Maria Noor1, Yaser Ishaq2, Asma Aftab3, Saadia Ata4, Salima Naveed Manji1 and Malik Adeel Anwar5*
1Department of Oral Medicine, FMH College of Medicine and Dentistry Lahore, Pakistan
2Department of Oral and Maxillofacial Surgery, Akhtar Saeed Medical and Dental College Lahore, Pakistan
3Department of Oral and Maxillofacial Surgery, Mayo Hospital Lahore, Pakistan
4Department of Orthodontics, Akhtar Saeed Medical and Dental College, Lahore, Pakistan
5Department of Oral Pathology, Fatima Memorial College of Medicine and Dentistry Lahore, Pakistan
*Corresponding Author:
Malik Adeel Anwar
Assistant Professor
Department of Oral Pathology
Fatima Memorial College of Medicine and Dentistry Lahore, Pakistan
Tell: +923214512366
E-mail: dr_adeel_anwar@yahoo.com
Received Date: June 05, 2020; Accepted Date: June 25, 2020; Published Date: June 30, 2020
Citation: Noor M, Ishaq Y, Aftab A, Ata S, Manji SN, et al. (2020) Diagnostic Accuracy of Lugol’s Iodine Staining in Detection of Safe Margins of Oral Squamous Cell Carcinoma. Health Sci J. 14 No. 3: 725.
DOI: 10.36648/1791-809X.14.3.725
Keywords
Oral squamous cell carcinoma; Diagnostic accuracy; Dysplastic epithelium; Malignant tissue; Safe margins; Involved margins; Lugol’s iodine; Staining pattern
Introduction
Oral squamous cell carcinoma (OSCC) is a malignant disease of oral mucosa characterized by rapid uncontrolled proliferation of mucosal cells [1]. South East Asia (India, Pakistan, Nepal and Bangladesh) has the high risk population for oral cancer [2]. According to World Health Organization data, there is risk of epidemic spread of OSSC in this region by 2020 [1]. Among the malignancies of oral cavity, most common is mucosal squamous cell carcinoma i.e. >90% [3].
The etiology of OSCC is genetic, but multiple extrinsic and intrinsic agents may be at work as predisposing factors in the causation of oral carcinoma [4,5]. The most important among these are alcohol, tobacco smoking, smokeless tobacco, betel nut, nutritional deficiency, immune deficiency disorders, chronic trauma, syphilis, radiations and viruses [6]. Various forms of smokeless tobacco like snuff, and betel quid with tobacco have been found to be most strongly associated with OSCC [5]. Clinically OSCC typically presents as a non-healing ulcer, persistent fungating growth or nodule. The most common sites of involvement are buccal mucosa, tongue, lip and alveolus [7,8].
Many OSCCs develop from preexisting lesions and conditions of the oral cavity. Such lesions are called premalignant lesions for example; leukoplakia, erythroplakia, palatal lesion of reverse cigar smoking, oral submucous fibrosis, discoid lupus erythematosus, and hereditary disorders such as dyskeratosis congenita and epidermolysis bullosa [9,10].
The prognosis of cancer treatment is inversely proportional to the stage of disease at diagnosis [4,8]. In spite of advances in modalities of early diagnosis small lesions often go unrecognized. As a result 60% of lesions are well advanced at the time of discovery [2]. Treatment of OSCC frequently requires surgery and adjuvant radiotherapy with high rate of morbidity and mortality. Although advanced techniques for the detection and treatment of this disease have developed, the overall survival rate of oral cancer is disappointingly low for the last 10 years and was reported as less than 50% in the literature [3]. This implicates that the treatment outcome depends not only on the early detection but improvement of surgical techniques and the behavior of the tumor itself. Recent statistical data, suggests the 5-year survival rate of stage I oral cancer patients was 66.2%, while for stage IV, the survival rate declined to only 22.2% [7,11]. Treating patients in their early stages of disease and adequate treatment of primary lesion is the most important factor in improving the survival rate. It was shown to reduce mortality from oral cancer by 32% in highrisk individuals [12].
