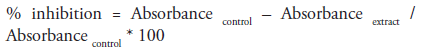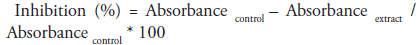Research Article - (2023) Volume 12, Issue 3
COMPARATIVE α-AMYLASE AND α-GLUCOSIDASE INHIBITORY POTENCY AND MODE OF INHIBITION OF AQUEOUS LEAF EXTRACTS OF Acalypha wilkesiana ‘green’ AND Acalypha wilkesiana ‘red’
Didunyemi OM*
Department of Chemical Science, Olusegun Agagu University of Science and Technology, Ondo State, Nigeria
*Correspondence:
Didunyemi OM, Department of Chemical Science, Olusegun Agagu University of Science and Technology, Ondo State,
Nigeria,
Email:
Received: 03-Apr-2023, Manuscript No. ipjbs-23-13668;
Editor assigned: 05-Apr-2023, Pre QC No. P-13668;
Reviewed: 19-Apr-2023, QC No. Q-13668;
Revised: 22-Apr-2023, Manuscript No. R-13668;
Published:
31-May-2023
Abstract
The objective of this study was to investigate comparatively the inhibitory potency and mode of inhibition of aqueous leaf extracts of Acalypha wilkesiana ‘green’ (A.w.g) and Acalypha wilkesiana ‘red’ (A.w.r) on alpha glucosidase from Saccharomyces cerevisiae and alpha amylase from Aspergillus oryzae with a view to establishing the better antidiabetic plant. This study employed para-nitrophenylglucopyranoside (PNPG) and starch as substrates while Acarbose was used as reference. We also qualitatively screened for important phytochemicals as well as the phenolic contents of the extracts. The extracts contained alkaloid, flavonoid, saponin, tannin, steroid, carotenoid and terpenoid while anthraquinone was absent. Aqueous extract of A.w.r had the higher total phenolics (total phenol: 35.75±3.39mgGAE/g and total flavonoid: 350.63±5.01mgQE/g). A.w.r expressed the higher inhibitory effect against alpha glucosidase and alpha amylase with IC50 values of 85.6μg/ ml and 75.6μg/ml respectively in a near competitive fashion while the A.w.g exhibited mixed non-competitive inhibition of alpha amylase and alpha glucosidase. We can therefore deduce from this study that aqueous extract of Acalypha wilkesiana ‘red’ is a more potent inhibitor of alpha amylase and alpha glucosidase.
Keywords
α-glucosidase; α-amylase; Total phenol; Flavonoids
Introduction
Acalypha wilkesiana Muell. Arg. otherwise known as Jacob’s coat, match me if you can, fire dragon or copper leaf belong to the family of Euphorbiaceae and to the genus Acalypha which consist of more than 500 species [1]. Acalypha wilkesiana is native to Fiji and near islands of south pacific. They are globally distributed with substantial occurrence in the tropics of Asia, America and Africa [2]. It has 4 – 8 inch heart shaped leaves presented with various mottled color combinations ranging from green with edged white patches to coppery green to copper, red or even pink-patched leaves, hence its ornamental feature. It is generally found across the regions of West Africa and widely spread in southern Nigeria [3]. The folkloric use of Acalypha wilkesiana has been established by several reports; [4] reported the antimicrobial activity of the plant, [5] reported the antioxidant activity of the plant. [6] reported antimalarial activity. [7] reported the usefulness of Acalypha wilkesiana in the management of diabetes. The findings of [8] were re-affirmed by [9]. These antidiabetic establishments were further strengthened by the in-vitro investigations [10], where they revealed the inhibitory potency of Acalypha wilkesiana against α-glucosidase.
