Key words
Stem cells, genetic engineering, bioethics, legal framework
Introduction
The rapid development of Cell and Molecular biology in the latest years have contributed to the understanding of the basic functions of stem cells and, consequently, of the way in which a perfect organism is developed from a single multivalent cell, as well as of the human organism’s ability to partially regenerate some of its tissues. A characteristic example of this is marrow transplantation that is widely used today in medicine, a technique developed after the identification of totipotent stem cells. [1]
The phenomenon of tissue regeneration is due to the ability of stem cells to remain undifferentiated for a long time period, without, however, loosing their ability to create new healthy cells in order to replenish the destroyed ones. [2] Through many years of study, it was found that embryonic stem cells are able to transform into various types of cells, including nerve cells, bowel cells, muscle cells, bone cells and cartilage cells. Research on human embryonic stem cells is considered, by many people, as the most significant scientific achievement of our century as it is expected to open new horizons so much from a medicine aspect as from the aspect of genetic engineering, giving hope to individuals who suffer from incurable or chronic, up to date, diseases. The most significant benefit of such autologous cell therapies is safety and lack of side-effects, since there is utilization of the potential of the human organism itself. However, genetic engineering, escalating human intervention upon nature, along with many promises, it also involves many risks. The therapeutic possibilities that the use of stem cells promises necessitates the establishment of rules of ethics which will adapt the specific biomedical evolutions to the fundamental moral values of today’s society. In the light of these new scientific developments, many nations, states, cultures and religious groups have come to reconsider their attitude towards this issue. At the same time, a great deal of controversy and pressure was put on policymaking. The applications of today’s technology that expand to stem cell study and use affect the quality of social life, constituting objects of regulatory intervention by the legislator. This activity has been developing rapidly in the last years, while the legislative choices of the various countries are substantially different, even inside the European Union. There are countries that choose to set limitation in any biomedical intervention such as France and Germany and countries which wish to intervene in as few as possible issues such as England. [3]
Nowadays legal and ethical constraints for the progress of research are posed, as the main –although not sole- source of stem cells are embryos, which come either from in-vitro fertilization techniques or from abortions and which are ultimately destroyed. This fact generates conflicting social opinions as to “when” human existence acquires a “face” and, consequently, human rights.
The present study explored the possibilities offered by genetic engineering, especially in the research field of embryonic stem cells. Subsequently, the possible therapeutic applications of their use were presented while there was an extended mention of the issue of embryonic stem cell research, which raises intense ethical and legal considerations.
Purpose
The purpose of the present study was to investigate ethical and legal issues that emerge in relation to the embryonic stem cells research, as well as the European –mainly- texts and the Member States legislative choices. Thus to show the degree of present uniformity among states in dealing with these, taking into consideration sociopolitical problems. Furthermore, to refer to the possibilities that genetic engineering offers, mainly in the field of embryonic stem cells, and to explore possible therapeutic applications with the use of stem cells.
Materials and Methods
We done an extensive review of Greek and international literature. The main literature search sources that were used are known and valid databases of biomedical studies, like: PubMed, Cochrane Library, CINAHL, Medline. Also, the scientific article search engine Google Scholar (https://scholar.google.com/) was chosen, as a widely used search tool.
An additional search was also performed in various websites, as well as in publications of UNICEF, of the E.U. of health committees, of the national biomedical committees of the E.U. states, as well as of the "Eurostemcell" partnership, which is funded by the E.U.
A further search was performed also in references of retrieved publications and in files of abstracts from national bioethics committees.
A necessary condition for publication search was the determination of indexing terms. The search was performed with the use of key-words (MeSH terms) like: stem-cells, bioethics, research, legislation, laws, etc. In order to increase the results of the search and the number of studies that were to be reviewed, synonym phrases and word combinations were used.
After the collection of literature, the articles were evaluated according to specific criteria.
The search was limited according to the following restrictions:
a) The publications were mainly articles published in international valid and current health magazines
b) The search referred to the years 2000-2012, aiming at the retrieval of articles that described the current situation (state-of-the-art), and
c) The language in which the articles were published was English and/or Greek.
The selection criteria of a study or article were its clarity, its closeness to the research issues of the present study, its year of publication (2000-2012), the validity of its data, the existence of valid references, its authors and their research work, the validity of the scientific magazine where it had been published.
The exclusion criteria of a study were, the originality of its content (i.e. not being a repetition of other studies), its reference to countries outside the European Union.
As a result of the aforementioned search, 28 articles were retrieved, which satisfied the criteria, the content of which is discussed in this study.
The nature of stem cells
The term “stem cells” refers to those undifferentiated cells that are characterized by: a) the ability for self-multiplying, and b) the ability to differentiate into types of cells of various tissues and organs. [4] They are found in all developmental phases of an organism, however, as development is completed, their biological dynamics decreases. Therefore, the earliest the developmental phase, the more significant their differentiation ability. [5]
During the first four days after fertilization, human stem cells are called totipotent because of their ability to develop into all types of cells, including membranes and tissues that are necessary for the support of the embryos’ development. Subsequently, there is separation of stem cells to those leading to supportive tissues and to those creating the rest of the embryo. These stem cells –in whole- are characterized as pluripotent. [6]
After completion of implantation in the uterus (in the beginning of the second week of pregnancy) the zygot is called an embryo. In the end of the eighth week the formation of organs and tissues is completed, the embryo acquires human characteristics and it is then referred to as a late embryo. Upon completion of development, some cells reserve a limited self-multiplying ability in reference to the type of differentiated cells they can produce and they are simply called stem cells.