Multiple screening and detection techniques have been developed in order to assist complete removal of malignant lesions [13]. Continuous research is being done to improve the operative techniques for surgical removal of OSCC. Special lights, magnification tools, like surgical microscopes and special stains like toluidine blue and Lugol’s iodine are such clinical aids [13]. Lugol’s iodine is been advocated in recent literature, for peroperative identification of tumor margins as it’s a quick, easy and inexpensive technique [14].
Lugol’s iodine was also found useful in the detection of patients at risk of developing malignant disease and those with lesions that were clinically suspicious of malignancy [15]. Vital staining with an iodine solution was found to determine the precise borders of dysplastic epithelium, and it was concluded that a 5 mm border of normal tissue peripheral to the iodine-positive area was adequate for the removal of the dysplastic epithelium [16]. The clinical application of Lugol’s iodine has been shown to be selective for staining of premalignant and malignant lesions.
The mechanism of iodine staining used to diagnose mucosal lesions is based on a phenomenon by which the iodine is absorbed by a coil of one of a normal chain of polysaccharide existing in the cytoplasm; the reaction is visualized as a brown color [17]. This reaction does not take place in dysplastic mucosa. That’s why normal mucosa is stained dark brown with Lugol’s iodine whereas abnormal or dysplastic mucosa does not pick up the color [18].
The present study was aimed at determination of the diagnostic accuracy of Lugol’s iodine staining in detection of safe margins of oral squamous cell carcinoma, taking histopathology as gold standard.
Materials and Methods
This study was conducted in the Department of Oral and Maxillofacial Surgery, King Edward Medical University (KEMU)/ Mayo Hospital, Lahore. Following the non-probability purposive sampling technique, a total of 50 diagnosed cases of OSCC were included after an informed consent. The ethical approval had been granted by the Institutional Ethical Review Board of KEMU. Inclusion criteria
• Diagnosed cases of OSCC of any age and gender.
• Patients fit for surgery from medical point of view.
• Patients with resectable disease.
• Patients who are not allergic to Lugol’s iodine
• Patient with no previous history of chemo or radiotherapy.
Exclusion criteria
• Patients with OSCC lesion on hard palate or attached gingiva
• Patients with history of thyrotoxicosis
• Patients with recurrent OSCC lesion
Data collection procedure
Patients were prepared for surgery under general anesthesia. Informed consent of the patients was taken for inclusion in study. Performa were filled regarding demographic details and site of lesion. Surgical treatment plan was decided by consultants according to a similar protocol for all patients. On surgical table, under general anesthesia, oral cavity was thoroughly washed with saline and 1% acetic acid and dried with gauze. Lugol’s iodine was applied for 1-2 min and excess stain was washed off. Area that picked up brown stain was considered safe by Lugol’s iodine and area that did not stain brown was considered as involved and resection of tumor was done with a border of normal tissue at least 5 mm around the involved margins. All resection margins were counter examined microscopically and safe or involved resection margins were noted by the histopathologist.
Data analysis
All collected data was entered and analyzed using Statistical Package for Social Sciences (SPSS) version 20.0. Quantitative data like age was presented as mean ± standard deviation. Qualitative variables like gender and safe margins of oral SCC with Lugol’s iodine and safe margins on histopathology were described in terms of frequencies and percentages. A 2×2 contingency table was generated to calculate sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of Lugol’s iodine in detection of safe margins of OSCC by taking histopathology as gold standard.
Results
Total 50 patients were included in this study. Patients were selected from the inpatient department of Oral and Maxillofacial surgery, Mayo Hospital Lahore. Mean age of all 50 patients was 52.2 ± 13.12 years. Minimum age of patients was 25 and maximum age of patients was 88 years. Mean age of male and female patients was 52.0 ± 13.5 and 52.4 ± 12.6 years respectively.
Gender distribution of patients shows that there were 62% male and 38% female patients. Male patients were predominant as compared to female patients (Table 1). Site of lesion was also noted. The most common site of presentation was buccal mucosa (40%) followed by tongue (24%), lower vestibule (18%), upper vestibule (8%) and lip (6%) (Figure 1).
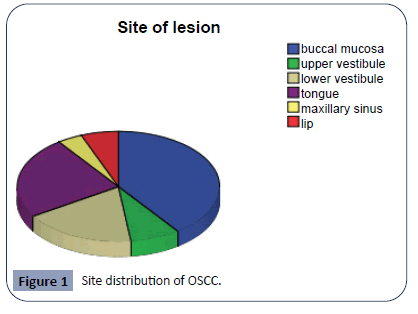
Figure 1: Site distribution of OSCC.