α-Glucosidase and α-amylase are important enzymes involved in the breakdown of large carbohydrate molecules and consequent increase in concentration of blood glucose especially after a carbohydrate-rich meal, whose uncontrolled actions can pose significant danger to the health of type 2 diabetics and borderline patients [11]. Dietary carbohydrate are composed of glucans; amylose with linear chain of α-1,4-glycosidic bonds and amylopectin with α-1,4 and α-1,6-glycosidic linkages [12]. Salivary and pancreatic amylases hydrolyze the α-1,4- glycosidic bonds in starch and shorter oligomers giving rise to maltose, maltotriose and dextrin (mixture of D-glucose polymers units linked by α-1,4 and α -1,6-linkages [13]. Products of hydrolysis by α-amylases are further hydrolysed into monosaccharides (glucose, fructose or galactose) by small intestinal membrane bound α-glucosidases such as Sucrase-isomaltase and Maltose glucoamylase hydrolyzing α-1,4-linkages to yield α-D-glucose. The N-terminal of Sucrase-isomaltase (NtSI) and C-terminal domain Sucrase-isomaltase (CtSI) cleave α-1,6-glycosidic linkage of amylopectin and α-1,2-linkages of sucrose with all cleavages releasing glucose end product which is further subjected to energy-yielding metabolisms. This chain of breakdown continues so long polysaccharides are available within the body system especially following a carbohydrate rich meal.
In a non-insulin dependent diabetic condition when the body is unresponsive to insulin, emanating from impaired stimulation of physiological responses necessary to facilitate trafficking of GLUT-4 from storage vesicles to the plasma membranes of skeletal muscle and adipose tissue consequently leading to impaired uptake of glucose from circulation, blood glucose concentration rises and hyperglycemia ensues [14]. The management of blood glucose level is one of the major target goals in the management of type 2-diabetes which can be achieved through regular exercise, healthy dieting and use of α-amylase and α-glucosidase inhibitors [15] among others. Inhibitors are proven agents of glucose level control. Synthetic inhibitors such as voglibose, acarbose, miglitol etc are not without side effects, a more reason why the search for plant based inhibitors is still a scientific interest [16, 17]. Acarbose, a microbial pseudotetrasaccharide is a potent inhibitor of α-glucosidase and α-amylase, clinically being useful especially in synergy with other antidiabetic agents to achieve suppression of post prandial hyperglycemia [18, 19], however the use of Acarbose is reportedly associated with a number of side effects such as diarrhea and abdominal discomfort [20]. Voglibose, an α-glucosidase inhibitor exerting its antidiabetic actions by reversible inhibition of the enzyme as well as facilitating the increased release glucagon-like-peptide-1 (an insulinotropic hormone) which has inhibitory action on glucagon, thus limiting fasting glucose level [21] had however been documented to have adverse reactions such as nausea and abdominal pain among others [22]. Natural product inhibitors, as opposed to voglibose and acarbose, are reported to possess significant inhibition towards α-glucosidase and even α-amylase with minimal or no side effects [23]. The medicinal plants Acalypha wilkesiana, as evident by reports [24] have established antidiabetic properties, however studies on the comparative potency of the red and green colored Acalypha wilkesiana with respect to inhibition of α-glucosidase and α-amylase and their modes of inhibition, to the best of our knowledge are absent in literature or scanty if available. Hence, the objective of this study.
MATERIALS AND METHODS
Plant materials
Our plant materials, leaves of Acalypha wilkesiana ‘green’ and Acalypha wilkesiana ‘red’ were obtained from same location ‘Olusegun Agagu University of Science and Technology (OAUSTECH)’ mega school campus, Okitipupa local government area of Ondo state, Nigeria in February, 2022 and authentication was done in plant biology department of OAUSTECH.
Chemicals and reagents
Alpha glucosidase from Saccharomyces cerevisiae, alpha amylase from Aspergillus oryzae, paranitrophenylglucopyranoside (PNPG), acarbose, starch, Folin-Ciocalteu reagent, gallic acid and quercetin were gotten from Sigma – Aldrich (St. Louis, MO. USA). Other reagents and chemicals used were of analytical grade and water was distilled.
Preparation of plant materials
Leaves of plants were cleaned via washing and spread for 14 days under shade to air-dry and subsequently ground to powder. 350g was soaked in 1 liter of distilled water for 48 hours. Extract was dried using rotary evaporator and yield was stored at 4oC prior to analysis.
