Keywords
Aedes aegypti; Breeding of mosquitoes
Introduction
Aedes aegypti is the most important mosquito vectors of dengue fever virus, and dengue hemorrhagic fever in almost all countries [1]. A. aegypti breeding sites are defined as any water-holding container in which immature stages of A. aegypti are found. A container is considered 'positive’ for A. aegypti when one or more larvae or pupae are present [2]. A. aegypti oviposition sites are typically in artificial, man-made containers such as flower vases, water storage drums or tanks, and discarded plastic or metal containers [3], buckets, tyres, and flower pots [4]. The surface area and volume can influence the suitability of a container for oviposition as adult females lay more eggs in larger and deeper containers [5]. The mosquitoes are also attracted by certain visual factors and prefer containers which have solid, dark colors and low reflectance. When larval habitats are abundant (i.e. during the rainy season) females often exhibit “skip oviposition” whereby they will bypass undesirable sites and lay their eggs in multiple sites [6]. Under less favorable conditions, the mosquito laid eggs in only a few locations. An artificial container’s construction material may play a role in determining the suitability of the aquatic environment [7]. Shading of the container may also have an impact on the water temperature which affects the suitability of the habitat and larval development rates [8]. Several studies have determined that in some instances only a few container types (<40%) may be responsible for more than 80% of the pupae, and presumably the adult population [9]. These “super-producing” containers vary by location. For instance, in cement, flower pots and vases are important sources of mosquito production due to their relative abundance and infrequent cleanings [10]. However, in homes where the water is frequently changed, it is unlikely that mosquito pupae will have sufficient time to develop and the vases and flower pots are thus less important sources of adult mosquitoes [11]. Abdalmagid et al. [12] and Seidahmed et al. [13] found the clay-pots (zeer) considered as a preference breeding habitats of A. aegypti in Kassala. Most arboviral disease outbreaks occur during the rainy season and are associated with environmental factors such as rainfall, humidity, and temperature. These play a significant role in the transmission of arboviruses [14].
Methods
Study area
Kassala State is the one of the eastern Sudan states of eleven localities (mahaliyas). With a total area of 55,374 km2, Kassala State lies between longitudes 34° 12' and 36° 57' E, and latitudes 15° 12' and 17° 12' N. It is bordered by Eritrea and Ethiopia to the east, Red Sea State to the north; Khartoum and River Nile states to the west and Gadarif State to the south west (Figure 1) [4]. Kassala City, lies 15°12′N, 34°19′E [12] and its population is 298,529. The total number of households in Kassala locality is 52853 according to 2008 census. The state is composed of eleven localities (mahaliyas), nine of which are primarily rural in composition while the other two localities, Kassala City and New Halfa, are urban areas. Kassala State has a total population of about 1.5 million people, one fifth lives Kassala City which is the capital of the state. The annual growth rate of the entire population stands at 2.5%. The average household size is 6.2 persons with a significant number of female-headed households. Drinking water sources are superficial, ground and deep ground waters.
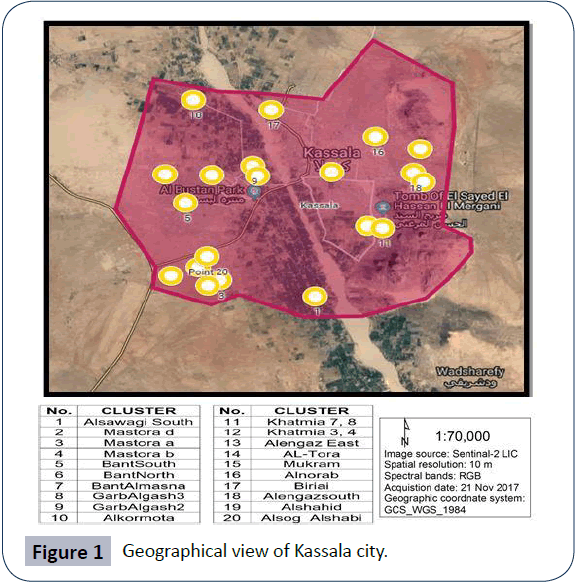
Figure 1: Geographical view of Kassala city.
Physical environment and climate
Over 80% of Kassala State consists of flat plains, whereas rocky outcrops and hilly terrain comprise the rest of the area. Alluvial and volcanic deposits cover the state and beneath these, clays lay basement complex formations that are only a poor repository for ground water. Water sources in the state tend to be distributed along the cracks in the geological formations and in the few areas where alluvial deposits accumulate. The largest of the state’s aquifers are the Gash Basin which has an estimated storage capacity of 600 million cubic meters of water and runs North, from the Eritrean highlands through Kassala City. Mean maximum temperature in Kassala State occurs in summer months with an average of 40°C in May and mean minimum temperature is 15°C in January. Kassala State falls within the arid and semi-arid region where rainfall is unreliable for domestic and economic uses. The average total annual rainfall is about 225 mm occurring dominantly between May to October while evaporation amounts to 2-2.5 mm. The main sources of water supply are surface (UNDP). Effective use of rainfall is, however, hampered by its short duration, uneven distribution and high rates of evaporation. Overall, a trend of long-term decline in rainfall has been observed in Kassala State since the 1940s and the current rate of depletion is calculated to stand at 2.6 mm per annum. Relative humidity varies from 27% in April to 60% in August in Kassala and from 27% to 48% in Aroma.
Design of entomological study
Across-sectional longitudinal entomological study was performed during the three seasons; dry (winter), hot (summer) and wet (autumn) for two consecutive years (2014 and 2015).
Sample size determination of households
The sample size was calculated using the following formula [15].
Sampling Techniques of Households
The sampling technique is a two stage cluster sampling technique. In the first stage, clusters (which are equivalent to a Popular Administrative Units (PAU) were selected using a probability proportionate to size sampling (PPS) technique. In the second stage, households were selected using a systematic random sampling technique. According to Sudan 2008 census data, Kassala locality has a total of 95 PAU. The smallest clusters defined by clear borders, is the PAU. The number of clusters to be chosen from selected PAU is calculated by dividing the sample size of 400 households by the number of households which can be covered in one day, which are 20 houses. So, the number of clusters is 400/20=20 clusters. The Probability Proportional to Size technique was used because the numbers of households vary in each cluster, so it gives all clusters the same probability to be chosen in the sample. Twenty clusters set apart that include all the localities were randomly selected. These clusters were Alkormota, Alengaz East, Alengazsouth, BantAlmasna, BantNorth, BantSouth, Biriai, GarbAlgash 2, GarbAlgash 3, Mastora a, Mastora b, Mastora d, Mukram, Khatmia Blocks (3,4), Khatmia Blocks (7,8), Alnorab, Alsawagi South, Alshahid, Alsog Alshabi and Altora.