Table 1 Gender distribution of patients.
| Gender |
Frequency |
Percent |
| male |
31 |
62.0 |
| female |
19 |
38.0 |
| Total |
50 |
100.0 |
Site of lesion was also observed in relation to gender of the patients. In male patients 12 (38.7%) had lesion on buccal mucosa, 6 (19%) had lesion on lower vestibule, 7 (22%) patients had lesion on tongue, 3 (9.6%) patients had lesion on upper vestibule and 1 (3.2%) had lesion on lip. Whereas in female patients 8 (42%) patient had lesion on buccal mucosa, only 1 (5.2%) had lesion on upper vestibule, 5 (26%) had lesion on tongue and 3 (15.7%) patients had lesion on lower alveolus (Table 2). It was observed that in male and female patients buccal mucosa is site on which lesions were frequently present as compared to other oral parts.
Table 2 Distribution of site of lesion in relation to gender of patients.
| Site of lesion |
Gender |
Total |
| Male |
Female |
| |
Buccal mucosa |
12 |
8 |
20 |
| Upper vestibule |
3 |
1 |
4 |
| Lower vestibule |
6 |
3 |
9 |
| Tongue |
7 |
5 |
12 |
| Maxillary sinus |
2 |
0 |
2 |
| Lip |
1 |
2 |
3 |
| Total |
31 |
19 |
50 |
Sensitivity of Lugol’s iodine is 90.9%, it means that it had accurately detected 10 margins which were involved by tumor. Specificity of Lugol’s iodine is 92.3% it means that it had accurately detected 36 margins which were not having any malignant lesion. Positive Predicative and Negative predictive value of Lugol’s iodine is 76.9% and 97.2% respectively (Table 3).
Table 3 Diagnostic accuracy of lugol’s iodine in detection of safe margins of OSCC.
| |
Margins on histopathology |
Total |
| Safe |
Involved |
| Margins on Lugol’s iodine |
Safe |
36(92.3%)TN |
1(9.0)FN |
37(74%) |
| Involved |
3(7.6%)FP |
10(90.9%)TP |
13(26%) |
| Total |
39 |
11 |
50(100%) |
Sensitivity=TP/(TP+FN)=90.9%
Specificity=TN/(TN+FP)=92.3%
Positive Predictive Value (PPV)=TP/(TP+FP)=76.9%
Negative Predictive Value (NPV)=TN/(TN+FN)=97.2%
Discussion
There are several reports in which the value of iodine staining in the detection of premalignant and malignant lesions of the oral mucosa is emphasized. Lugol’s iodine was found to be an effective mean of reducing the likelihood of unsatisfactory surgical margins in the resection of OSCC [15]. In a study based on immunohistochemical examination, YOKOO et al probed the relationship between epithelial dysplasia unstained with iodine and the expression of proliferating cell nuclear antigen (PCNA), the p53 tumor suppressor gene, and the presence of glycogen in the dysplastic tissues. They reported that epithelium that was stained with iodine could be categorized as normal epithelia, whereas epithelium that was unstained with iodine showed both moderate and severe dysplasia [18]. In 2011, Umeda et al studied the recurrence rate of Oral squamous cell carcinoma and found out that recurrence rate was significantly low in patients who had Lugol’s iodine guided resection of tumor. They reported that the Lugol-unstained area seems to represent the extent of malignant or premalignant lesions precisely, and taking surgical margin 5 mm away from the border of the stained and unstained area resulted in reduced recurrences [19]. In this study they followed up, for three years, 93 cases of OSSC tongue after Lugol’s iodine assisted resection and none of the patients showed recurrence.
In a similar study KURITA et al showed that a 5 mm safety margin from the Lugol-unstained area was adequate for the complete removal of the dysplastic tissue [16]. In their study 18 cases were diagnosed of having malignancy on stain and 16 out of 18 showed consistent histopathological finding. Only two cases were false positive and they had a histologic diagnosis of lichen planus [16]. Staining with Lugol’s iodine is related to the glycogen component of cells. Normal stratified epithelium has abundant glycogen content in spinous layer, however in carcinoma the amount of glycogen is decreased due to increased glycolysis. Yajima et al investigated 33 cases of SCC who had iodine unstained lesions. They analyzed telomerase activity in the lesions by a fluorescence technique and histologically confirmed that iodine unstained areas has mild to severe degree of dysplasia [20].
