs 14: S6-S10.
- Werdin F, Tennenhaus M, Schaller HE, Rennekampff HO (2009) Evidence-based Mmanagement strategies for treatment of chronic wounds. Eplasty 9: e19.
- Akinyele TA, Akinpelu DA, Okoh AI (2011) In vitro antilisterial properties of crude aqueous and n-hexane extracts of the husk of Cocos nucifera. Afr J Biotechnol 10: 8117-8121.
- Pankey GA, Sabath LD (2004) Clinical relevance of bacteriostatic against bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clinical Infec Dis 38: 864-870.
- Suffredinni IB, Paciencia MLB, Varella AD, Younes RN (2006) Antibacterial activity of Brazillian Amazon plant extract. Brazilian J Infec Dis 10: 400-402.