In conclusion, the stem cells that remain after our birth help in keeping the number of differentiated cells in the tissues where they are located stable, in case of destruction of some cells because of injury, disease or cellular death. [7]
Categories of stem cells
Adult stem cells
They are undifferentiated cells in tissues or cells of the human body from where they can be isolated. These stem cells are in small quantities and they are difficult to be spotted in the organism. [8] In contrast to the embryonic cells, adult stem cells are already differentiated to some degree, i.e. they cannot transform into all cell types but mostly in cells of the tissue where they come from. Cells procured from the umbilical cord of newborns belong to this category.
Most known groups of adult stem cells are those of the bone marrow and of the late embryo. The late embryo stem cells are isolated either from the tissues or from the reproductive organs of aborted embryos and they are procured after abortion or miscarriage. Their ability to multiply is smaller than that of embryonic stem cells. [9]
Another group of adult stem cells is progenitor stem cells which have a limitless ability of self-multiplying but also a big difficulty in their maintenance in an undifferentiated state.
Embryonic stem cells
These stem cells are procured from developing embryos that are in the blastocyst stage i.e. they are created and developed outside the human body (in vitro) and they cannot develop further unless they are transplanted in the uterus. In the stage of 5 days the internal cell mass of the blastocyst – where the stem cells are procured from – can generate all the tissues of the human body. However, this leads to the destruction of the blastocyst, hence to the destruction of the developing embryo, as well. [10]
Embryonic stem cells are more flexible than the adult ones, as they have the ability to produce any kind of cells of the human body. They are also easier to collect, isolate and maintain in the laboratory. They also have the ability to reproduce for a long time period, remaining in an undifferentiated state, before they are activated in order to produce differentiated specialized types of cells. It should be noted that undifferentiated cells cannot be directly used in tissue transplantation, as there is a risk for creation of tumors that are called teratomas.
The main sources that embryonic stem cells are procured from are: [11]
1) Supernumerary embryos that were created with artificial insemination techniques but ultimately they were not used. These fertilized ova, which are “surplus” and are stored in artificial insemination banks, could be a significant source of embryonic stem cells for research purposes.
2) The in vitro fertilization technique, which can create: a) embryos that are used exclusively for research, b) cloned embryos through therapeutic cloning. [12] Therapeutic cloning constitutes creation of human embryos through transfer of body cell nucleui for utilization in research and for therapeutic purposes. [13] Cloning of human embryos for therapeutic purposes aims at isolation of primitive stem cells and not human birth.
Stem cells of all forms are isolated with the appropriate laboratory methods and, subsequently, they are cultivated in controlled environments so that the cells remain healthy, divided and undifferentiated. Their multiplication process follows, resulting, in only a few months, to the creation of millions of undifferentiated cells, which are called stem cell lines and they are directly available for research and development of new therapies.
Therapies - Applications
The use of stem cells can bring about, in the near future, significant developments in four scientific fields:
1. In the field of basic research, it could contribute to understanding of the complicated organic processes that occur during embryonic development. Through laboratory observation of stem cells and verification of the factors that determinate the process of cell differentiation, scientists are able to understand serious diseases mainly due to cell specialization or cellular proliferation abnormalities like cancer. [14]
2. In the field of pharmacology, it can incur radical changes in the methodology of medicine development and testing. Medicine design and preparation could become more effective and efficient as it will be possible to test the effectiveness of new medicines first on specialized cell lines. Thus, there will be a final approval for conducting clinical tests only for those medicines that are considered safe for cell growth and have beneficial results. [15]
3. The field of genetic engineering in connection with the use of stem cells, constitutes a new technique for the cure of genetic diseases. Gene therapy aims at isolation of stem cells from patients with genetic diseases, their genetic repair, their ex-vivo proliferation and finally their transplantation back to the same organism in order to produce genetically corrected cells. [15]
4. Activation of undifferentiated stem cells, so that they can generate specialized types of cells, leads to the creation of a renewable source of cells and tissues that could be used as implants in many diseases that are due to deregulation of cell function or to loss of a type of cells or tissue. [16]
For example, type Ι (juvenile) diabetes is due to loss of pancreas cells that produce insulin, and treatment –as it is today- is restricted to the alleviation of symptoms. The possibility of re-growth of the missing or destroyed cells and of their implantation into the pancreas would be a therapeutic management of the disease. [17] Another characteristic example is Parkinson’s disease, which is caused by the loss of a specific type of nerve cells. In this case also, stem cells could offer a tangible possibility of cure.
Finally, the aforementioned method can significantly contribute to the solution of the problem in reference to the lack of donors as well as to the creation of tissues that are genetically identical (histocompatibility) to the recipients.
Ethic and legal dimension
Bioethics is an inter-scientific discipline, as it is related to sciences like genetics, medicine, law, philosophy, sociology and theology, a fact that complicates the clear definition of the principles that govern it. One of the most complicated problems that bioethics has to encounter nowadays, is medical research on stem cells. Thus, in most states bioethics councils or committees have been established, in order to weigh the consequences of scientific and technological innovations, which they evaluate according to generally acceptable principles, taking into account the significance of the changes they incur to the living conditions of individuals.
Developments in embryonic stem cell research have not only brought about hope, but also controversies in relation to the ethical aspect of the whole issue. This activity is considered to offend basic values of society, posing, at the same time, new dilemmas and questions in respect to the content and the assurance of basic constitutional principles like respect of human dignity, protection of life, and freedom of sciences.