Although there are some limitations to use of Lugol’s iodine, for example, the stain is not picked up by orthokeratinized epithelium of hard palate, yet it’s a cost effective and non-technique sensitive method with results comparable to fancy investigation modalities like fluorescent lights and frozen sections [4]. Epstein et al evaluated the efficacy of Lugol’s iodine staining for detection of oral squamous cell carcinoma in suspected lesions. They prospectively analysed 59 cases and counter confirmed the results of staining by histopathology. They reported that sensitivity, specificity, PPV and NPV of stain were 87.5%, 84.2%, 92.1 and 76.2 respectively [20]. They also reported that glycogen content of epithelium cells is inversely proportional to the degree of keratinization and inflammation. This may limit the use of Lugol’s solution in more keratinized tissue and might explain the false negative results in a study. Therefore stain uptake in such areas should be interpreted cautiously.
One of initial published reports on diagnostic accuracy of Lugol’s iodine is by Shiozaki et al. This screening study of 178 patients describes Lugol’s iodine as more efficient in detecting malignant and dysplastic tissue than visual and endoscopic examination [21].
The current study describes the diagnostic efficacy of Lugol’s iodine in detection of safe margins of oral squamous cell carcinoma. Patients with biopsy proven lesions of OSCC were included in this study, as done by Epstein at el. in their study of 59 patients with 97 suspicious lesions [20]. Mean age in current study was 52.2 ± 13.1 years. Minimum age of patients was 25 and maximum age of patients was 88 years respectively. Mean age of male and female patients was 52.06 ± 13.58 and 52.42 ± 12.68 years respectively and Male patients were predominant as compared to female patients with ratio of male to female is 1:6. This is comparable to study of McMahona et al published in 2012, who reported similar gender distribution in patient of OSCC [22]. However the most common site of occurrence in their study was floor of mouth (n=37) where as in our setup buccal mucosa is documented as most common site of involvement in both genders i.e. 38% and 42% in males and females respectively. Moreover, McMahona et al reported a very low incidence of OSCC of buccal mucosa (n=2) compared to our study (n=20).This can be attributed to the intimate contact of buccal mucosa to the predisposing factor of OSCC which is betel, paan and naswar in our region.
In our study, Sensitivity of Lugol’s iodine is 90.9% it means that it had accurately detected 10 margins which were involved by malignant lesions. Specificity of Lugol’s iodine is 92.3% which means that it had accurately detected 36 margins which were not involved by lesion (safe margins). These results are comparable to study of McMahona et al. but higher than the values reported by Epstein et al. who reported 87.5% sensitivity and 84.2% specificity. PPV and NPV reported by them are 92.1 and 76.2 respectively whereas in our study its 76.9% and 97.2% respectively [20,21]. In current study, 7.6% were false positive and 1% were false negative but McMahona et al found 4% false negative results but no false positive results in their study [22].
Problems with studies of Lugol’s iodine was that, in past most studies were conducted on carcinoma of cervix and esophagus. However in last decade, studies have been done on carcinoma of oral cavity but these are mostly for correlation of recurrence of primary lesion with iodine staining rather than for surgical margins. Although current study, study of McMahona et al and few other authors have emphasized on usefulness of iodine solution for taking safe margins but more extensive randomized controlled trial with good bias control (histopathologist who examines the slides) is required on the subject. Hence, in view of the limited published data, further research is needed to reinforce these results in other populations using different study designs, before practitioners can be confident that sensitivity and specificity is improved significantly over conventional surgical techniques.
Conclusion
Lugol’s iodine is one of the diagnostic adjuncts that can be used for detection of safe margins of oral squamous cell carcinoma, during surgical excision. As it is a simple, cost effective, noninvasive, technique with good diagnostic accuracy, it may be performed as a routine on-table diagnostic adjunct to enhance the prognostic value of surgical procedure. But further research is needed to establish the use of Lugol’s iodine as a Standard Operative Procedure.