Entomological Studies
Entomological survey of immature Aedes spp
Entomological surveys were carried out at 0600 h until at 1800 h and conducted in three seasons; dry (winter), hot (summer) and wet (autumn) in 20 households every season on both sides of Kassala city (East Algash (Alengaz East, Alengazsouth, Biriai, Mukram, Khatmia Blocks 3and 4, Khatmia Blocks 7 and 8, Alnorab, Altora) and west Algash (Alkormota, BantAlmasna, BantNorth, BantSouth, GarbAlgash 2, GarbAlgash 3, Mastora a, Mastora b, Mastora d, Alsawagi South, Alshahid, Alsog Alshabi) in each of the 20 clusters in 2014 and 2015. All the water holding containers inside the households and around the households were inspected for immature stages of A. aegypti. All larvae and pupae were collected and numbers per container were recorded on entomological survey forms (in/outdoor). Aedes spp. breeding sites were identified using a spotlight in any water-holding container where immature stages of Aedes spp. were detected. A container is considered ‘positive’ for Aedes spp. when one or more larvae or pupae were present. Different types of containers were examined depending on size. For large water storage containers (>100 L) such as large clay pots, water tanks, and barrels, water was emptied gradually, using a pipette and a sieve as a filter for collection of immature stages and were then counted and classified to larvae and pupae. For small water containers (less than 20 L) e.g. vase and small clay-pots, water were directly filtered using fine mesh in white-enamel trays [16]. If the container is large (>100 L) and has high number of larvae/ pupal, they were collected by sweeping the surface using a hose pipe with a net. Larvae and pupae were kept in plastic cups with 250 ml of water and labeled indicating location, date, time, house number, type of container, number of sample, indoor or outdoor collection, then, transported to the laboratory for identification according to the methods described by Rueda [17], Cutwa et al. [18] and Tun-Lin et al. [19].
Entomological survey of female Aedes spp
Collection of resting Aedes spp. from indoor using pyrethrum knockdown technique: Indoor resting female Aedes spp. was collected from the 20 houses in 20 cluster using pyrethrum knockdown collection (PKC) technique. One room in each house was randomly selected in each cluster on a seasonal basis in dry and wet seasons (winter, summer, and autumn) in the years 2014 and 2015. Collections were carried out from 0600 until 1500 h. Two white cloth sheets (3.6 X 3.6 m) were used to cover the floor of the room. The cloth sheets were placed from wall to the opposite and were made to overlap with each other at the centre of the room to avoid escape of falling mosquitoes. The rooms had no open eaves and the windows and doors were properly closed. The whole room was sprayed with pyrethrum. After 10 minutes according to WHO [20], the doors and windows were opened, and the cloths were folded from edge to edge to ensure that all fallen mosquitoes were gathered together at the centre. The sheets were, then, unfolded and all Aedes spp. were collected in winter vials [21] and covered by cotton wool and labeled indicating location, date, time of collection, house number and cluster number. They were placed in a box lined with a moist towel, and transported to the laboratory for identification using Tun-Lin et al. [19] and Rueda [17] methods.
Collection of resting Aedes spp. using light traps: Light traps for female Aedes spp. collection were set overnight within randomly selected cluster sites and selected house for approximately 12 h from 1800 h to 0600 h according to Lines et al. [22]. Five houses were selected randomly in each cluster during (winter, summer, and autumn) in each year of 2014 and 2015. Two light traps were deployed by hanging them approximately 1.5 m above the ground, one indoors (inside room) and another outdoors (10-20 m away from houses), in each of the 20 clusters (1 night/cluster/ seasons) taking into consideration feeding habits of Aedes spp. according to Lines et al. [22]. The captured Aedes spp. were separated from other flies trapped and placed in a box lined with a moist towel to keep them soft, and labeled indicating location, date, time of collection and house number prior to transporting to the laboratory.
Collection of resting Aedes spp. using mouth aspirator: Collection of female Aedes spp. using mouth aspirator once a day was performed in the 20 clusters for all seasons inside or outside houses. The aspiration was performed over walls, under furniture, and inside closets. Other likely adult mosquito resting sites such as water holding containers e.g. Clay-pots, barrels, and cemented storage basins were also sampled. Female Aedes spp. collected samples were kept in winter vials, closed by cotton wool and labeled indicating location, date, time of collection and house number and, then, identified.
Results
Prevalence of immature stages of A. aegypti in indoor breeding sites from residential areas during 2014/2015
The mean total number (94.97 ± 27.48) of immature stages of A. aegypti during 2014 and 2015 was significantly (P ≤ 0.05) higher at Garb Algash3 and the lowest (0.30 ± 0.30) at Alengaz South and Alshahid (Table 1). The highest mean number (22.16 ± 9.64) of the 3rd larval instar was significantly (P ≤ 0.05) higher at Alkormota while none was recovered at Alengaz South and Alnorab, and the highest mean number (61.47 ± 19.43) of the 4th larval instar was significantly (P ≤ 0.05) higher at Garb Algsh3 compared with (0. 03 ± 0.30) at Alengaz South and Altora (2.02 ± 1.08). In Mastora (a,b,d) and Alsog Alshabi, no developmental stages were recorded.
Table 1 Mean (±SE) total number of immature stages of A. aegypti collected indoor from breeding sites at residential areas during 2014/2015 in Kassala City.