Large social groups, depending mainly on their religious and/or philosophical beliefs, consider the use of embryonic stem cells as unacceptable. The existence of possible alternative research methods further reinforces this position. In contrast, it is believed that, if there are even simple indications that a research method may lead to control of some diseases, then it should be allowed. Controversies about research on these cells also come from the existing techniques that are applied for the creation and use of stem cell lines.
The opponents of stem cells cloning talk of turning human into a tool, as they believe that every embryo constitutes a potentially living organism, while they judge the creation of a living organism exclusively for purposes of research and/or therapeutic purposes as ethically unacceptable. [12] Moreover, there is fear for establishment of agreements – before or after conception – for resorting to abortion with the sole purpose of stem cell isolation, leading to commercialization of embryos and to abuse of women’s personality, offending her human value.
Moreover, there are many possibilities for the existence of financial rewards behind these agreements. Therefore, human life is exploited in order to achieve alien purposes, which opposes the principle of respect of human dignity. Even the use of cadaveric embryonic tissue, as a source of embryonic stem cells, poses the issue of complicity in the process of abortion.
It is also argued that the lift of the prohibition of “therapeutic” cloning could constitute the beginning of a process that will ultimately lead to the acceptance of reproductive cloning. On the other hand, the creation of a cloned human embryo intended to be finally destroyed is an action that reduces the value of human life itself, because of the treatment of a human embryo as a simple object and because of the intentional removal of human life.
According to most National Bioethics Committees, one of the necessary conditions for the procurement of stem cells from an adult person is his/her previous informing and valid consent. However, the National Bioethics Committees of Cyprus and Greece judge the prohibition of stem cells procurement from underage persons for experimental purposes right, while they accept it only for therapeutic purposes. Moreover, in favor of the protection of the personality and confidentiality of the donor, as well as of the recipient of body stem cells, maintaining anonymity of the donor is considered compulsory, as is the case in organ transplantation. [18,19]
Along with the ethical reservations, there are many legal reservations that must be taken into account before stem cell therapies are clinically available. These relative legal issues mainly concern matters of intellectual property and of law enforcement which sometimes clash in state level. Finally, a major social issue appears to be the dispersal of benefits of stem cell research.
The main arguments of the two different approaches in relation to the ethical aspect of research on and use of stem cells are summarized in Table 1. [20]

The ethical dilemmas discussed above have led to the search of new techniques for production of “ethical” stem cells, like:
1. The modification of adult stem cells in order to produce cell lines similar to those of embryonic stem cells. This method does not require destruction of embryos.
2. The production of stem cells –identical to the embryonic ones- from simple skin cells, the fibroblasts. Recently, researchers managed to “reprogram” the fibroblasts, turning them into embryonic stem cells.
3. The production of stem cell lines from “embryos” that are 2 days old, which are created with artificial insemination. In this phase, the fertilized ovum has been divided into eight cells (blastomeres). In artificial insemination centres, often one of these blastomeres is removed in order to undergo all the necessary antenatal examinations. If the results are desirable, the embryo, which consists now of seven cells, is implanted into the uterus and pregnancy progresses naturally. Although this technique does not require destruction of the embryo, it exposes it to some risks, therefore it is not for sure compatible with the laws and regulative restrictions of each country. [21]
Oviedo Convention
The most significant milestone in the field of bioethics, in European level, is the Council of Europe Convention for protection of human rights and dignity of the human being with regard to the applications of Biology and Medicine (Convention on Human Rights and Biomedicine) which was signed in April of 1997 in the city of Oviedo in Spain.
This Convention established specific terms, the most significant of which is the protection of the human being (article 2 of the Convention). Also, according to article 16 of the Convention, in cases of research on human beings, the following conditions must apply: 1) There must not be any alternative of comparative effectiveness to that of research on humans, 2) There must be a proportionality standard between possible risks and anticipated results 3) The research program must have been accepted by the competent body after the necessary estimation of its scientific value, 4) The persons on which the research is performed must have been fully informed on the lawful rights for their protection, 5) The free consent of the persons involved must be ensured. [22]
As far as in vitro research on embryos is concerned, according to article 18 of the Convention it is considered allowed on condition that the sufficient protection of the embryo is ensured, while the creation of human embryos for research purposes is prohibited.
The text of the Convention is legally binding, which means that the states that have signed and ratified the Convention have to adapt their national legislation to the respective principles that it provides. It must be noted that the respective Convention does not regulate the issue of research on embryos in whole but it renders each country responsible to regulate the issue separately in its internal legal order. Germany and the United Kingdom did not sign the Convention, while Greece validated it with Law 2619/1998 and Cyprus with Law 31(ΙΙΙ)/2001. [23]
European legislation and international practice
The fact that biomedicine development has preceded bioethics, as well as legislation, has only recently led the governments of various states to the creation of corresponding legal frameworks. Contrary to the bioethics approach, what is important in law is not the time when human life begins according to medicine, but the time when, according to its own principles, the embryo is considered as a legal body, which has rights and obligations, regulated by the institution, as well as by common law.
It is remarkable that the E.U. has not yet applied a supranational normative regulation for research on embryos. The only European Legislation that there is today, and must be applied also to stem cells, is the Directive 2004/23/EU of the European Parliament and of the Council on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells.