Alpha amylase inhibition assay
We adopted the assay procedure of McCue as modified by [25]. Briefly 250μl of different concentrations of the extract (20, 40, 60, 80 and 100μg/ml) was added to same quantity of 0.02M sodium phosphate buffer of pH 6.9 containing α-amylase solution (0.5mg/ml) in a test tube. Preincubation at 25oC for 10 mins was done, followed by addition of 250μl of 1% starch in 0.02M sodium phosphate buffer (pH 6.9) at timed interval and incubated further for 10 mins at room temperature. 0.5ml of dinitrosalicylic acid (DNSA) was introduced in order to terminate the reaction. All tubes were incubated at boiling temperature and then cooled to room temperature followed by addition of 5ml of distilled water to dilute reaction mixtures. ‘Control’ contained same content except that extract was replaced with distilled water. Absorbance measurement was carried out at 540nm using spectrophotometer. Result of inhibition was expressed in percentage and calculated using the formula;
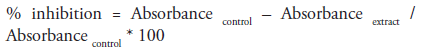
Concentration of extract inhibiting half population of enzyme (IC50) was determined graphically.
Mode of inhibition of alpha amylase
Assay of mode of inhibition of α-amylase by aqueous extract of Acalypha wilkesiana ‘green’ and Acalypha wilkesiana ‘red’ was carried out by a modified method of [26]. 250μl of the extracts was added to 250μl of α-amylase solution and preincubated at 25oC for 10 minutes in one set of test tubes. α-Amylase added to 250μl of pH 6.9 phosphate buffer was also preincubated in a different set of test tubes. To start the reaction, 250ul of varying and increasing concentration of starch solution (1.0 – 5.0mM) were added to the two sets of reaction tubes. All reaction mixtures were incubated at room temperature for 10 mins. 0.5ml of DNSA was added to halt the reactions and boiling for 5 minutes followed. Quantity of released reducing sugar was measure by spectrophotometry and extrapolated from standard maltose curve and then converted to reaction velocities (V). A Lineweaver Burk plot or double reciprocal plot of the inverse of reaction velocities (1/V) versus inverse of substrate concentrations (1/[S]) was generated and the mode of inhibition of α-amylase by the aqueous extracts of both plants were thus determined (Nelson and Cox, 2008) [27].
Alpha glucosidase inhibition assay
The assay protocol [28] was adopted here with slight modification as reported [29]. 250μl of varying concentrations (1.0 – 5.0mM) of the extracts were introduced into test tubes, 50ul of alpha glucosidase (1U/ml) from Saccharomyces cerevisiae was added into all the test tubes. 30ul of 5.0 mM para-nitrophenyl-α- D-glucopyranoside (PNPG) already prepared in 20 mM phosphate buffer (pH 6.9) was added to start the reaction. Incubation of reaction mixtures was done at 37oC for 60 minutes and stoppage of reaction by adding 1ml of 0.1M sodium carbonate (Na2CO3). Enzyme (α-glucosidase) activity was determined by measuring the released yellow colored p-nitrophenol from PNPG at a wavelength of 405nm using spectrophotometer. Results were presented as percentage of blank control.
Concentration of extracts inhibiting 50% activity of enzyme (IC50) was determined graphically.
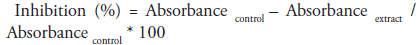
Mode of inhibition of alpha glucosidase
The α-glucosidase inhibitory mode of the extracts was carried out as described [30]. Briefly two different sets of test tubes were prepared. 50μl of extracts’ concentrations (0, 85.6 &171.2μg/ml for the A.w.r and 0, 106.8 & 213.6μg/ml for the A.w.g) were preincubated with 100ul of α-glucosidase solution at 25oC for 10 mins in one set of test tubes while in the other set, α-glucosidase was preincubated with 50μl of pH 6.9 phosphate buffer. 50μl of PNPG at increasing concentrations (1.0 – 5.0mM) was then introduced into the test tubes to begin reactions. Mixtures were incubated at room temperature for 10 mins after which 0.5ml of Na2CO3 was added to stop the reaction. Using paranitrophenol standard curve, amount of reducing sugar released was determined by spectrophotometry and converted to reaction velocities. A line weaver Burk plot of inverse of velocities (1/V) against inverse of substrate concentrations (1/[S]) was plotted and mode of inhibition of α-glucosidase by extracts determined (Nelson and Cox, 2008).
Determination of total phenol
The total phenol contents of the extracts was estimated using the Folin-Ciocalteu colorimetric method of [31], a method which relies on electron transfer from phenolic compounds in alkaline medium giving rise to phosphotungstic/phosphomolybdenum complex indicated by a blue coloration where the depth of color is directly proportional to the concentration of phenolics. 200ul of extract was mixed with 1000ul Folin-Ciocalteu reagent. After 4 mins, 1000ul of 7.5% sodium carbonate (Na2CO3) was introduced. Mixture was then incubated at 25oC for 2 hours. Absorbance was measured at 760nm and total polyphenol in the extracts was expressed as mg of gallic acid equivalent per gram of extract (mgGAE/g).
