Research Article - (2022) Volume 16, Issue 3
Differential Inhibitory Effects of Cytotoxic Agents on Lymphoma Cell Proliferation and Tumor Growth
Qiping Zheng*,
Lichun Sun,
Jinlei Ye,
Mengli Yang,
Shilei Wang,
Ying Chen,
Xiaojing Zhang,
Chen Chen and
Songhai Shen
Shenzhen Academy of Peptide Targeting Technology at Pingshan and Shenzhen Tyercan Bio-Pharm Co, Ltd, Guangdong, 518118, China
Department of Haematological Laboratory Science, Jiangsu Key Laboratory of Medical Science and Laboratory Medicine, School of Medicine, Jiangsu Univer, China
Department of Medicine, School of Medicine, Tulane University Health Sciences Center, New Orleans, USA
*Correspondence:
Qiping Zheng, Shenzhen Academy of Peptide Targeting Technology at Pingshan and Shenzhen Tyercan Bio-Pharm Co, Ltd, Guangdong, 518118,
China,
Email: ,
Received: 04-Apr-2022, Manuscript No. Iphsj-22-12703;
Editor assigned: 06-Apr-2022, Pre QC No. Iphsj-22-12703 (PQ);
Reviewed: 20-Apr-2022, QC No. QC No. Iphsj-22-12703;
Revised: 25-Apr-2022, Manuscript No. Iphsj-22-12703(R);
Published:
03-May-2022, DOI: 10.36648/1791-809X.16.4.936
Abstract
In the last few decades, many biomolecules obtained from nature exhibit great potential in antitumor application, some of them have made great contributions to clinical treatment. Ansamitocin P3 and paclitaxel are tubulin inhibitors while camptothecin is a Topo I inhibitor. These molecules are known to induce apoptosis of cancer cells and either has been used or show great potential in application of tumor targeted therapy. In this study, we investigated the roles and mechanisms of ansamitocin P3, paclitaxel and camptothecin in inducing apoptosis of human histolytic lymphoma U937 cells. CCK8 assay was performed on U937 cells and the results showed that ansamitocin P3 exhibits great antiproliferative activities with a half-maximal inhibitory concentration (IC 50) at 0.18 ± 0.04 nM, while the IC50 of camptothecin and paclitaxel was 25.09 ± 2.64 and 6.06 ± 1.24 nM respectively. Results of apoptosis analysis by flow cytometry indicated that both early and late apoptotic cells increased significantly after treated with above three biomolecules. Cell cycle analysis results suggested that ansamitocin P3 and paclitaxel arrested cells in the G2/M Phase, while camptothecin arrested cells in G2/M phase at low concentration but S phase at high concentration. Mechanistically, we have performed expression analysis using qRT-PCR and found that ansamitocin P3 and paclitaxel may induce apoptosis of cells by down regulating expression of PCNA and BCL-2, while cells treated with camptothecin showed upregulated P21 but down regulated BCL-2 expression. Moreover, to investigate the in vivo anti-tumor activity of ansamitocin P3, we have shown data in a xenograft tumor model that ansamitocin P3 greatly inhibited tumor growth with little side effect. Taken together, our results suggest the strongest antitumor activity of ansamitocin P3 on lymphoma U937 cells. Ansamitocin P3 has great potential in tumor targeted therapy.
Keywords
Lymphoma cells; Ansamitocin P3; Paclitaxel; Camptothecin; Apoptosis;
Tumor growth
Introduction
Lymphoma is a malignant tumor originating from the lymphatic
hematopoietic system. Although it is more likely to occur in lymph
nodes, it can invade almost any tissues and organs in the body
due to flow of the lymphatic system, making the treatment effect
unsatisfactory, especially in non-Hodgkin lymphoma [1, 2]. In
recent years, there has been extensive research on tumor targeted
therapy. By targeting tumor cells, drugs are able to attack tumors
accurately, achieve ideal efficacy with little damage to normal
cells [3]. As shown in the research of antibody-drug conjugate
(ADC), as a key aspect of tumor targeted drugs, small molecules
should have a suitable half maximal inhibitory concentration
(IC50) value [4, 5], so the selection of small molecules is critical
and also challenging for drug development.