The aim of the directive 2004/23/EC is the protection of human health and setting minimum standards for processes concerning manipulation of human tissues and cells. This directive also highlights the obligation of member states to adopt measures for reserving high levels of quality and safety for materials of human origin and that a member state shall not be prevented “from maintaining or introducing more stringent protective measures”. [24]
Through the safety and quality requirements that are mentioned in this directive, selection criteria are created, in respect to:
• the donors and recipients
• the testing laboratories involved in the “donation”
• the processes concerning the procurement of cells and tissues and their forwarding to cell banks
• the processing of cells and tissues
• their maintenance and distribution
• the quality system and encoding that is generally applied.
The 2004/23/EU directive also determines the requirements that the certified research laboratories must meet, the terms under which each research program must be conducted, checking mechanisms for imports and exports of the biological materials they handle, while, finally, it gives guidelines on the ethical aspect of the issue, mainly in respect of the databases that must be observed by stem cell banks. Among others, it also determines:
(a) Specifications for the process of laboratory preparation of tissues and cells,
(b) The need for authorization or licensing of the respective research laboratories, as well as (c) the responsibility for supervision and continuous compliance of all the processes included in such an activity.
The directive in question requires that the member-states create and observe a record of authorized laboratories that deal with manipulation of human cells and tissues, as well as the establishment of relative audit and inspection units in national level.
The directive also reflects significant helpful suggestions, e.g.:
-tissue/cell donation must be voluntary and non profitable,
-the consent of the directly involved and the provision of information must be mandatory,
-confidentiality and data protection donors and recipients must be ensured. [24]
The similar directive 2006/17/EC sets some additional safeguards and technical requirements in respect to the operation of cryopreservation banks and the procurement, maintenance and testing of human tissues and cells.
In October of 2006, the directive 2006/86/EC came into force – as an auxiliary document– for the application of directive 2004/23/EC regarding traceability requirements, notification of serious adverse reactions and events, and certain technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells. For harmonization purposes, Cyprus Republic has issued a Regulatory Administrative Act 438/2008, while Greece, with a Presidential Decree (26/2008) has incorporated all the aforementioned directives into the Greek law.
Finally, in the E.U. also applies the Directive 2001/20/EC, which refers to the clinical trials of medicines, the main research field in embryonic stem cells. [25] Except from research in new medicine, there are efforts so that the biological “basic” research that directly interests biomedicine– especially the one that is applied on embryos in vitro – is inspected by an experts group in terms of ethics at the stage of approval of research protocols. This inspection does not deal with the scientific validity of the protocol, but it concentrates on the compliance of the specific research planning with the current legislation.
While the international community as a whole condemns, almost unanimously, cloning for reproductive purposes, the sensitivities of the different peoples very as to the freedom of research on human embryos. Even within the borders of the E.U. there is diversity, as there are some states with very strict laws and others that adopt a more liberal attitude.
Generally, in the E.U. level, we could discern three legislative tendencies in reference to the issue of research on embryos:
(a) In some states the creation of supernumerary embryos from which stem cells could be procured and, even more, the creation of embryos exclusively for research purposes is forbidden. In Germany, for example, the creation of an embryo when there is no intention of immediate implantation is forbidden, while in Ireland the possible supernumerary embryos are compulsorily transferred into the woman’s vagina, where they find premature death. Finally, it should be noted that, in countries like Austria [26], Norway [27], Poland [28] and Italy [29] very conservative and stringent restrictions apply.
(b) There are countries where not only research on supernumerary embryos is allowed, but also the creation of embryonic stem cells, as well as therapeutic cloning, with Sweden [30] and United Kingdom [31,32] being the characteristic examples, where a more liberal policy has been adopted since 1990.
(c) On the contrary, countries like the Czech Republic [33], France [34], Spain [35], Greece [36], the Netherlands [37], Finland [38] and Denmark [39] have adopted a more modest and flexible legislation, allowing stem cell isolation only from supernumerary embryos, while they forbid the creation of embryos exclusively for research purposes.
The internationalized character of biomedical research as well as the “isolation” conditions under which it is practiced, make its ex post control difficult, even impossible, since transferring the conduction of such research in the territory of another country the possible legal penalties may be avoided.
As a result, in the last years there has been a dramatic increase of medical tourism to states where non-confirmed therapies with the use of stem cells are implemented. There are now many private clinics that promise immediate therapeutic results that are not based on a proven scientific justification, withholding, at the same time, the possibility of serious side-effects. This way, excessive expectations are cultivated by the patients and an unethical approach to disease treatment is uncontrollably applied, with a view to profit.
Finally, it should be noted that the E.U. is funding, today, research on stem cells mainly coming from persons and on stem cells from supernumerous embryos, excluding however, funding of research on embryos that are exclusively intended for this purpose. It also promotes inter-border cooperation, active research and the creation of European networks for the construction of a united European Place of Research. It should be noted that the funds that are received from the E.U. for research purposes pertinent to stem cells have no relation to nor they affect the national legal frameworks on the specific issue in every member-state of the E.U.