Determination of total flavonoid content
We adopted the Aluminium chloride (AlCl3) reagent method as described [32]. Briefly, 1 ml of extract was mixed with 1 ml of AlCl3 (2%) in methanol. Reaction mixtures were incubated at 25oC for 10 mins and immediately followed by absorbance measurement at 430nm using spectrophotometer. Result was expressed as mg of quercetin equivalent per gram of extract (mgQE/g).
Phytochemical screening
Investigations for the presence of phytochemicals in the aqueous leaf extracts of Acalypha wilkesiana ‘green’ and Acalypha wilkesiana ‘red’ were done by the standard procedures of Sofowora (1993) and Trease and Evans (2002).
Statistical analysis
Assays were carried out in triplicates and results were expressed as mean ± standard deviation (SD). Analysis of data was carried out using graph pad prism (graphpad software Inc. San Diego, USA) and statistical difference between groups was done by one way analysis of variance (ANOVA) and Duncan multiple range test. Values were considered significant at p < 0.05.
DISCUSSION
Physiological activities necessary to mobilize GLUT-4 carrier vesicles to cell membranes of mycocytes and adiposites for internalization of GLUT-4 molecules and subsequent insulin-regulated uptake of glucose for metabolisms may be distorted as a result of unresponsiveness to insulin, defects in insulin actions and/or insulin secretory processes, thus resulting in persistent elevated glucose level [33]. Post prandial hyperglycemia (PPHG) is characteristic of type-2- diabetes and risk factor for macrovascular and microvascular complications [34]. It’s close monitoring and control is critical in the clinical management of diabetes and related complications. Among a number of management strategies available in literature, the use of inhibitors of α-amylase and α-glucosidase is key, as inhibitors are understood to inhibit or delay the degradation of glucose polymers in the gastrointestinal environment and limit post prandial glucose excursion in type-2-diabetes [35]. The unbearable side effects associated with the use of synthetic drugs (α-glucosidase and α-amylase inhibitors) triggered the search for medicinal plant-based inhibitors with promising level of potency and less side effects.
In this study, we evaluated and compared the inhibitory potency and mode of inhibition of aqueous extracts of Acalypha wilkesiana ‘green’ (A.w.g) and Acalypha wilkesiana ‘red’ (A.w.r) against α-glucosidase and α-amylase. Across the tested concentrations (20 – 100μg/ml), the aqueous extract of A.w.r showed higher inhibition (in percentage) towards α-amylase, although statistically insignificant (p < 0.05) when compared to A.w.g except at 80μg/ml and 100μg/ml. Values of extracts are significantly different (p < 0.05) from that of acarbose at all concentrations with the exemption of 80μg/ml where A.w.r favorably compares with the standard (Fig.1.)
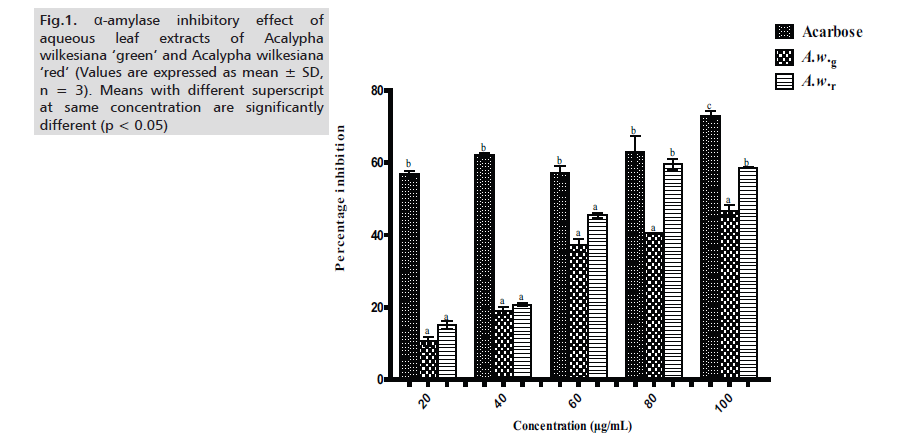
Figure 1: α-amylase inhibitory effect of aqueous leaf extracts of Acalypha wilkesiana ‘green’ and Acalypha wilkesiana ‘red’ (Values are expressed as mean ± SD, n = 3). Means with different superscript at same concentration are significantly different (p < 0.05)
A.w.r also showed promising inhibition of α-glucosidase and values were significantly higher (p < 0.05) than those of A.w.g except at 20μg/ml and 60μg/ml. However, inhibition values of the two extracts were significantly lower (p < 0.05) when compared to acarbose with the exception of 20μg/ml, in which case A.w.r favorably compares with acarbose (Fig.2.)