In the long history of antitumor research, a lot of active ingredients
extracted from plants and microorganisms have exhibited strong
antitumor activities [6, 7]. The microtubules (MTs), which
play an essential role in mitosis, have been commonly used as
targets for cancer therapy [8-10]. Ansamitocin P3 is an analogue
of maytansine discovered in Nocardia species [11]. It has been known to disrupt the assembly of microtubules by binding to
tubulin at the same site with vinblastine [12]. After treatment
with ansamitocin P3, cells were arrested in mitotic phase and
apoptosis was induced. Although ansamitocin P3 shows great
cytotoxicity, it has not been approved for clinical purpose due
to its severe side effects and narrow therapeutic spectrum [13].
Paclitaxel, another molecule isolated from Pacific yew tree Taxus
brevifolia, is one of the most widely used drug against many
kinds of cancers [14-16]. As a microtubule-stabilizing drug, it can
make chromosomes non-segregation, cause mitotic arrest and
induce apoptosis of cells [17, 18]. Since first authorized in the
treatment of ovarian and breast cancer by FDA, paclitaxel has
been approved for treatment of many kinds of Tumors [19, 20].
However, the high toxicity and low solubility of paclitaxel make
it imperfect as a drug candidate; so, nanotechnology was then
established to improve the solubility and safety of paclitaxel [21, 22].
Camptothecin (CPT), which is a pentacyclic alkaloid extracted
from Camptotheca acuminata, also exhibits good antitumor
effect and has been used in tumor therapy for decades [23-25]. It
is a Topo inhibitor which can disrupt the replication of DNA and
lead to apoptosis [26]. Irinotecan, a camptothecin-derived drug,
has been approved in the treatment of several advanced cancers
since 1994 [27, 28]. However, the application of camptothecin
was also limited due to its heavy toxicity and poor solubility [24, 29].
With the development of biomolecule conjugation technology
and tumor targeting therapy, a lot of biomolecules show great
potential to be developed as targeted antitumor drugs [30]. In
this study, we compared the antitumor effect of ansamitocin
P3, paclitaxel and camptothecin on lymphoma U937 cells,
investigated the potential mechanisms relating to apoptosis, and
explored the inhibition effect of ansamitocin P3 in the xenograft
tumor model, so as to identify appropriate therapeutic molecules
suitable for targeting lymphoma or other cancer cells.
Materials and Methods
Cell lines and cell culture
Human histolytic lymphoma U937 cells were purchased from
Shanghai Cell Bank of the Chinese Academy of Sciences and
cultured in RPMI-1640 medium supplied with 10% fetal bovine
serum and 1% penicillin-streptomycin, and all of these reagents
were supplied by Gibco (Thermo Fisher Scientific). Cells were
incubated at 37℃ and maintained in the condition of 5% CO2.
Chemicals
Ansamitocin P3 (purity > 98%), paclitaxel (purity 99.97%) and
camptothecin (purity 99.69%) were purchased from MCE (Med
Chem Express, NJ, USA). PBS, RNase A was obtained from
Solarbio (Solarbio Life Science, Beijing, China). Matrigel basement
membrane matrix was supplied by BD Biosciences.
Cell Counting Kit-8 assay
The survival rate of cells after treated with biomolecules was
assessed with Cell Counting Kit-8 colorimetric assay (Dojindo, Kumamoto, Japan). First, U937 cells were seeded into 96-well
plates at 8 × 103 cells per well, then incubated with different
concentrations of ansamitocin P3, paclitaxel and camptothecin
(from 10-13 M to 10-6 M) for 72 hours. After adding 10 μl of Cell
Counting Kit-8 solution into each of the wells for two hours, the
OD values of the cells were recorded at 450 nm with the micro
plate reader (Biotek, USA).
Cell cycle analysis
Cells were seeded into six-well plates for 2 × 105 cells per well,
and treated with different concentrations of drugs for 24 hours.
After collected and washed with PBS, cells were fixed with 70%
ethanol at -20℃ for several hours to days, then centrifuged at
4000 rpm for 2 minutes. Cell pellets were then resuspended in
500 μl PBS containing 0.25% Triton-X 100 and incubated on ice
for 15 minutes. Then repeated centrifugation, and added 500 μl
PBS with 10 μg/ml RNase A and 20 μg/ml PI to resuspend cells
and incubated in the dark place at room temperature for 30
minutes. Finally, cell cycle was analysed using the flow cytometer
(Beckman, USA). Data were analyzed with ModFit LT 5.0 (Trial
version).