| |
Indoor breeding sites |
| Residential area |
NO |
Immature stages of A. aegypti |
| Larva3 |
Larva4 |
Pupae |
Total |
| Alkrmota |
36 |
22.16 ± 9.64a |
35.44 ± 11.97abc |
11.66 ± 4.072a |
69.27 ± 23.32abc |
| Alengaz East |
30 |
6.33 ± 4.43abc |
7.40 ± 6.59bc |
14.36 ± 12.02a |
28.10 ± 22.76bcd |
| Alengazsouth |
30 |
0c |
0.30 ± 0.30c |
0a |
0.30 ± 0.30d |
| BantAlmsna |
24 |
15.12 ± 6.75abc |
34.91 ± 13.69abc |
7.62 ± 4.26a |
57.66 ± 18.42abcd |
| BantNorth |
24 |
6.54 ± 4.24abc |
24.08 ± 14.59abc |
1.58 ± 1.04a |
32.20 ± 19.33abcd |
| BantSouth |
24 |
10.87 ± 5.43abc |
41.58 ± 17.65abc |
6.37 ± 2.95a |
58.83 ± 24.60abcd |
| Biriai |
30 |
10.10 ± 3.12abc |
41.90 ± 13.82abc |
7.53 ± 2.86a |
59.53 ± 18.60abcd |
| GarbAlgash2 |
36 |
20.16 ± 5.56ab |
45.69 ± 17.74ab |
7.86 ± 4.07a |
73.72 ± 24.31ab |
| GarbAlgash 3 |
36 |
20.38 ± 6.38ab |
61.47 ± 19.43a |
13.11 ± 3.77a |
94.97 ± 27.48a |
| Mastora a |
36 |
0c |
0c |
0a |
0d |
| Mastora b |
36 |
0c |
0c |
0a |
0d |
| Mastora d |
36 |
0c |
0c |
0a |
0d |
| Mukram |
30 |
1.76 ± 0.73c |
19.60 ± 8.93abc |
1.73 ± 0.96a |
23.10 ± 10.31bcd |
| khatmia3,4 |
30 |
4.16 ± 2.66bc |
30.16 ± 11.74abc |
3.90 ± 1.55a |
38.23 ± 14.35abcd |
| khatmia7,8 |
30 |
8.56 ± 3.27abc |
18.83 ± 9.24abc |
8.60 ± 5.92a |
36.00 ± 16.76abcd |
| ALnorab |
30 |
0c |
0c |
0a |
0d |
| Alsawagi South |
30 |
2.91 ± 1.97bc |
6.16 ± 5.83bc |
1.30 ± 0.74a |
10.38 ± 7.33bcd |
| Alshahid |
36 |
0.27 ± 0.27c |
0c |
0.02 ± 0.02a |
0.30 ± 0.30d |
| Alsog Alshabi |
36 |
0c |
0c |
0a |
0d |
| ALtora |
36 |
2.77 ± 1.67bc |
2.02 ± 1.08c |
1.58 ± 1.11a |
6.38 ± 3.40cd |
Mean separation was based on values transformed to log10 (x?+1).
Means (±SE) with the same letter in each column are not significantly different at 5% level according to REGWQ range test.
Total= Larvae 3+Larvae 4+Pupae.
NO=Number of observations in each residential area.
Prevalence of immature stages of A. aegypti in outdoor breeding sites in residential areas during 2014/2015
The highest female of the 3rd larval instar (29.80 ± 10.27) was significantly (P ≤ 0.05) higher at Biriai and the lowest (0.64 ± 0.45) was at Alengaz south and Alnorab (0.78 ± 0.78). In Biriai area, the highest female of the 4th larval instar (87.71 ± 24.78) was significantly (P ≤ 0.05) higher while the highest female of pupae (23.02 ± 8.41) was significantly (P ≤ 0.05) higher at Biriai area (Table 2). The highest total female of immature (140.54 ± 38.10) was significantly (P ≤ 0.05) higher at Biriai while none was recorded at Alnorab.
Table 2 Mean (±SE) total number of immature stages of A. aegypti collected outdoor from breeding sites at residential areas during 2014/2015 in Kassala City.
| Residential area |
Outdoor breeding sites |
| Immature stages of A. aegypti |
| Larva3 |
Larva4 |
Pupae |
Total |
| Alkrmota |
6.33 ± 2.03bc |
32.97 ± 13.88b |
6.85 ± 3.28b |
46.16 ± 18.10bc |
| Alengaz East |
1.73 ± 1.51bc |
2.83 ± 2.12b |
1.16 ± 1.05b |
5.73 ± 4.63bc |
| Alengazsouth |
0.64 ± 0.45c |
1.71 ± 1.19b |
0.19 ± 0.13b |
2.54 ± 1.77c |
| BantAlmsna |
5.35 ± 2.88bc |
31.97 ± 18.75b |
7.85 ± 5.02b |
45.19 ± 26.47bc |
| BantNorth |
3.71 ± 1.23bc |
39.09 ± 16.77b |
7.83 ± 3.50b |
50.64 ± 20.25bc |
| BantSouth |
4.00 ± 1.66bc |
26.69 ± 10.29b |
7.50 ± 3.41b |
38.19 ± 14.66bc |
| Biriai |
29.80 ± 10.27a |
87.71 ± 24.78a |
23.02 ± 8.41a |
140.54 ± 38.10a |
| GarbAlgash2 |
4.97 ± 1.72bc |
20.14 ± 6.69b |
5.92 ± 2.46b |
31.04 ± 9.90bc |
| GarbAlgash 3 |
5.30 ± 1.67bc |
24.76 ± 10.33b |
4.16 ± 2.16b |
34.23 ± 13.63bc |
| Mastora a |
0c |
0b |
0b |
0c |
| Mastora b |
0c |
0b |
0b |
0c |
| Mastora d |
0c |
0b |
0b |
0c |
| Mukram |
1.11 ± 0.85c |
3.95 ± 2.79b |
1.14 ± 0.79b |
6.21 ± 4.41bc |
| Khatmia3,4 |
7.40 ± 2.32c |
37.90 ± 14.99b |
5.28 ± 2.33b |
50.59 ± 18.47bc |
| Khatmia7,8 |
14.61 ± 5.03b |
42.69 ± 13.96b |
9.38 ± 2.96b |
66.69 ± 20.37b |
| ALnorab |
0.78 ± 0.78c |
1.73 ± 1.73b |
0.59 ± 0.59b |
3.11 ± 3.11c |
| Alsawagi South |
6.80 ± 1.96bc |
15.23 ± 4.78b |
3.95 ± 1.34b |
26.00 ± 7.3bc |
| Alshahid |
0c |
0b |
0b |
0c |
| Alsog Alshabi |
0c |
0b |
0b |
0c |
| ALtora |
3.40 ± 1.30bc |
12.71 ± 4.26b |
3.04 ± 1.13b |
19.16 ± 6.27bc |
Mean separation was based on values transformed to log10 (x?+1).
Means (±SE) with the same letter in each column are not significantly different at 5% level according to REGWQ range test.
Total=Larvae 3+Larvae 4+Pupae.
Number of observations in each residential area=42.