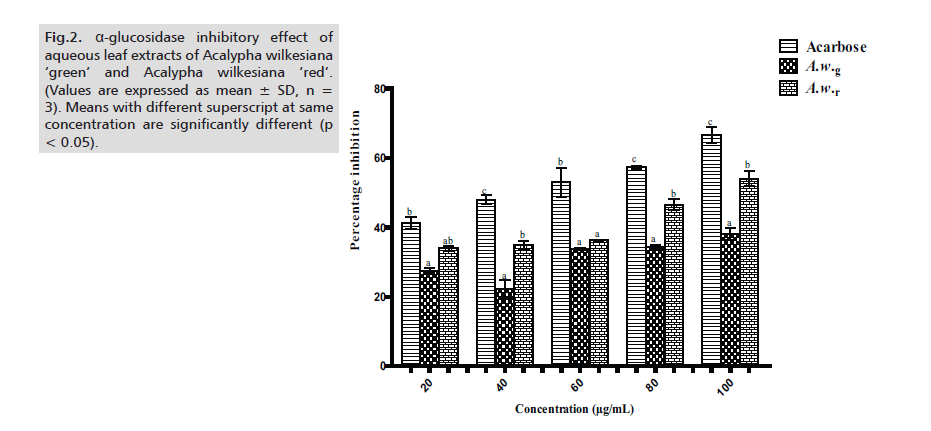
Figure 2: α-glucosidase inhibitory effect of aqueous leaf extracts of Acalypha wilkesiana ‘green’ and Acalypha wilkesiana ‘red’. (Values are expressed as mean ± SD, n = 3). Means with different superscript at same concentration are significantly different (p < 0.05).
The potency of the extracts was further ascertained by determining the IC50 values of the inhibition of α-amylase and α-glucosidase. For both enzymes, A.w.r showed higher inhibitory potency (with IC50 values of 76.11±2.23μg/ml and 85.59±2.34μg/ml respectively against α-amylase and α-glucosidase) than A.w.g (Tabs. 1-3.) As for the modes of inhibition, from the line weaver Burk plot generated. A.w.r displayed a near competitive mode of inhibition against α-amylase and α-glucosidase (Figs. 3. and 4.), which is an indication that its active components compete with the substrate for the active site on both enzymes thereby inhibiting the degradation of large glucans. In the case of A.w.g, extract displayed mixed non-competitive mode of inhibition (Figs. 3. and 4.), suggesting that its active components do not compete with the substrate for the active site on the enzymes; rather it binds to other sites on the enzymes to limit the breakdown of carbohydrate.
Extract Phytochemicals |
A.w.g |
A.w.r |
| Alkaloid |
+ |
+ |
| Flavonoid |
+ |
+ |
| Steroid |
+ |
+ |
| Tannin |
+ |
+ |
| Saponin |
+ |
+ |
| Anthraquinone |
- |
- |
| Carotenoid |
+ |
+ |
| Cardiac glycoside |
- |
+ |
| Terpenoid |
+ |
+ |
+ = Present, - = Absent
Tab.1. Phytochemicals in aqueous extracts of Acalypha wilkesiana ‘green’ (A.w.g) and Acalypha wilkesiana ‘red’ (A.w.r)
Extracts |
Total Phenol (mgGAE/g) |
Total flavonoid (mgQE/g) |
| A.w.g |
21.61±1.26 |
247.02±9.10 |
| A.w.r |
35.75±3.99 |
350.63±5.01 |
Values are expressed as mean ± standard deviation – SD. (n = 3)
Tab.2. Total phenol and flavonoid contents of aqueous leaf extracts of A. w.g and A.w.r
| |
IC50 (µg/ml) |
| Samples |
α-amylase α-glucosidase |
| Acarbose |
46.33±1.20a 60.50±2.52a |
| A.w.g |
102.53±2.71c 106.81±2.92c |
| A.w.r |
76.11±2.23b 85.59±2.34b |
The values are expressed as means ± SD of triplicate tests. Means on same column sharing different superscripts are significantly different (ð?? < 0.05). Tab.3. IC50 values for ð?¼-amylase and ð?¼-glucosidase inhibition of aqueous extracts of Acalypha wilkesiana ‘green, and Acalypha wilkesiana ‘red’.