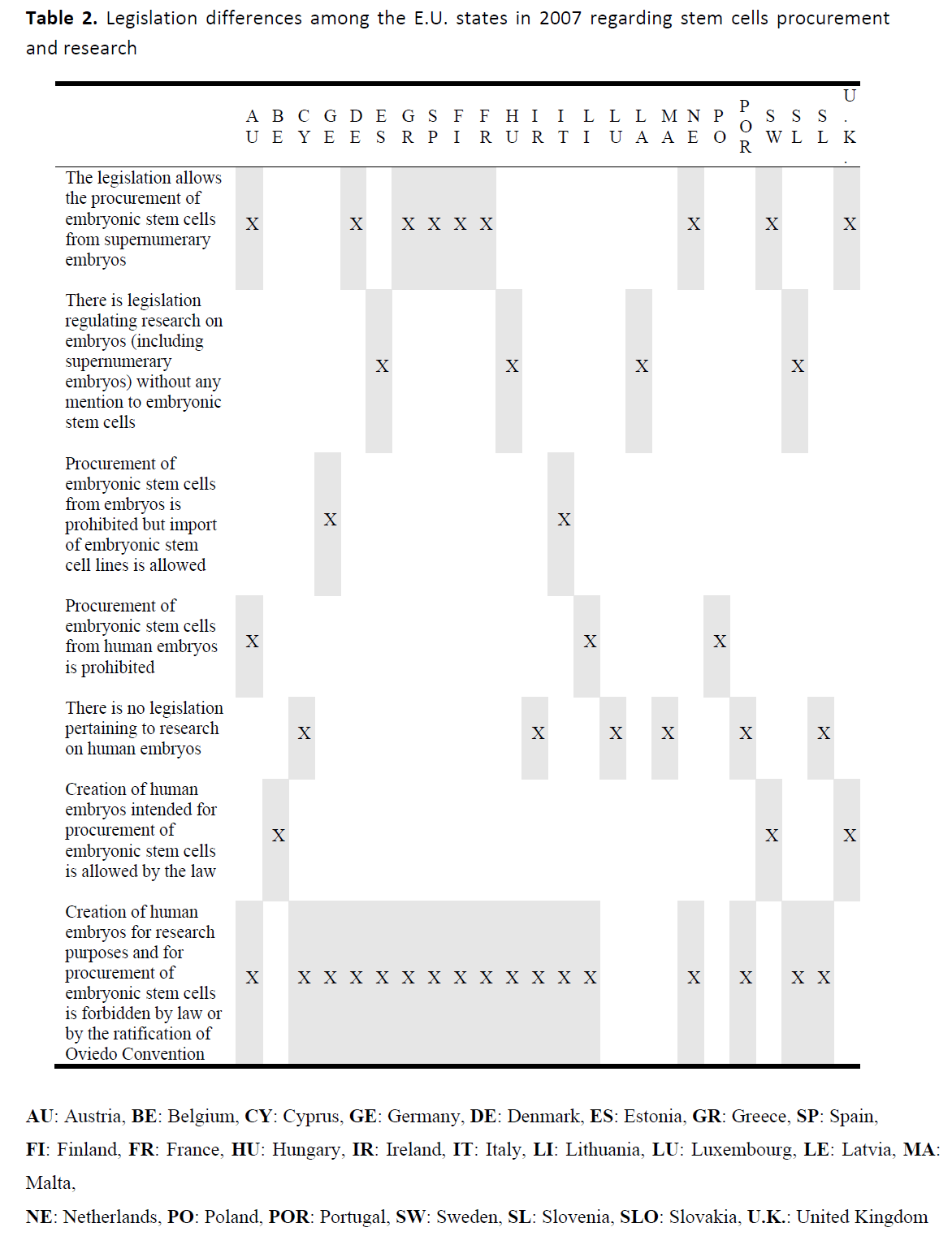
Legislation differences among the E.U. states – in year 2007 – in regard to stem cells procurement and research, are recorded in Table 2. [40]
Conclusions
The genetic technological revolution that is happening nowadays, gives the humankind many promises and hopes for the future of our health. A twofold characteristic example is therapeutic cloning, for the cure of diseases that are incurable until today and for operations such as transplantations, basically eliminating the problem of histocompatibility.
The most important bioethical dilemma today in relation to the research on and use of stem cells concerns three different but interconnected aspects: a) the collection of embryonic stem cells, which results in destruction of fertilized ova, b) therapeutic cloning, and c) the critical question of “when” life is supposed to begin.
In regard to the legal and ethical implications of the use of and research on embryonic stem cells, the policies and legislations that the states follow and apply are diversely stringent, depending mainly on their cultural and religious traditions. State intervention for valid informing of public opinion is also significant, so that the possibilities and prospects of biomedical research and use of stem cells are discussed.
The need for the protection of fundamental human rights has been acknowledged in a national, European, and international level, resulting in drawing and signing the Oviedo Convention, which specifies some basic principles that should govern human activity in regard to biomedical interventions. The ultimate purpose of this Convention was the sufficient protection of fundamental rights and principles, such as respect for human life and protection of human dignity.
Given that the applications of stem cell research are expected to intensify and to possibly cover a wider range of therapies in the near future, alleviating millions of people from many diseases that are considered today as incurable, it would be wise, before taking sides on the specific issue, to think of the benefits, as well as of the possible risks, so that the decision made will be the most advantageous for humanity.
References
- Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA [Internet]. 2008 [cited 2010 Jan 3]; 299(8):925–936. Available from: https://jama.jamanetwork.com/article.aspx-articleid=181511.
- Ying QL, Nichols J, Chambers I, Smith A. BMP Induction of Id Proteins Suppresses Differentiation and Sustains Embryonic Stem Cell Self-Renewal in Collaboration with STAT3. Cell. 2003; 115 (3):281–292.
- Savvakis C. Law and Society in the 21st century, new technologies and constitutional rights. Sakoulas Publications, Athens, 2004.
- Vidalis TK. Public Law and Policies in Biomedicine. Sakoulas Publications, Athens, 2007.