Seasonal prevalence of immature stages of A. aegypti in indoor breeding sites in water containers during 2014 and 2015
The abundance of immature stages was significance (P ≤ 0.05) higher in clay-post than most of the breeding sites (104.8 ± 33.3) during 2014 and 2015 in winter season (Table 3). The female of the 3rd larval instar in winter season was significantly (P ≤ 0.05) higher in clay-pots (22.1 ± 6.6), followed by barrels (9.8 ± 4.2) while none was recorded in cement, flower-vase, jerrycans, and water-tanks. In winter season the highest female of the 4th instar larvae was (significantly (P ≤ 0.05) higher in clay-pots (58.5 ± 18.7), followed by barrels (13.8 ± 4.3). The highest female of pupae (24.2 ± 12.7) was significantly (P ≤ 0.05) higher in claypots, followed by barrels (4.7 ± 2.6), while none was recorded in cement, flower-vase, jerrycans, and water-tanks in winter (Table 3). In summer, there was a significant difference (P ≤ 0.05) in mean total female of immature in clay-pots (105.1 ± 27.8) followed by barrels (53.3 ± 17.4) during 2014 and 2015. In summer, the mean female (22.8 ± 6.9) of the 3rd instar larvae was significantly (P ≤ 0.05) higher in clay-pots, followed by barrels (12.7 ± 7.8) and the lowest was in flower-vase (2.7 ± 2.4). None was recorded in jerrycans and water-tanks. The highest mean number of the 4th larval instar (71.8 ± 19.7) was significantly (P ≤ 0.05) higher in claypots, followed by barrels (34.1 ± 11.5). The clay–pots recorded significantly (P ≤ 0.05) higher (10.5 ± 10.0) in summer followed by barrels (6.6 ± 2.9) but none was no recorded in jerrycans and water-tanks. In autumn, the mean total female of immature stages (121.7 ± 25.1) was significantly (P ≤ 0.05) higher in claypots followed by barrels (40.4 ± 9.8) but none was recorded in water-tanks. In autumn, the mean number (24.3 ± 5.3) of the 3rd larval instar was significantly (P ≤ 0.05) higher in clay-pots, followed by barrels (10.3 ± 2.9) and the lowest in jerrycans (2.6 ± 1.5), but none in water-tanks. The highest female of the 4th instar larvae (80.7 ± 18.1) was significantly (P ≤ 0.05) higher in clay-pots, followed by barrels (27.3 ± 8.1), while for pupae, the clay–pots had significantly (P ≤ 0.05) higher female (16.7 ± 5.1) followed by flower-vase (3.5 ± 2.4) and none in water-tanks in autumn.
Table 3 Mean (±SE) number of immature stages of A. aegypti collected indoor from water containers in different seasons during 2014 / 2015 in Kassala City.
| Immature A. aegypti |
Indoor breeding sites |
| Clay-pots |
Barrels |
Cement containers |
Flower vase |
Jerrycans |
Water-tanks |
| Winter (30) |
| Larva 3 |
22.1 ± 6.6a |
9.8 ± 4.2ab |
0.0 ± 0.0c |
0.0 ± 0.0b |
0.0 ± 0.0b |
0.0 ± 0.0c |
| Larva 4 |
58.5 ± 18.7ab |
13.8 ± 4.3bc |
0.0 ± 0.0e |
0.0 ± 0.0e |
0.3 ± 0.2ed |
0.0 ± 0.0e |
| Pupae |
24.2 ± 12.7a |
4.7 ± 2.6b |
0.0 ± 0.0b |
0.0 ± 0.0b |
0.0 ± 0.0b |
0.0 ± 0.0b |
| Total |
104.8 ± 33.3a |
28.3 ± 9.0b |
0.0 ± 0.0c |
0.0 ± 0.0c |
0.3 ± 0.2c |
0.0 ± 0.0c |
| Summer (40) |
| Larva 3 |
22.8 ± 6.9a |
12.7 ± 7.8b |
0.7 ± 0.4c |
2.7 ± 2.4c |
0.0 ± 0.0c |
0.0 ± 0.0c |
| Larva 4 |
71.8 ± 19.7ab |
34.1 ± 11.5bc |
5.9 ± 3.9ed |
0.2 ± 0.2e |
0.2 ± 0.2e |
0.0 ± 0.0e |
| Pupae |
10.5 ± 10.0a |
6.6 ± 2.9b |
3.8 ± 2.6bc |
0.4 ± 0.3c |
0.0 ± 0.0c |
0.0 ± 0.0c |
| Total |
105.1 ± 27.8a |
53.3 ± 17.4b |
10.3 ± 5.7cd |
3.3 ± 2.7d |
0.2 ± 0.2d |
0.0 ± 0.0d |
| Autumn (37) |
| Larva 3 |
24.3 ± 5.3a |
10.3 ± 2.9b |
4.1 ± 2.4c |
3.9 ± 2.7c |
2.6 ± 1.5c |
0.0 ± 0.0c |
| Larva 4 |
80.7 ± 18.1ab |
27.3 ± 8.1bc |
10.8 ± 6.1d |
6.2 ± 5.3d |
2.6 ± 1.6d |
0.0 ± 0.0d |
| Pupae |
16.7 ± 5.1ab |
2.8 ± 0.8cd |
3.4 ± 2.1d |
3.5 ± 2.4d |
1.2 ± 1.0d |
0.0 ± 0.0d |
| Total |
121.7 ± 25.1a |
40.4 ± 9.8bc |
18.3 ± 10.1cd |
13.7 ± 9.9d |
6.5 ± 3.8d |
0.0 ± 0.0d |
Mean separation was based on values transformed to log10 (x?+1).
Means (±SE) with the same letter in each row are not significantly different at 5% level according to REGWQ range test. Values in parenthesis=number of observations.
Total=Larvae3+Larvae4+Pupae.
Seasonal prevalence of immature stages of A. aegypti in outdoor breeding sites in water containers during 2014 and 2015
In winter season of 2014 and 2015, the mean total female of immature stages of A. aegypti was significantly (P ≤ 0.05) higher in outdoor clay-pots (84.8 ± 17.6) and the lowest female was in barrels (1.5 ± 1.1) while there were none in flower-vases (Table 4). The mean female (19.7 ± 9.6) of the 3rd instar larvae was significantly (P ≤ 0.05) higher in outdoor cement, followed by outdoor clay-pots (12.5 ± 2.3), and there was none in flowervase. For the 4th instar larvae in winter season, the highest female was significantly (P ≤ 0.05) different in outdoor clay-pots with a mean of (59.2 ± 13.6) from that of other outdoor containers. For pupae, the mean number female (13.1 ± 3.4) was significantly (P ≤ 0.05) higher in outdoor clay-pots, followed by cement containers (9.0 ± 4.6) and there was none in barrels and flower-vase. In summer season, the mean total female of immature stages was significantly (P ≤ 0.05) higher in outdoor clay-pots (98.8 ± 18.8) while none was recorded in barrels, flower-vase, generator barrels and tyres. The mean female of the 3rd larval instar was significantly (P ≤ 0.05) higher in outdoor cement containers and clay-pots (15.1 ± 6.4) (14.9 ± 3.7), respectively, but none was recorded in other containers. For the 4th larval instar, the highest mean was in outdoor clay-pots (70.2 ± 14.6) followed by cement (33.3 ± 15.7). In summer season, the mean number of pupae was significantly (P ≤ 0.05) higher in outdoor clay-pots (13.7 ± 3.1) compared to other containers (Table 4). In autumn, the mean total female of immature stages was significantly (P ≤ 0.05) higher in outdoor clay-pots (199.7 ± 36.1) and the lowest in barrels (3.7 ± 3.7). The 3rd larval instar from outdoors clay-post was significantly (P ≤ 0.05) higher, followed by barrels (12.7 ± 3.9), and there were none in flower-vase and generators barrels. In autumn season, the highest mean female of the 4th instar larvae (147.6 ± 27.8) was significantly (P ≤ 0.05) higher in outdoor clay-pots, followed by cement containers (50.9 ± 20.4), while for pupal stage, the female in outdoor clay–pots was significantly (P ≤ 0.05) higher (32.4 ± 6.8) followed by cement containers (15.6 ± 7.8) and the lowest in barrels (0.9 ± 0.9).