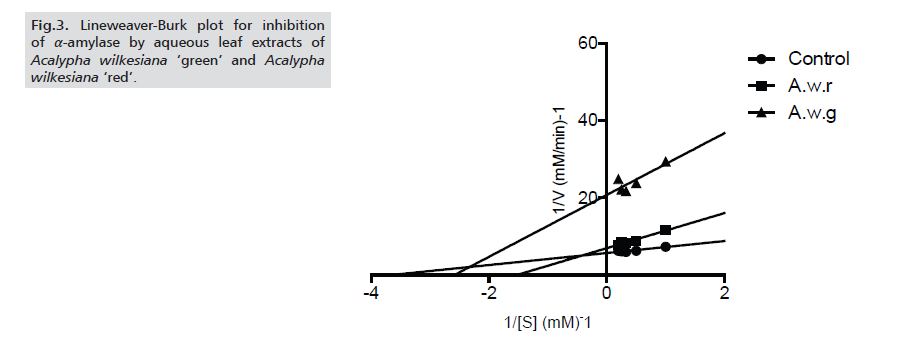
Figure 3: Lineweaver-Burk plot for inhibition of α-amylase by aqueous leaf extracts of Acalypha wilkesiana ‘green’ and Acalypha wilkesiana ‘red’.
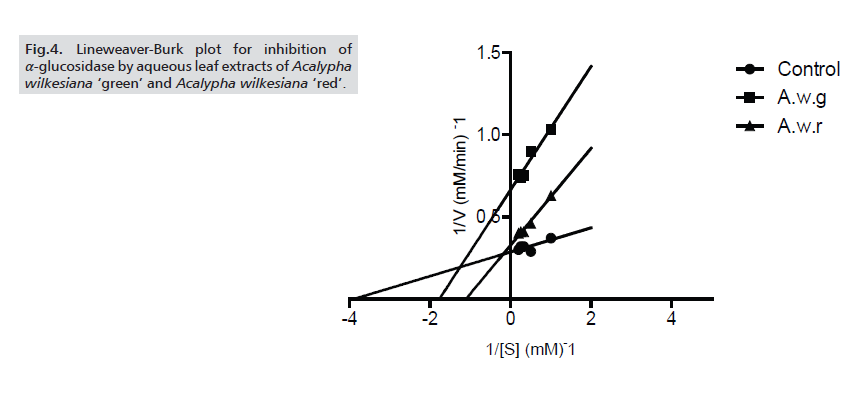
Figure 4: Lineweaver-Burk plot for inhibition of α-glucosidase by aqueous leaf extracts of Acalypha wilkesiana ‘green’ and Acalypha wilkesiana ‘red’.
The significantly higher (p < 0.05) values of acarbose than the extracts is an indication of mild potency of the extracts against the enzymes, which is in agreement with previous reports that excessive inhibition of pancreatic α-amylase could trigger abnormal bacterial fermentation of undigested carbohydrates, as such mild inhibition, offered by most plant derived inhibitors is well appreciable. The inhibitory effects of these extracts could be a reflection of their total phenolic contents as their IC50 values directly correlate with their phenolic contents. The A.w.r had higher total phenol and flavonoid values (Tab.2.) Phenolic compounds are reportedly able to inhibit carbohydrate degrading enzymes due to their protein-binding properties. Our finding is thus in conformity with other reports that potential inhibitors of α-glucosidase and α-amylase belong to the flavonoid class. The presence of other phytochemicals such as terpenoid, saponin, tannin etc in the extracts of A.w.r and A.w.g (Tab.1.) could have also contributed to their inhibitory propertieswhich is in line with the reports.