Apoptosis analysis
For apoptosis analysis, cells were seeded into six-well plates at 2 ×
105 cells per well, and treated with ansamitocin P3, camptothecin
and paclitaxel at different concentrations for 48 hours. After
collected and washed with PBS, cells were suspended in 100 μl
binding buffer and incubated with 5 μl FITC-AV and 5 μl PI (binding
buffer, FITC-AV, PI were supplied within the detection kit) for 15
minutes. Finally, after 400 μl binding buffer was added, samples
were detected on the flow cytometer. Data were analyzed with
FlowJo V10 (Trial version).
mRNA expression analysis
Total RNA was extracted with Total RNA Kit (OMEGA, USA) and
measured with Nano Drop micro volume spectrophotometer
(Thermo Fisher Scientific, Waltham, MA, USA). Then reverse
transcription was conducted with 1 μg RNA using the cDNA
Synthesis kit (Genecopoeia, USA). Quantitative real-time PCR
was performed by CFX Connect Real-Time System (Bio-Rad, USA)
with 2 μl cDNA template and 2 μl primer according to the SYBR
Green qPCR Mix 2.0 Kit (Genecopoeia, USA). GAPDH primers
were purchased from Genecopoeia and other primers were
supplied by Sangon Biotech. Primer sequences are each sample
was tested triplicate in three tubes. The reaction procedure was
set as follows: initial denaturation at 95℃ for 30 seconds, then
repeat 40 cycles at 95℃ for 10 seconds to denature and 65℃
for 30 seconds as annealing/extension, fluorescent intensity was
detected at the end of every cycle. The data was analyzed with
the comparative threshold cycle (2−ΔΔCt) and taking GAPDH as
control (Table 1).
| |
Primer sequence |
| PCNA |
Forward |
TTAGCTCCAGCGGTGTAAAC |
| Reverse |
TTTGGACATACTGGTGAGGTTC |
| P63 |
Forward |
CGTGTTATTGATGCTGTGCG |
| Reverse |
GAAGTCATTCCACTCATCTCGG |
| P21 |
Forward |
TGTCACTGTCTTGTACCCTTG |
| Reverse |
GCGTTTGGAGTGGTAGAAATC |
| BCL-2 |
Forward |
TTGTGGCCTTCTTTGAGTTCGGTG |
| Reverse |
GGTGCCGGTTCAGGTACTCAGTCA |
Table 1. Primer sequences of PCNA, P63, P21 and BCL-2.
Xenograft mouse tumor model
Five weeks-old female SCID mice were purchased from Beijing
Vital River Laboratory Animal Technology Co., Ltd. in China and
reared in SPF condition. Before the experiment, mice were raised
for one week to adapt to the new environment. U937 cells (1 × 106) were resuspended in PBS with Matrigel (ratio 1:1, total
volume 100 μl) and injected subcutaneously into the right flank
of each mouse. Tumor volumes were calculated as 0.5 × Length
× Width2, When the tumor size reached 70-120 mm3, mice were
divided into the control group and the treatment/experimental
(ansamitocin P3) group. Mice in the experimental group received
ansamitocin P3 (0.4 mg/kg, 100 μl) by tail vein injection while mice
in the control group were given 100 μl normal saline containing
10% alcohol. Mice were treated every two weeks for four weeks.
Tumor measurements and bodyweights were recorded twice a
week. The protocol was approved by Shanghai Medicilon IACUC
(approval number: TEK2103P).
Statistical analysis
IC50 values were acquired and analyzed by Graph Pad 8.3.0. All
experiments were done at least three times and mean ± SD was
calculated. Statistical significance was determined by t-test, p <
0.05 was recognized as significant.
Results
Differential cytotoxic activity of ansamitocin P3,
camptothecin, and paclitaxel on U937 cells
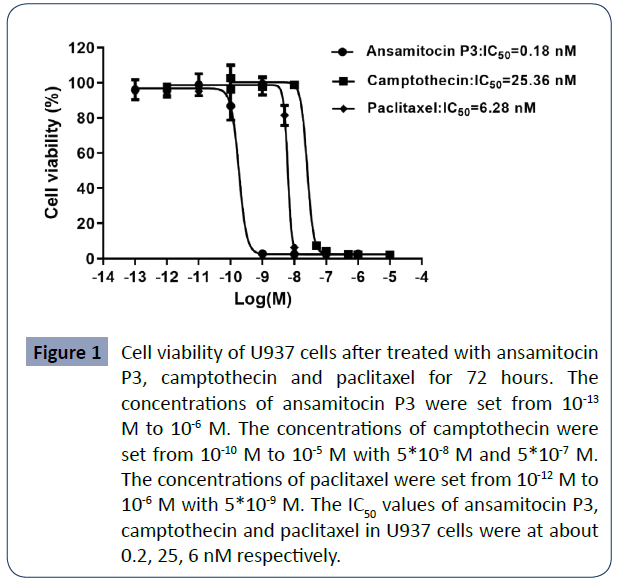
To compare their cytotoxicity, we measured the cell viability of
U937 cells using the CCK8 assay kit after cells were treated with
above three compounds for 72 hours. The results indicated that
treatment with these drugs can significantly and differentially
inhibit U937 cell proliferation. ansamitocin P3, camptothecin
and paclitaxel all inhibited the cell viability of U937 in a dosedependent
manner. When increased the concentration of the
compounds, the viability of U937 cells rapidly decreased to
extremely low level. With the concentration of ansamitocin
P3, camptothecin, and paclitaxel at 1 nM, 50 nM and 10 nM,
the cell viabilities of U937 cells reduced to about 2%, 7%, and
5% respectively. In all groups, the cell viability reduced to
about 2% when the compounds were at highest concentration.