- Commission of the European Communities. Commission Staff Working Paper: Report on Human Embryonic Stem Cell Research. [Internet] 2003 [cited 2010 Dec 12]. Available from https://ec.europa.eu/research/press/2003/pdf/sec2003-441report_en.pdf.
- Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nature Biotechnology. 2007; 25(7): 803–816.
- National Bioethics Commission of Greece. Texts on Bioethics. Sakoulas Publications, Athens, 2002.
- Rippon HJ, Bishop AE, Embryonic stem cells, (Review), Tissue Engineering and Regenerative Medicine Centre, Investigative Science, Imperial College London, London, UK, 2004.
- Law Association of North Greece. Artificial Insemination and Genetic Technology: the ethical-legal dimension. Sakoulas Publications, Athens, 2003.
- Wade N. "Some Scientists See Shift in Stem Cell Hopes". New York Times. [Internet]. 2006 Aug 14 [cited 2010 Jan 7]; Available from: https://www.nytimes.com/2006/08/14/washington/14stem.html-_r=1.
- Commission of the European Communities. Commission Staff Working Paper: Report on Human Embryonic Stem Cell Research. [Internet] 2003 [cited 2010 Dec 12]. Available from https://ec.europa.eu/research/press/2003/pdf/sec2003-441report_en.pdf
- Mallios Ε. Cloning embryos for therapeutic purposes. The Institution. 2002; 2: 40
- Sartor M, Antonenas V, Garvin F, Webb M, Bradstock, KF. Recovery of viable CD34+ cells from cryopreserved hemopoietic progenitor cell products, Bone Marrow Transplant. 2005;36(3):199-204.
- Greek National Commission of Bioethics. Essays on Bioethics. Sakoulas Publications, Athens, 2002.
- Vidalis TK. Public Law and Policies in Biomedicine, Sakoulas Publications, Athens, 2007.
- Greek National Commission of Bioethics. Essays on Bioethics, Sakoulas Publications, Athens, 2002.
- Locatelli F, Nöllke P, Zecca M, Korthof E, Lanino E, Peters C et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005; 105: (1):410-419.
- Cyprus National Bioethics Committee: Opinion on the creation and use of biobanks and human origin biosamples archives for research purposes. 23/06/2009. [Internet] 2009 [cited 2010 Dec 12]. Available from: https://www.bioethics.gov.cy/Law/cnbc/cnbc.nsf/All/834211FEA75D0839C225760A003A72E3/$file/ ΓΝΩΜΗ%20ΕΕΒΚ%20ΓΙΑ%20ΒΙΟΤΡΑΠΕΖΕΣ_23%2006%202009.pdf
- Greek National Bioethics Commission: A guide for Research Ethics Committees for Biological Research 19/06/2008. [Internet] [cited 2010 Dec 12]. Available from: https://www.bioethics.gr/document.php-category_id=55&document_id=808.
- Cyprus Research Promotion Foundation. The controversy over stem cells, Research Magazine Ipsipetis 2008; 22:11.
- Cyprus Research Promotion Foundation. The controversy over stem cells, Research Magazine Ipsipetis 2008; 22:12.
- Andorno R. “The Oviedo Convention: A European Legal Framework at the Intersection of Human Rights and Health Law”. Journal of International Biotechnology Law, 2005; 2(4): 133-143.
- Gratton Β. Survey on the National Regulations in the European Union regarding Research on Human Embryos, July 2002.
- Directive 2004/23/EC of the European Parliament and Council. Official Journal no. L 102 07/04/2004; p. 0048 – 0058.
- Directive 2001/20/EC of the European Parliament and Council. Official Journal no. L 121/34; 01/05/2001 p. 0034 – 0044.
- Fortpflanzungsmedizingesetz Geändert Wird (Fortpflanzungsmedizingesetz-Novelle 2004 – FMedGNov 2004), Reproductive Medicine Act Amendment 2004 – FmedGNov 2004 [Internet] 2004 [cited 2010 Dec 17]. Available from: https://www.ris.bka.gv.at/Dokument.wxe-Abfrage=BgblAuth&Dokumentnummer=BGBLA_2004_I_163.
- Law 56, Chapter 3 On the medical use of biotechnology [Internet] 1994;[cited 2010 Dec 17]; Available from:https://unesdoc.unesco.org/images/0013/001342/134277e.pdf
- Law on family planning, protection of human fetuses, and the conditions under which pregnancy termination is permissible. International Digest of Health Legislation [Internet]. 1993 [cited 2010 Dec 17]; 44(2):253-255.) Available from: https://www.hsph.harvard.edu/population/abortion/POLAND.abo.htm
- Law 40, Regulation of Medically Assisted Human Reproduction, Legge 24 Febbraio 2004, n. 40, Norme in materia di procreazionemedicalmenteassistita, G. U. N. 45 24-2-2004 [Internet]. 2004 [cited 2010 Dec 17]. Available from: https://www.guritel.it/free-sum/ARTI/2004/02/24/sommario.html
- Lag 39, Lag omgenetiskintegritetm.m. Swedish Code of Statutes no 2006: 351, Genetic Integrity Act [Internet] 2006 [cited 2010 Dec 17]; Available from: https://www.riksdagen.se/sv/DokumentLagar/Lagar/Svenskforfattningssamling/sfs_sfs-2006-351/
- Human Fertilisation and Embryology Act [Internet] 2008; [cited 2010 Dec 17]. Available from: https://www.legislation.gov.uk/ukpga/2008/22/contents.