28388
References
- Parkin DM, Pisani P, Ferlay J (1999) Estimates of worldwide incidence of 25 major cancers in 1990. Int J Cancer 80: 827-841.
- Siddiqui IA, Farooq MU, Siddiqui RA, Rafi SM (2006) Role of toluidine blue in early detection of oral cancer. Pak J Med Sci 22: 184-187.
- Kalmar JR (2006) Advances in the detection and diagnosis of oral precancerous and cancerous lesions. J Oral Maxillofacial Surg Clin N Am 18: 465-482.
- Epstein JB, Silverman S, Epstein JD, Lonky SA, Bride MA (2008) Analysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blue. Oral Oncol 44: 534-544.
- Kuriakose MA, Sharan R (2006) Oral cancer prevention. J Oral Maxillofacial Surg Clin N Am 18: 493-511.
- Neville BW, Damm DD, Allen CM (2009) Oral and Maxillofacial Pathology (3rd Edn). Philadelphia: W B Saunders.
- Chol SY, Kahyo H (1991) Effect of cigarette smoking and alcohol consumption in the etiology of cancer of the oral cavity, pharynx and larynx. Int J Epidemiol 20: 878-885.
- Franchesci S, Levi F, Lucchini F, Negri E, P, et al. (1994) Trends in cancer mortality in young adults in Europe, 1955-1989. Eur J Cancer 30A: 2096-2118.
- Das C, Schantz SP, Shillitoe EJ (1993) Antibody to a mutagenic peptide of herpes simplex virus in young adult patients with cancer of the head and neck. Oral Surg Oral Med Oral Pathol 75: 610-614.
- Siddiquee BH, Alauddin M, Choudhury AA, Akhtar N (2006) Head and neck squamous cell carcinoma (HNSCC) 5 year study at BSMMU. Bangladesh Med Res Counc Bull 32: 43-48.
- Hart AK, Karakla OW, Pitman KT, Adams JF (1999) Oral and oro pharyngeal cell carcinoma in young adults: a report on 13 cases and review of the literature. Otolaryngol Head Neck Surg 120: 828-833.
- Eliezri YD, Israel HA, Pochal WF (1989) Treatment of an oral erythroplastic squamous cell carcinoma with Moh's micrographic surgery. Oral Surg Oral Med Oral Pathol 67: 249-254.
- Epstain JB, Scully C, Spinelli J (1992) Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med 21: 160–163.
- Kouzu T, Takahashi H, Onozawa K, Kuga K, Miyajima T, et al. (1975) Experiences on endoscopical esophageal staining. Prog Digest Endosc 6: 41–44.
- Kurita H, Kurashina K (1996) Vital staining with iodine solution in delineating the border of oral dysplastic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81: 275-280.
- Yajima Y, Noma H, Furuya Y, Nomura T, Yamauchi T, et al. (2004) Quantification of telomerase activity of regions unstained with iodine solution that surround oral squamous cell carcinoma. Oral Oncol 40: 314-320.
- Yokoo K, Noma H, Inoue T, Hashimoto S, Shimono M (2004) Cell proliferation and tumour suppressor gene expression in iodine ined area surrounding oral squamous cell carcinoma. Int J Oral unstaMaxillofac Surg 33: 75-83.
- Umeda M, Shigeta T, Takahashi H, Minamikawa T, Komatsubara A, et al. (2011) Clinical evaluation of Lugol’s iodine staining in the treatment of stage I–II squamous cell carcinoma of the tongue. Int J Oral Maxillofac Surg 40: 593-596.
- Yajima Y, Noma H, Furuya Y, Nomura T, Yamauchi T, et al. (2004) Quantification of telomerase activity of regions unstained with iodine solution that surround oral squamous cell carcinoma. Oral Oncol 40: 314-320.
- Shiozaki H, Tahara H, Kobayashi K, Yano H, Tamura S, et al. (1990) Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer 66: 2068-2071
- McMahona J, Devine JC, McCaulb JA, McLellan DR, Farrowa A (2010) Use of Lugol’s iodine in the resection of oral and oropharyngeal squamous cell carcinoma. B J Oral Maxfac Surg 48: 84-87