Half-maximal proliferation inhibitory concentrations (IC50) of
ansamitocin P3, camptothecin, and paclitaxel in U937 cells
were at about 0.18 ± 0.04, 25.09 ± 2.64 and 6.06 ± 1.24 nM
respectively, suggesting that these compounds have strong
antitumor activities with ansamitocin P3 showed the strongest
antitumor effect (Figure 1).
Figure 1 Cell viability of U937 cells after treated with ansamitocin
P3, camptothecin and paclitaxel for 72 hours. The
concentrations of ansamitocin P3 were set from 10-13 M to 10-6 M. The concentrations of camptothecin were
set from 10-10 M to 10-5 M with 5*10-8 M and 5*10-7 M.
The concentrations of paclitaxel were set from 10-12 M to
10-6 M with 5*10-9 M. The IC50 values of ansamitocin P3,
camptothecin and paclitaxel in U937 cells were at about
0.2, 25, 6 nM respectively.
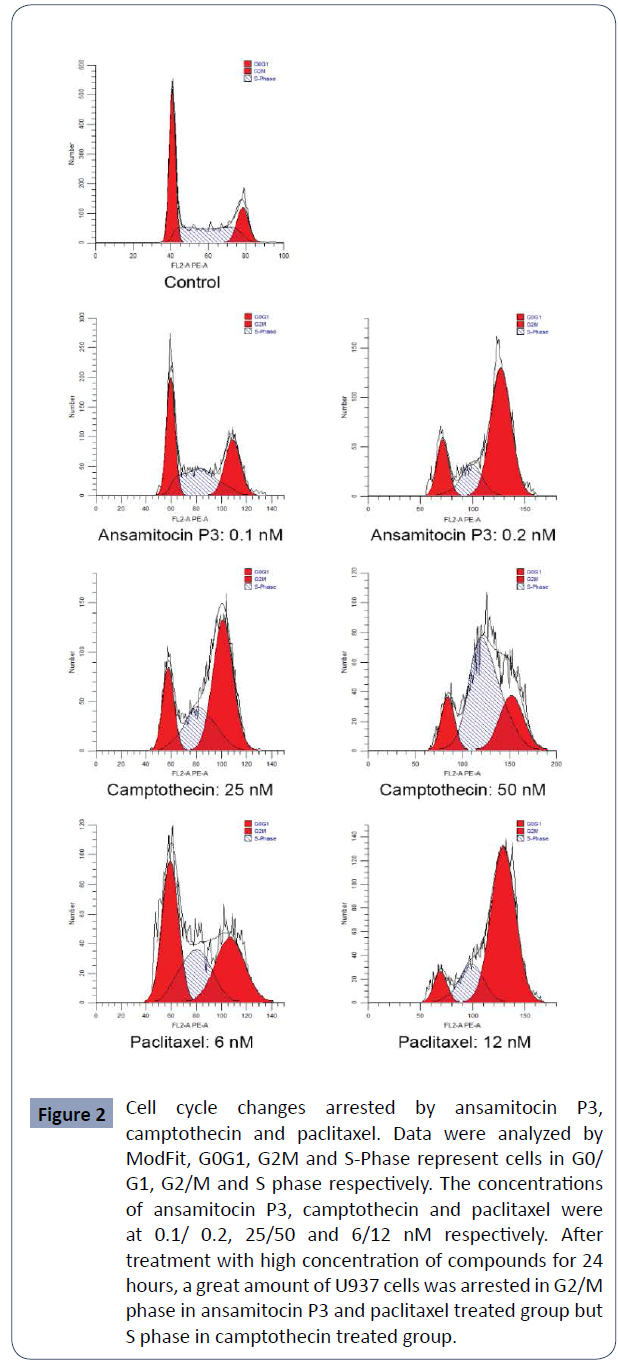
Cells were arrested in G2/M or S phase after
treatment with above compounds
In order to evaluate their effect on cell cycle, cells were treated
with the above three compounds in three concentrations near
IC50 for 24 hours and analyzed by flow cytometry. it is obvious that
after treated with ansamitocin P3 and paclitaxel, large amounts of
cells accumulated in G2/M phase. However, in the camptothecin
group, it showed different effect in two concentrations, most
cells were arrested in G2/M phase at 25 nM but in S phase at 50
nM (Figure 2).