Table 4 Mean (±SE) number of immature stages of A. aegypti collected outdoors from water containers in different seasons during 2014/2015 in Kassala City.
| Immature A. aegypti |
Outdoor breeding sites |
| Out-clay-pots |
Barrels |
Cement containers |
Flower-vases |
Generators barrels |
Tyres |
| Winter (40) |
| Larva 3 |
12.5 ± 2.3a |
0.7 ± 0.5c |
19.7 ± 9.6a |
0.0 ± 0.0c |
0.6 ± 0.6c |
0.7 ± 0.5c |
| Larva 4 |
59.2 ± 13.6a |
0.8 ± 0.6ed |
26.3 ± 10.7cd |
0.0 ± 0.0e |
0.8 ± 0.8e |
1.2 ± 0.9ed |
| Pupae |
13.1 ± 3.4a |
0.0 ± 0.0b |
9.0 ± 4.6b |
0.0 ± 0.0b |
0.1 ± 0.1b |
0.2 ± 0.2b |
| Total |
84.8 ± 17.6a |
1.5 ± 1.1c |
55.0 ± 23.7b |
0.0 ± 0.0c |
1.5 ± 1.5c |
2.0 ± 1.3c |
| Summer (40) |
| Larva 3 |
14.9 ± 3.7a |
0.0 ± 0.0c |
15.1 ± 6.4a |
0.0 ± 0.0c |
0.0 ± 0.0c |
0.0 ± 0.0c |
| Larva 4 |
70.2 ± 14.6a |
0.0 ± 0.0e |
33.3 ± 15.7cd |
0.0 ± 0.0e |
0.0 ± 0.0e |
0.0 ± 0.0e |
| Pupae |
13.7 ± 3.1a |
0.0 ± 0.0c |
5.0 ± 2.4bc |
0.0 ± 0.0c |
0.0 ± 0.0c |
0.0 ± 0.0c |
| Total |
98.8 ± 18.8a |
0.0 ± 0.0d |
53.4 ± 23.8bc |
0.0 ± 0.0d |
0.0 ± 0.0d |
0.0 ± 0.0d |
| Autumn (40) |
| Larva 3 |
19.7 ± 3.6a |
0.2 ± 0.2c |
12.7 ± 3.9b |
0.0 ± 0.0c |
0.0 ± 0.0c |
4.2 ± 1.4bc |
| Larva 4 |
147.6 ± 27.8a |
2.6 ± 2.6d |
50.9 ± 20.4bc |
0.0 ± 0.0d |
0.0 ± 0.0d |
8.4 ± 2.7cd |
| Pupae |
32.4 ± 6.8a |
0.9 ± 0.9cd |
15.6 ± 7.8bc |
0.0 ± 0.0d |
0.0 ± 0.0d |
2.3 ± 1.0cd |
| Total |
199.7 ± 36.1a |
3.7 ± 3.7d |
79.2 ± 29.5b |
0.0 ± 0.0d |
0.0 ± 0.0d |
14.9 ± 4.3bcd |
Mean separation was based on values transformed to log10 (x?+1).
Means (±SE) with the same letter in each row are not significantly different at 5% level according to REGWQ range test. Values in parenthesis=number of observations.
Total=Larvae3+Larvae4+Pupae.
Prevalence of immature stages of A. aegypti in breeding sites in residential areas during 2014 and 2015 in Kassala City
The mean total female of immature stages of A. aegypti (106.79±23.88) during 2014 and 2015 was significantly (P ≤ 0.05) higher at Biriai and the lowest at Alshahid (0.14 ± 0.14) (Table 5). The mean female of the 3rd instar larvae (21.59 ± 6.20) during 2014 and 2015 was significantly (P ≤ 0.05) higher at Biriai followed by Garb Algash 3 (12.26 ± 3.17), and the lowest was in Alnorab (0.45 ± 0.45), while for the 4th instar larvae, the highest mean female (68.62 ± 15.70) was significantly (P ≤ 0.05) higher at Biriai followed by Garb Algash3. The mean female of the pupae (16.56 ± 5.10) during 2014 and 2015 was significantly (P ≤ 0.05) higher at Biriai and the lowest at Alnorab (0.34 ± 0.34).
Table 5 Mean (±SE) number of immature stages of A. aegypti collected from breeding sites in different residential areas during 2014/2015 in Kassala City.
| Residential Areas |
No |
Immature stages of A. aegypti |
| Larva 3 |
Larva 4 |
Pupae |
Total |
| Alkrmota |
78 |
13.64 ± 4.63ab |
34.11 ± 9.23bc |
9.07 ± 2.57ab |
56.83 ± 14.48bc |
| AlengazEste |
72 |
3.65 ± 2.04bcd |
4.73 ± 2.99cd |
6.66 ± 5.05b |
15.05 ± 9.85cd |
| AlengazSouth |
72 |
0.37 ± 0.26d |
1.12 ± 0.71d |
0.11 ± 0.08b |
1.61 ± 1.04d |
| BantAlmsna |
66 |
8.90 ± 3.08bcd |
33.04 ± 12.86bc |
7.77 ± 3.53ab |
49.72 ± 18.04bc |
| BantNorth |
66 |
4.74 ± 1.71bcd |
33.63 ± 11.87bc |
5.56 ± 2.28b |
43.93 ± 14.6bcd |
| BantSouth |
66 |
6.50 ± 2.25bcd |
32.10 ± 9.13bc |
7.09 ± 2.41ab |
45.69 ± 12.87bcd |
| Biriai |
72 |
21.59 ± 6.20a |
68.62 ± 15.70a |
16.56 ± 5.10a |
106.79 ± 23.88a |
| GarbAlgash 2 |
78 |
11.98 ± 2.84abc |
31.93 ± 8.99bc |
6.82 ± 2.28b |
50.74 ± 12.57bc |
| GarbAlgash 3 |
78 |
12.26 ± 3.17ab |
41.70 ± 10.69b |
8.29 ± 2.14ab |
62.26 ± 14.95b |
| Mastora a |
78 |
0d |
0d |
0b |
0d |
| Mastora b |
78 |
0d |
0d |
0b |
0d |
| Mastora d |
78 |
0d |
0d |
0b |
0d |
| Mukram |
72 |
1.38 ± 0.58cd |
10.47 ± 4.12cd |
1.38 ± 0.61b |
13.25 ± 5.06cd |
| Khatmia 3,4 |
72 |
6.05 ± 1.75bcd |
34.68 ± 9.97bc |
4.70 ± 1.50b |
45.44 ± 12.26bcd |
| khatmia7,8 |
72 |
12.09 ± 3.23abc |
32.75 ± 9.06bc |
9.05 ± 2.99ab |
53.90 ± 13.81bc |
| ALnorab |
72 |
0.45 ± 0.45d |
1.01 ± 1.01d |
0.34 ± 0.34b |
1.81 ± 1.81d |
| ALsawagi South |
78 |
5.01 ± 1.40bcd |
11.05 ± 3.73bcd |
2.73 ± 0.80b |
18.79 ± 5.26bcd |
| Alshahid |
78 |
0.12 ± 0.12d |
0d |
0.01 ± 0.01b |
0.14 ± 0.14d |
| Alsog Alshabi |
78 |
0d |
0d |
0b |
0d |
| Altora |
78 |
3.11 ± 1.03bcd |
7.78 ± 2.41cd |
2.37 ± 0.79b |
13.26 ± 3.77cd |
Mean separation was based on values transformed to log10 (x?+1).