- Human Tissue Act [Internet] 2004; [cited 2010 Dec 17]. Available from: https://www.legislation.gov.uk/ukpga/2004/30/contents.
- Law 206 on the Protection of Biotechnological Inventions and on the Amendment to Act No. 132/1989 of Coll, Zákonazedne 21. -ervna 2000, o ochran-biotechnologickýchvynález-aozm-n-zákona -. 132/1989 Sb. 21 June 2000;, [cited 2010 Dec 17]. Available from: https://www.wipo.int/wipolex/en/text.jsp-file_id=126151.
- Law 2004-800 on Bioethics, (Loi n. 2004-800 du 6 Août 2004 relative à la bioéthique) 6 August 2004; [cited 2010 Dec 17]. Available from: https://ec.europa.eu/research/biosociety/pdf/french_law.pdf and https://www.legifrance.gouv.fr/affichTexte.do-cidTexte=JORFTEXT000000441469&dateTexte.
- Law 14, de Investigaciónbiomédica. 2007; [cited 2010 Dec 17]. Available from: https://www.boe.es/boe/dias/2007/07/04/pdfs/A28826-28848.pdf
- Law 3305 on Medically assisted reproduction techniques, Official Gazette of the Hellenic Republic, 27 January 2005; [cited 2010 Dec 17]. Available from: https://www.nurs.uoa.gr/fileadmin/nurs.uoa.gr/uploads/Nomothesia_Nosilefton/Nomoi/Nomos_3305_FEK_A__172005.pdf
- Act containing rules relating to the use of gametes and embryos (Embryos Act) - [cited 2010 Dec 17]. Available from: https://www.healthlaw.nl/humsub.pdf
- Act 488/1999 Medical Research 2010; [cited 2010 Dec 17]. Available from: https://www.finlex.fi/pdf/saadkaan/E9990488.PDF
- Law 427 on amending the Law on artificial fertilization in connection with medical treatment, diagnosis, and research (Research on embryonic stem cells) 10 June 2003; [cited 2010 Dec 17]. Available from: https://unesdoc.unesco.org/images/0013/001342/134277e.pdf
- The European Group on Ethics in Science and New Technologies to the European Commission. Recommendations on the ethical review of hESC FP7 research projects. Opinion No 22. European Communities, 2007; [cited 2010 Dec 17]. Available from: https://ec.europa.eu/bepa/european-group-ethics/docs/publications/opinion_22_final_follow_up_en.pdf
3037
References
- Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA [Internet]. 2008 [cited 2010 Jan 3]; 299(8):925–936. Available from: https://jama.jamanetwork.com/article.aspx-articleid=181511.
- Ying QL, Nichols J, Chambers I, Smith A. BMP Induction of Id Proteins Suppresses Differentiation and Sustains Embryonic Stem Cell Self-Renewal in Collaboration with STAT3. Cell. 2003; 115 (3):281–292.
- Savvakis C. Law and Society in the 21st century, new technologies and constitutional rights. Sakoulas Publications, Athens, 2004.
- Vidalis TK. Public Law and Policies in Biomedicine. Sakoulas Publications, Athens, 2007.
- Commission of the European Communities. Commission Staff Working Paper: Report on Human Embryonic Stem Cell Research. [Internet] 2003 [cited 2010 Dec 12]. Available from https://ec.europa.eu/research/press/2003/pdf/sec2003-441report_en.pdf.
- Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nature Biotechnology. 2007; 25(7): 803–816.
- National Bioethics Commission of Greece. Texts on Bioethics. Sakoulas Publications, Athens, 2002.
- Rippon HJ, Bishop AE, Embryonic stem cells, (Review), Tissue Engineering and Regenerative Medicine Centre, Investigative Science, Imperial College London, London, UK, 2004.
- Law Association of North Greece. Artificial Insemination and Genetic Technology: the ethical-legal dimension. Sakoulas Publications, Athens, 2003.
- Wade N. "Some Scientists See Shift in Stem Cell Hopes". New York Times. [Internet]. 2006 Aug 14 [cited 2010 Jan 7]; Available from: https://www.nytimes.com/2006/08/14/washington/14stem.html-_r=1.
- Commission of the European Communities. Commission Staff Working Paper: Report on Human Embryonic Stem Cell Research. [Internet] 2003 [cited 2010 Dec 12]. Available from https://ec.europa.eu/research/press/2003/pdf/sec2003-441report_en.pdf
- Mallios Ε. Cloning embryos for therapeutic purposes. The Institution. 2002; 2: 40
- Sartor M, Antonenas V, Garvin F, Webb M, Bradstock, KF. Recovery of viable CD34+ cells from cryopreserved hemopoietic progenitor cell products, Bone Marrow Transplant. 2005;36(3):199-204.
- Greek National Commission of Bioethics. Essays on Bioethics. Sakoulas Publications, Athens, 2002.
- Vidalis TK. Public Law and Policies in Biomedicine, Sakoulas Publications, Athens, 2007.
- Greek National Commission of Bioethics. Essays on Bioethics, Sakoulas Publications, Athens, 2002.
- Locatelli F, Nöllke P, Zecca M, Korthof E, Lanino E, Peters C et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005; 105: (1):410-419.