CONCLUSION
From this study, we can conclude that aqueous extracts of A.w.r and A.w.g have antidiabetic properties, conforming to the reports of Ikewuchi and Ikewuchi (2010). The α-glucosidase and α-amylase inhibitory activities might be due to the presence of important phytochemicals such as flavonoid, saponin, terpenoid etc acting synergistically. We could also conclude that A.w.r had a higher inhibitory potency on α-amylase and α-glucosidase activities, as reflected by the IC50 values which could possibly be due to the higher phenolic contents detected in the extract.
References
- Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006; 107: 449-455.
Indexed at, Google Scholar, Crossref
- Anokwuru CP, Sinisi A, Samie A, et al. Antibacterial and antioxidant constituents of Acalypha wilkesiana.Nat Prod Res.2015; 29(12): 1180 ‐ 1183.
Indexed at, Google Scholar, Crossref
- Apostolidis E, Kwon YI, Shetty K. “Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension,” Innovative Food Science and Emerging Technologies.2007; 8(1): 46–54.
Indexed at, Google Scholar, Crossref
- Asano N. Sugar-mimicking glycosidase inhibitors: bioactivity and application: A Review. Cell Mol Life Sci.2009; 66: 1479 – 1492.
Indexed at, Google Scholar, Crossref
- Ayoola GA, Coker HB, Adesegun SA, et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop J Pharm Res.2008; 7: 1019 – 1024.
Indexed at, Google Scholar, Crossref
- Dabhi AS, Bhatt NR, Shah MJ. Voglibose: an alpha glucosidase inhibitor. Journal of clinical and diagnostic research.2013; 7(12): 3023.
Indexed at, Google Scholar, Crossref
- El-Manawaty M, Gohar LAMIAA. In vitro alpha glucosidase inhibitory activity of Egyptian plant extracts as an indication for their antidiabetic activity. Vitro.2018; 11: 360-367.
Indexed at, Google Scholar, Crossref
- Forcados GE, Chinyere CN, Shu ML. Acalypha wilkesiana: Therapeutic and Toxic Potential. Journal of Medical & Surgical Pathology.2016; 1: 3.
Indexed at, Google Scholar, Crossref
- Fujisawa T, Ikegami H, Inoue K. Effect of two alpha glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabol. 2005; 54: 387-390.
Indexed at, Google Scholar, Crossref
- Göke R, Larsen PJ, Mikkelsen JC. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosc. 2015; 7: 2294–2300.
Indexed at, Google Scholar, Cross Ref
- Gotep JG, Agada GOA, Gbise DS. Antibacterial activity of ethanolic extract of Acalypha wilkesiana leaves growing in Jos, Plateau State, Nigeria. Mal J Microbiol. 2010; 6: 69 - 74.
Indexed at, Google Scholar, Crossref
- Hanefeld M, Fischer S, Julius U. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39: 1577–1583.
Indexed at, Google Scholar, Crossref
- Ikewuchi JC, Ikewuchi CC. Hypocholesterolemic effect of aqueous extract of Acalypha wilkesiana ‘Godseffiana’ Muell Arg on rats fed egg yolk supplemented diet: Implications for cardiovascular risk management. Res J Sci Technol. 2010; 2: 78 - 81.
Indexed at, Google Scholar
- Kahn CR. The molecular mechanism of insulin action. Annual review of medicine.1985; 36(1): 429-451.
Indexed at, Google Scholar, Cross Ref
- Kazeem MI, Adamson JO, Ogunwande IA. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed research international. 2013.
Indexed at, Google Scholar, Crossref
- Kim YM, Jeong YK, Wang MH. “Inhibitory effect of pine extract on 𝛼-glucosidase activity and postprandial hyperglycemia. Nutrition.2005; 21(6). 756–761.
Indexed at, Google Scholar, Crossref
- Kwon YI, Apostolidis E, Kim Y. Health benefits of traditional corn, beans and pumpkin; in vitro studies for hyperglycemia and hypertension management. J Med Food.2007; 10: 266–275.
Indexed at, Google Scholar, Crossref
- Kwon YI, Apostolidis E, Shetty K. Inhibitory potential of wine and tea against a-amylase and a-glucosidase for management of hyperglycemia linked to type 2 diabetes. J. Food Biochem. 2008; 32: 15-31.