Means (±SE) with the same letter in each column are not significantly different at 5% level according to REGWQ range test.
Total=Larvae3+Larvae4+Pupae.
No=number of observations.
Seasonal prevalence of females A. aegypti collected from residential areas by different methods 2014/2015 in Kassala City
The mean female of females A. aegypti collected in winter season was significantly (P ≤ 0.05) higher (17.50 ± 7.99) at Garb Algash3 area followed by Garb Algash 2 (13.17 ± 6.94) and Alkrmota (13.00 ± 5.57), and the lowest mean female of females in winter season was at Alnorab area (1.67 ± 0.76), and there was none recorded at Alshahid, Alsog Alshabi, and Mastora (a, b, and d) (Table 6). In summer season, the female of females was significantly (P ≤ 0.05) higher at Alkrmota (9.00 ± 3.56) followed by Khatmia Blocks 3, 4 (8.33 ± 2.44), and the lowest mean number in summer was at Alshahid (0.50 ± 0.5), while there was none at Alsog Alshabi, and Mastora (a, b, and d).
Table 6 Mean (±SE) total number of females A. aegypti collected in different residential areas during seasons of 2014 and 2015 in Kassala City.
| Residential areas |
Females A. aegypti |
| Winter |
Summer |
Autumn |
| Alkrmota |
13.00 ± 5.57ab |
9.00 ± 3.56ab |
7.67 ± 2.64a |
| GarbAlgash 2 |
13.17 ± 6.94ab |
5.50 ± 2.19abcd |
6.67 ± 2.23a |
| GarbAlgash 3 |
17.50 ± 7.99a |
7.50 ± 2.54abc |
3.17 ± 1.64ab |
| BantNorth |
6.33 ± 3.92abc |
0.83 ± 0.54abc |
1.17 ± 0.83ab |
| BantSouth |
2.00 ± 1.37bc |
0.83 ± 0.54bcd |
3.33 ± 1.11ab |
| BantAlmasna |
3.83 ± 1.45abc |
3.33 ± 1.33abcd |
1.50 ± 1.15ab |
| Mukram |
2.17 ± 1.80bc |
1.83 ± 1.47abcd |
2.83 ± 1.38ab |
| Alengaz East |
3.00 ± 2.16bc |
2.33 ± 1.17abcd |
4.50 ± 1.59ab |
| Khatmia 3,4 |
5.83 ± 1.87abc |
8.33 ± 2.44a |
6.67 ± 2.68a |
| Biriai |
3.50 ± 2.22bc |
4.67 ± 2.58abcd |
5.00 ± 3.20ab |
| Khatmia 7,8 |
4.50 ± 1.82abc |
3.67 ± 2.28abcd |
6.83 ± 2.96a |
| Alengaz south |
2.17 ± 1.80bc |
0.83 ± 0.65bcd |
0.00 ± 0.00b |
| ALnorab |
1.67 ± 0.76bc |
1.17 ± 0.60abcd |
2.83 ± 1.42ab |
| ALtora |
2.83 ± 1.38abc |
4.33 ± 1.48abcd |
6.00 ± 3.15ab |
| Alsog Alshabi |
0.00 ± 0.00c |
0.00 ± 0.00d |
00.00 ± 00.00b |
| Alshahid |
0.00 ± 0.00c |
0.50 ± 0.50cd |
0.83 ± 0.54ab |
| Alsawagi |
5.17 ± 1.78abc |
7.00 ± 2.37abc |
7.50 ± 1.99a |
| Mastora a |
0.00 ± 0.00c |
0.00 ± 0.00d |
0.00 ± 0.00b |
| Mastora b |
0.00 ± 0.00c |
0.00 ± 0.00d |
0.00 ± 0.00b |
| Mastora d |
0.00 ± 0.00c |
0.00 ± 0.00d |
0.00 ± 0.00b |
Mean separation was based on values transformed to log10 (x?+1).
Means (±SE) with the same letter in each row are not significantly different at 5% level according to REGWQ range test.
Number of observations in each residential area=6.
The mean female of females in autumn season was significantly (P ≤ 0.05) higher at Alkrmota (7.67 ± 2.64) followed by Alsawagi (7.50 ± 1.99), Alkhatmia 7, 8 (6.83 ± 2.96), and Garb Algash2 (6.67 ± 2.23), and the lowest at Alshahid area (0.83 ± 0.54), and there was none recorded at Alsog Alshabi, and Mastora (a, b, and d). The mean female of females collected by mouth aspirator was significantly (P ≤ 0.05) higher at Garb Algash3 (15.33 ± 7.99) followed by Alkrmota (14.17 ± 4.48), and Birai (12.33 ± 1.54), while the lowest was at Alshahid (0.50 ± 0.50), and there was none at Alsog Alshabi, and Mastora (a, b, and d).
The mean female of females collected by light traps was significantly (P ≤ 0.05) higher at Alsawagi (3.83 ± 2.10) and the lowest at Khatmia Blocks 7, 8 (0.67 ± 0.67), the mean female of females collected by light traps at Khatmia Blocks 3, 4 was (2.83 ± 1.80), Garb Algash3 (2.00 ± 1.29) and Garb Algash 2 (1.67 ± 0.80). The mean female of females collected by knockdown was significantly (P ≤ 0.05) higher at Alkrmota (15.50 ± 2.05), followed by Khatmia Blocks 3, 4 (13.00 ± 1.34), and Garb Algash2 (12.00 ± 1.63), and the lowest mean number at Alshahid area (0.83 ± 0.54). None was recorded at Alengaz south, Alsog Alshabi, and Mastora (a, b, and d) (Table 7).