- Cyprus National Bioethics Committee: Opinion on the creation and use of biobanks and human origin biosamples archives for research purposes. 23/06/2009. [Internet] 2009 [cited 2010 Dec 12]. Available from: https://www.bioethics.gov.cy/Law/cnbc/cnbc.nsf/All/834211FEA75D0839C225760A003A72E3/$file/ ΓΝΩΜΗ%20ΕΕΒΚ%20ΓΙΑ%20ΒΙΟΤΡΑΠΕΖΕΣ_23%2006%202009.pdf
- Greek National Bioethics Commission: A guide for Research Ethics Committees for Biological Research 19/06/2008. [Internet] [cited 2010 Dec 12]. Available from: https://www.bioethics.gr/document.php-category_id=55&document_id=808.
- Cyprus Research Promotion Foundation. The controversy over stem cells, Research Magazine Ipsipetis 2008; 22:11.
- Cyprus Research Promotion Foundation. The controversy over stem cells, Research Magazine Ipsipetis 2008; 22:12.
- Andorno R. “The Oviedo Convention: A European Legal Framework at the Intersection of Human Rights and Health Law”. Journal of International Biotechnology Law, 2005; 2(4): 133-143.
- Gratton Β. Survey on the National Regulations in the European Union regarding Research on Human Embryos, July 2002.
- Directive 2004/23/EC of the European Parliament and Council. Official Journal no. L 102 07/04/2004; p. 0048 – 0058.
- Directive 2001/20/EC of the European Parliament and Council. Official Journal no. L 121/34; 01/05/2001 p. 0034 – 0044.
- Fortpflanzungsmedizingesetz Geändert Wird (Fortpflanzungsmedizingesetz-Novelle 2004 – FMedGNov 2004), Reproductive Medicine Act Amendment 2004 – FmedGNov 2004 [Internet] 2004 [cited 2010 Dec 17]. Available from: https://www.ris.bka.gv.at/Dokument.wxe-Abfrage=BgblAuth&Dokumentnummer=BGBLA_2004_I_163.
- Law 56, Chapter 3 On the medical use of biotechnology [Internet] 1994;[cited 2010 Dec 17]; Available from:https://unesdoc.unesco.org/images/0013/001342/134277e.pdf
- Law on family planning, protection of human fetuses, and the conditions under which pregnancy termination is permissible. International Digest of Health Legislation [Internet]. 1993 [cited 2010 Dec 17]; 44(2):253-255.) Available from: https://www.hsph.harvard.edu/population/abortion/POLAND.abo.htm
- Law 40, Regulation of Medically Assisted Human Reproduction, Legge 24 Febbraio 2004, n. 40, Norme in materia di procreazionemedicalmenteassistita, G. U. N. 45 24-2-2004 [Internet]. 2004 [cited 2010 Dec 17]. Available from: https://www.guritel.it/free-sum/ARTI/2004/02/24/sommario.html
- Lag 39, Lag omgenetiskintegritetm.m. Swedish Code of Statutes no 2006: 351, Genetic Integrity Act [Internet] 2006 [cited 2010 Dec 17]; Available from: https://www.riksdagen.se/sv/DokumentLagar/Lagar/Svenskforfattningssamling/sfs_sfs-2006-351/
- Human Fertilisation and Embryology Act [Internet] 2008; [cited 2010 Dec 17]. Available from: https://www.legislation.gov.uk/ukpga/2008/22/contents.
- Human Tissue Act [Internet] 2004; [cited 2010 Dec 17]. Available from: https://www.legislation.gov.uk/ukpga/2004/30/contents.
- Law 206 on the Protection of Biotechnological Inventions and on the Amendment to Act No. 132/1989 of Coll, Zákonazedne 21. -ervna 2000, o ochran-biotechnologickýchvynález-aozm-n-zákona -. 132/1989 Sb. 21 June 2000;, [cited 2010 Dec 17]. Available from: https://www.wipo.int/wipolex/en/text.jsp-file_id=126151.
- Law 2004-800 on Bioethics, (Loi n. 2004-800 du 6 Août 2004 relative à la bioéthique) 6 August 2004; [cited 2010 Dec 17]. Available from: https://ec.europa.eu/research/biosociety/pdf/french_law.pdf and https://www.legifrance.gouv.fr/affichTexte.do-cidTexte=JORFTEXT000000441469&dateTexte.
- Law 14, de Investigaciónbiomédica. 2007; [cited 2010 Dec 17]. Available from: https://www.boe.es/boe/dias/2007/07/04/pdfs/A28826-28848.pdf
- Law 3305 on Medically assisted reproduction techniques, Official Gazette of the Hellenic Republic, 27 January 2005; [cited 2010 Dec 17]. Available from: https://www.nurs.uoa.gr/fileadmin/nurs.uoa.gr/uploads/Nomothesia_Nosilefton/Nomoi/Nomos_3305_FEK_A__172005.pdf
- Act containing rules relating to the use of gametes and embryos (Embryos Act) - [cited 2010 Dec 17]. Available from: https://www.healthlaw.nl/humsub.pdf
- Act 488/1999 Medical Research 2010; [cited 2010 Dec 17]. Available from: https://www.finlex.fi/pdf/saadkaan/E9990488.PDF
- Law 427 on amending the Law on artificial fertilization in connection with medical treatment, diagnosis, and research (Research on embryonic stem cells) 10 June 2003; [cited 2010 Dec 17]. Available from: https://unesdoc.unesco.org/images/0013/001342/134277e.pdf
- The European Group on Ethics in Science and New Technologies to the European Commission. Recommendations on the ethical review of hESC FP7 research projects. Opinion No 22. European Communities, 2007; [cited 2010 Dec 17]. Available from: https://ec.europa.eu/bepa/european-group-ethics/docs/publications/opinion_22_final_follow_up_en.pdf