Figure 2 Cell cycle changes arrested by ansamitocin P3,
camptothecin and paclitaxel. Data were analyzed by
ModFit, G0G1, G2M and S-Phase represent cells in G0/
G1, G2/M and S phase respectively. The concentrations
of ansamitocin P3, camptothecin and paclitaxel were
at 0.1/ 0.2, 25/50 and 6/12 nM respectively. After
treatment with high concentration of compounds for 24
hours, a great amount of U937 cells was arrested in G2/M
phase in ansamitocin P3 and paclitaxel treated group but
S phase in camptothecin treated group.
Percentages of cells in different cell cycle phase When the
concentration of ansamitocin P3 and paclitaxel increased
from 0.1 nM to 0.4 nM and from 3 nM to12 nM respectively,
percentage of cells in G2/M phase increased from about 30% to
more than 60% while cells in G0/G1 and S phase were reduced.
For camptothecin ranged from 12.5 nM to 25 nM, percentage
of cells in G0/G1 phase reduced from about 30% to 15%, and
percentage of cells in G2/M phase increased from about 45% to
60%. However, when the concentration of camptothecin reached
50 nM, percentage of cells in S phase increased to about 60%
while cells in G2/M phase reduced to about 30% (Table 2).
| |
Concentration (nM) |
G0/G1 phase (%) |
S phase (%) |
G2/M phase (%) |
| Control |
|
44.82 ± 2.16 |
36.67 ± 2.33 |
18.52 ± 0.17 |
| Ansamitocin P3 |
0.1 |
36.10 ± 1.53 |
34.06 ± 1.21 |
29.84 ± 0.33 |
| 0.2 |
16.03 ± 0.66 |
15.58 ± 2.21 |
68.40 ± 2.86 |
| 0.4 |
6.02 ± 2.91 |
18.62 ± 2.62 |
75.37 ± 5.53 |
| Camptothecin |
12.5 |
30.16 ± 3.34 |
35.60 ± 1.43 |
34.24 ± 1.91 |
| 25 |
16.95 ± 3.22 |
27.83 ± 0.48 |
55.23 ± 2.74 |
| 50 |
10.65 ± 3.42 |
70.82 ± 11.24 |
18.54 ± 7.81 |
| Paclitaxel |
3 |
40.56 ± 2.90 |
33.32 ± 8.81 |
26.12 ± 5.92 |
| 6 |
32.70 ± 9.02 |
33.70 ± 7.09 |
33.61 ± 1.93 |
| 12 |
5.59 ± 1.80 |
27.01 ± 12.30 |
66.41 ± 10.50 |
Table 2. Percentages of cells in different phases of cell cycle after
treatment with compounds for 24 hours.