Table 7 Mean (±SE) total female of A. aegypti collected using different methods during 2014 and 2015 in Kassala City.
| Residential areas |
Females A. aegypti |
| Mouth Aspirator |
Light trap |
Knock down |
| Alkrmota |
14.17 ± 4.48a |
0.00 ± 0.00b |
15.50 ± 2.05a |
| GarbAlgash2 |
11.67 ± 6.91a |
1.67 ± 0.80ab |
12.00 ± 1.63a |
| GarbAlgash 3 |
15.33 ± 7.99a |
2.00 ± 1.29ab |
10.83 ± 3.26abc |
| BantNorth |
4.50 ± 1.77ab |
0.00 ± 0.00b |
3.83 ± 3.83de |
| BantSouth |
4.00 ± 1.15abc |
0.00 ± 0.00b |
2.17 ± 1.05bcde |
| BantAlmasna |
4.33 ± 1.48abc |
0.00 ± 0.00b |
4.33 ± 1.05abcde |
| Mukram |
4.00 ± 1.57abc |
0.00 ± 0.00b |
2.833 ± 1.80de |
| Alengaz East |
4.17 ± 1.19abc |
0.00 ± 0.00b |
5.67 ± 2.04abcde |
| khatmia3,4 |
5.00 ± 0.89ab |
2.83 ± 1.80ab |
13.00 ± 1.34a |
| Biriai |
12.33 ± 1.54a |
0.00 ± 0.00b |
0.83 ± 0.83ed |
| khatmia7,8 |
5.83 ± 1.35ab |
0.67 ± 0.67ab |
8.50 ± 3.12abcd |
| Alengazsouth |
3.00 ± 1.71bcd |
0.00 ± 0.00b |
0.00 ± 0.00e |
| ALnorab |
1.83 ± 0.70bcd |
0.00 ± 0.00b |
3.83 ± 1.14abcde |
| ALtora |
3.83 ± 1.19abc |
0.00 ± 0.00b |
9.33 ± 2.19ab |
| Alsog Alshabi |
0.00 ± 0.00d |
0.00 ± 0.00b |
0.00 ± 0.00e |
| Alshahid |
0.50 ± 0.50cd |
0.00 ± 0.00b |
0.83 ± 0.54ed |
| Alsawagi |
8.17 ± 2.02ab |
3.83 ± 2.10a |
7.67 ± 1.61abc |
| Mastora a |
0.00 ± 0.00d |
0.00 ± 0.00b |
0.00 ± 0.00e |
| Mastora b |
0.00 ± 0.00d |
0.00 ± 0.00b |
0.00 ± 0.00e |
| Mastora d |
0.00 ± 0.00d |
0.00 ± 0.00b |
0.00 ± 0.00e |
Mean separation was based on values transformed to log10 (x?+1).
Means (±SE) with the same letter in each row are not significantly different at 5% level according to REGWQ range test.
Number of observations in each residential area=6.
The mean female of females collected in winter season was significantly (P ≤ 0.05} higher by mouth aspirator (6.98 ± 1.87) followed by knockdown (5.00 ± 1.12), and lowest by light traps (1.03 ± 0.44), also the mean female of females collected in summer season was significantly (P ≤ 0.05) higher by knockdown (4.55 ± 0.88), followed mouth aspirator (4.33 ± 0.79), and the lowest by light traps (0.38 ± 0.27). The mean female of females collected in autumn was significantly (P ≤ 0.05) higher by knockdown (5.63 ± 0.99), followed by mouth aspirator (4.10 ± 0.66), and the lowest by light traps (0.25 ± 0.15). No significance difference between mouth aspiration and knockdown (Table 8).
Table 8 Mean (±SE) total female of females A. aegypti using different methods in the seasons during 2014 and 2015 in Kassala city.
| Methods of collection |
Females A. aegypti |
| Winter |
Summer |
Autumn |
| Mouth Aspirator |
6.98 ± 1.87a |
4.33 ± 0.79a |
4.10 ± 0.66a |
| Light traps |
1.03 ± 0.44b |
0.38 ± 0.27b |
0.25 ± 0.15b |
| Knockdown |
5.00 ± 1.12a |
4.55 ± 0.88a |
5.63 ± 0.99a |
Mean separation was based on values transformed to log10 (x?+1).
Means (±SE) with the same letter in each column are not significantly different at 5% level according to REGWQ range test.
Number of observations in each residential area=40.
Discussion
In Kassala City, immature A. aegypti stages were collected from indoor and outdoor containers but by far the highest numbers were from outdoor containers in autumn season than other seasons. This agrees with the finding of Jansen et al. [23] who found a major reason in outdoor containers because nobody cares for cleaning these containers which are usually filled with water in the rainy seasons. On the other hand, there was no significant difference in breeding sites (indoor/outdoor) among immature stages of A. aegypti. This finding disagrees with Cupp et al. [24] who found outdoor and indoor breeding habits common among mosquito vectors. However, it is unclear whether mosquitoes that rest outdoors prefer certain vegetation for resting sites. Households, therefore, stored water in clay-pots and barrels for long periods especially during the winter seasons which allowed breeding of A. aegypti.
Due to shortage of water supply, the residents stored water in various containers for duration and these containers constituted the major mosquito breeding sources. Containers that retained water for long periods of time make good or suitable breeding habitats for mosquitoes such as the different water holding artificial containers. The result revealed that the clay-pots in indoor and outdoor were preferred breeding site. This finding agrees with Abdalmagid et al. [12] who found the clay-pots considered as preferable breeding habitats of A. aegypti in Kassala. Immature and mature A. aegypti were not recovered from some clusters like Alsog Alshabi, Mastora (a, b, and d). This is probably due to the fact that the residents use small clay-pots (zeer) and fetch water daily, which helps reduce suitable breeding or resting sites. This finding agrees with that of Barrera et al. [11] who found similar situation in homes where water is frequently changed. Vases and flower pots are less important breeding sites because larvae and pupae will not have ample time to develop before these containers winter up.
Many methods are available for the collection of females A. aegypti. The mouth aspirator was used to collect resting mosquitoes indoors and outdoors. Knockdown method for indoor collection and light traps for outdoor collection are also used. Most female A. aegypti were collected from sides of claypots using mouth aspirator technique. Mouth aspirator and knockdown methods were found significantly more efficient than light traps. This is probably because of the manual interference of residents of the light traps position or they use repellants with the traps. Similar findings were obtained by Schoeler et al. [25] who found that the mean number of mosquitoes collected per sampling period was significantly higher using backpack-aspirator than any other collecting methods or trap types. Moreover, this finding supported by the other statement of Schoeler et al. [25] who mentioned that historically the simplest and most effective sampling method for females dengue mosquito vectors has been the direct aspiration of mosquitoes from human collectors, known as human-landing collections.