Indexed at, Google Scholar, Crossref
- Matsuda H, Morikawa T, Yoshikawa M.“Antidiabetogenic constituents from several natural medicines,”. Pure and Applied Chemistry.2002; 74(7):1301–1308.
Indexed at, Google Scholar, Crossref
- Matsui T, Ogunwande IA, Abesundara KJM. “Anti-hyperglycemic potential of natural products”. Mini-Reviews in Medicinal Chemistry.2006; 6(3): 349–356.
Indexed at, Google Scholar Crossref
- McCue PP, Shetty K. “Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro”. Asia Pacific Journal of Clinical Nutrition.2004; 13(1): 101–106.
Indexed at, Google Scholar
- Odoh UE, Ndubuokwu RI, Inya-Agha SI. Antidiabetic activity and phytochemical screening of Acalypha wilkesiana Mull Arg roots in Alloxan induced diabetic rats. Scientific Research and Essay.2014; 9(7): 204-212.
Indexed at, Google Scholar, Crossref
- Ogbuehi I, Adikwu E, Oputiri D. Effect of" Acalypha wilkesiana" Muell Arg Leaf Extract on the Oxidative Indices, Liver Enzymes and Liver Integrity of Rats Infected with" Plasmodium berghei". British Journal of Pharmacology and Toxicology. 5(2): 68 – 74.
Indexed at, Google Scholar, Crossref
- Oyebode OA, Erukainure OL, Koorbanally NA. Acalypha Wilkesiana ‘Java White’: Identification of Some Bioactive Compounds by Gc-Ms and Their Effects on Key Enzymes Linked to Type 2 Diabetes. Acta Pharmaceutica. 2014; 68(4): 425-439.
Indexed at, Google Scholar, Crossref
- Parkin C, Brooks N. Is postprandial glucose control important? Is it practical in primary care settings?Clin Diabetes.2002; 20:71–76.
Indexed at, Google Scholar, Crossref
- Riley HP. Families of Flowering plants of Southern Africa.University of Kentucky Press, U. S. A. 1963; 73.
Indexed at, Google Scholar, Crossref
- Sallau AB, Yakubu RN, ABDULLAHI S. In vitro effect of terpenoids-rich extract of Momordica charantia on alpha glucosidase activity. Vitae. 2015; 25(3): 148-153.
Indexed at, Google Scholar, Crossref
- Saltiel AR, Kahn CR."Insulin signaling and the regulation of glucose and lipid metabolism".Nature.2001;414(6865): 799–806.
Indexed at, Google Scholar, Crossref
- Shobana S, Sreerama YN, Malleshi NG. “Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) Seed coat phenolics: mode of inhibition of 𝛼- glucosidase and pancreatic amylase,” Food Chemistry.2009; 115(4): 1268–1273.
Indexed at, Google Scholar, Crossref
- Sofowora A. Medicinal Plants and Traditional Medicinal in Africa.2nd Ed. Sunshine House, Ibadan, Nigeria: Spectrum Books Ltd. Screening Plants for Bioactive Agents.2003; 134 – 156.
Indexed at, Google Scholar, Crossref
- Sule OJ, Elekwa I, Ayalogu EO. Effect of Acalypha wilkesiana Muell Arg. On Haematological Parameters in Wistar Albino Rats. Int J Biol Med Res. 2012; 3: 1234-1237.
Indexed at, Google Scholar
- Tadera K, Minami Y, Takamatsu K. “Inhibition of 𝛼-glucosidase and 𝛼-amylase by flavonoids”. Journal of Nutritional Science and Vitaminology.2006; 52(2): 149–153.
Indexed at, Google Scholar, Crossref
- Trease GE, Evans WC. Pharmacognosy.15th Ed. London: Saunders Publishers.42–44. 221–229, 246–249, 304–306, 331–332, 391–393.
- Wang H, Du YJ, Song HC.𝛼-Glucosidase and 𝛼- amylase inhibitory activities of guava leaves. Food Chemistry.2010; 123(1): 6–13.
Indexed at, Google Scholar, Crossref
- Yafang S, Gan Z, Jinsong B. Total phenolic content and antioxidant capacity of rice grains with extremely small size. Afr J Agric Res.2011; 6(10): 2289 -2293.
Indexed at, Google Scholar