High abundance of females A. aegypti were recorded in Alkormota and GarbAlgash3 than other areas and that may be due to the availability of suitable resting sites where they remain at rest during the period preceding the onset of activities. Most of residents keep clay-pots (zeer) indoor and outdoor where the females prefer resting due to suitable humidity and darkness. Diaz-Nieto et al. [26] found that mosquitoes are generally found in areas where the air is relatively static and the humidity is high and during day time they prefer to rest in dark places avoiding light.
Conclusion
A. aegypti populations were found in very high densities in some residential areas of Kassala City, and immature stages were found breeding in all water containers mainly clay-pots which were considered the most preferable containers for breeding.
Recommendations
Control of mosquitoes should target the indoor clay-pots (Zirrs) as the main and breeding sites of A. aegypti, and Intervention should be applied to reduce vector of dengue virus in Kassala City, targeting other water holding containers, also it is important to create awareness among people in Kassala City to prevent mosquitoes from breeding in water containers.
Acknowledgement
The authors thank the DAAD scholarship for funding this research and the Ministry of Higher Education and Scientific Research. Thanks are due to Red Crescent for financial support. They also thank people of Kassala City for willingly collaborating with the project.
24736
References
- Getachew D, Tekie H, Gebre-Michael T, Balkew M, Mesfin A (2015) Breeding sites of Aedes aegypti: potential dengue vectors in Dire Dawa, East Ethiopia. Interdiscip Perspect Infect Dis 2015: 1-8.
- Manrique-Saide P, Che-Mendoza A, Rizzo N, Arana B, Pilger D, et al. (2011) Operational guide for assessing the productivity of Aedes aegypti breeding sites. Special Programme for Research and Training in Tropical Diseases. Geneva, Switzerland: TDR. World Health Organization 10: 1-30.
- Focks DA (2003) Review of entomological sampling methods and indicators for dengue vectors: Geneva WHO 1: 1-38.
- Himatt S, Osman KE, Okoued SI, Seidahmed OE, Beatty ME, et al. (2015) Sero-Prevalence of dengue infections in the Kassala State in the eastern part of the Sudan in 2011. J Infect Public Health 8: 487-492.
- Harrington LC, Ponlawat A, Edman JD, Scott TW, et al. (2008) Influence of container size, location, and time of day on oviposition patterns of the dengue vector, Aedes aegypti, in Thailand. Vector-Borne Zoonotic Dis 8: 415-424.
- Reiskind MH, Lounibos LP (2013) Spatial and temporal patterns of abundance of Aedes aegypti L. (Stegomyia aegypti) and Aedes albopictus in southern Florida. Med Veterinary Entomol 27: 421-429.
- Day JF (2016) Mosquito oviposition behavior and vector control. Insects, MDPI, Basel, Switzerland 7: 65-69.
- Vezzani D, Schweigmann N (2002) Suitability of containers from different sources as breeding sites of Aedes aegypti (L.) in a cemetery of Buenos Aires City, Argentina. Memorias do Instituto Oswaldo Cruz 97: 789-792.
- Barrera R, Amador M, Clark GG (2006) Use of the pupal survey technique for measuring Aedes aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am J Trop Med Hyg 74: 290-302.
- Vezzani D (2007) Review: artificial container-breeding mosquitoes and cemeteries: a perfect match. Trop Med Int Health 12: 299-313.
- Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, et al. (2008) Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Veterinary Entomol 22: 62-69.
- Abdalmagid MA, Alhusein SH (2008) Entomological investigation of Aedes aegypti in Kassala and Elgadarief States, Sudan. Sudanese J Public Health 3: 77-80.
- Seidahmed OME, Siam HAM, Soghaier MA, Abubakr M, Osman HA, et al. (2012) Dengue vector control and surveillance during a major outbreak in a costal Red Sea area in Sudan. Eastern Mediterranean Health J 18: 1217-1224.
- Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, et al. (2001) Climate variability and change in the United States: potential impacts on vector-and rodent-borne diseases. Environ Health Perspect 109: 223-225.
- Cochran WG (2007) Sampling techniques. John Wiley and Sons (3rdedn) New York, NY: Wiley: pp: 444-450.
- Dibo MR, Favaro EA, Parra MCP, Santos TCD, Cassiano JH, et al. (2013) Evaluation of two sweeping methods for estimating the number of immature Aedes aegypti (Diptera: Culicidae) in large containers. Revista da Sociedade Brasileira de Medicina Trop 46: 502-505.
- Rueda LM (2004) Pictoral keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmissions. Magnolia Press Auckland. News Land, first publication. pp. 1-60.
- Cutwa MM, O’Meara GF (2006) Psummerographic guide to common mosquitoes of Florida. Florida Med Entomol Laboratory 1: 1-83.
- Tun-Lin W, Lenhart A, Nam VS, Rebollar-Tellez E, Morrison AC, et al. (2009) Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop Med Int Health 14: 1143-1153.
- WHO (1992) Vector resistance to pesticides. Fifteenth report of the expert committee on vector biology and control. In: WHO Technical Report Series 818: 1-55.
- Onyido AE, Deezia NPL, Obiukwu MO, Amadi ES (2009) Ecology of man-biting mosquitoes in the development site of Nnamdi Azikiwe University Awka, Anambra State South Eastern Nigeria. Internet J Health 9: 3-10.
- Lines JD, Curtis CF, Wilkes TJ, Njunwa KJ (1991) Monitoring human-biting mosquitoes (Diptera: Culicidae) in Tanzania with light-traps hung beside mosquito nets. Bull Entomol Res 81: 77-84.
- Jansen CC, Beebe NW (2010) The dengue vector Aedes aegypti: what comes next? Microbes Infect 12: 272-279.
- Cupp EW, Zhang D, Yue X, Cupp MS, Guyer C, et al. (2004) Identification of reptilian and amphibian blood meals from mosquitoes in an eastern equine encephalomyelitis virus focus in central Alabama. Am J Trop Med Hyg 71: 272-276.
- Schoeler GB, Schleich SS, Manweiler SA, Sifuentes VL, Joy JE (2004) Evaluation of surveillance devices for monitoring Aedes aegypti in an urban area of northeastern Peru. J Am Mosquito Control Assoc 20: 6-11.
- Diaz-Nieto LM, Macia A, Perotti MA, Beron CM (2013) Geographical limits of the southeastern distribution of Aedes aegypti (Diptera, Culicidae) in Argentina, PLoS Negl Trop Dis 7: 1-6.