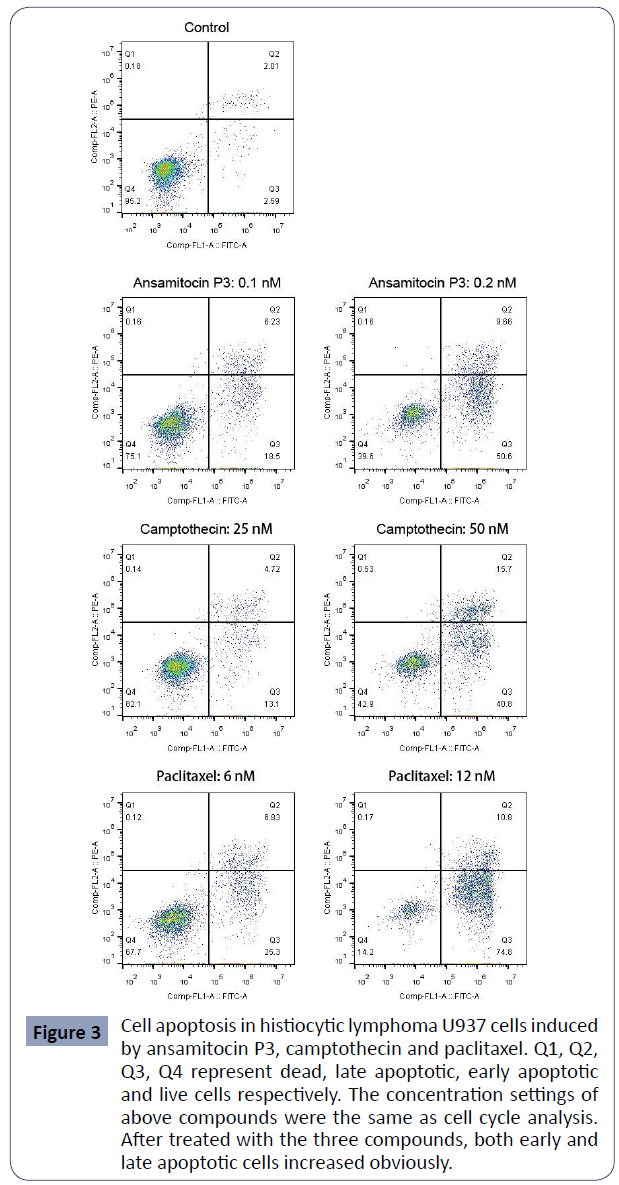
U937 cell apoptosis induced by ansamitocin P3,
camptothecin and paclitaxel
After treated with the above three compounds for 48 hours,
percentage of apoptotic U937 cells was detected by flow
cytometry. As shown in (Figure 3), the percentages of early (Q3)
and late (Q2) apoptotic cells both increased obviously in the
treated group, indicating the great pro-apoptotic effect of these
compounds on U937 cells.
Figure 3 Cell apoptosis in histiocytic lymphoma U937 cells induced
by ansamitocin P3, camptothecin and paclitaxel. Q1, Q2,
Q3, Q4 represent dead, late apoptotic, early apoptotic
and live cells respectively. The concentration settings of
above compounds were the same as cell cycle analysis.
After treated with the three compounds, both early and
late apoptotic cells increased obviously.
The percentages of apoptotic cells of U937 after treated with
different concentrations of the three compounds were. In
the control group, the total percentage of the apoptotic cells was about 4.43 ± 0.25%. In ansamitocin P3 treated group with
the concentration of drug ranged from 0.1 nM to 0.4 nM, the
percentage of apoptotic cells increased from 24.83 ± 0.13% to
83.31 ± 0.47%. Meanwhile, for camptothecin and paclitaxel
ranged from 12.5 nM to 50 nM, and from 3 nM to 12 nM, the
percentage of apoptotic cells increased from 5.54 ± 1.78% to 54.65
± 2.62% and from 7.17 ± 0.20% to 82.23 ± 4.77% respectively.
These results demonstrated that all three compounds were able to induce cell apoptosis and that increase of apoptotic cells was
positively correlated with drug concentration (Table 3).
| |
Concentration |
Early apoptotic cells (%) |
Late apoptotic cells (%) |
Total Apoptotic cells (%) |
| (nM) |
| Control |
|
2.55 ± 0.06 |
1.88 ± 0.19 |
4.43 ± 0.25 |
| Ansamitocin P3 |
0.1 |
18.55 ± 0.07 |
6.28 ± 0.06 |
24.83 ± 0.13 |
| 0.2 |
54.90 ± 6.08 |
10.23 ± 0.81 |
65.13 ± 6.89 |
| 0.4 |
74.10 ± 0.85 |
9.21 ± 0.38 |
83.31 ± 0.47 |
| Camptothecin |
12.5 |
3.43 ± 1.13 |
2.11 ± 0.65 |
5.54 ± 1.78 |
| 25 |
12.10 ± 1.41 |
5.04 ± 0.45 |
17.14 ± 0.97 |
| 50 |
37.80 ± 4.24 |
16.85 ± 1.63 |
54.65 ± 2.62 |
| Paclitaxel |
3 |
4.80 ± 0.64 |
2.38 ± 0.45 |
7.17 ± 0.20 |
| 6 |
27.35 ± 2.90 |
7.51 ± 0.96 |
34.86 ± 3.85 |
| 12 |
73.10 ± 2.40 |
9.13 ± 2.37 |
82.23 ± 4.77 |
Table 3. Percentages of apoptotic cells in U937 after treated with the
compounds.
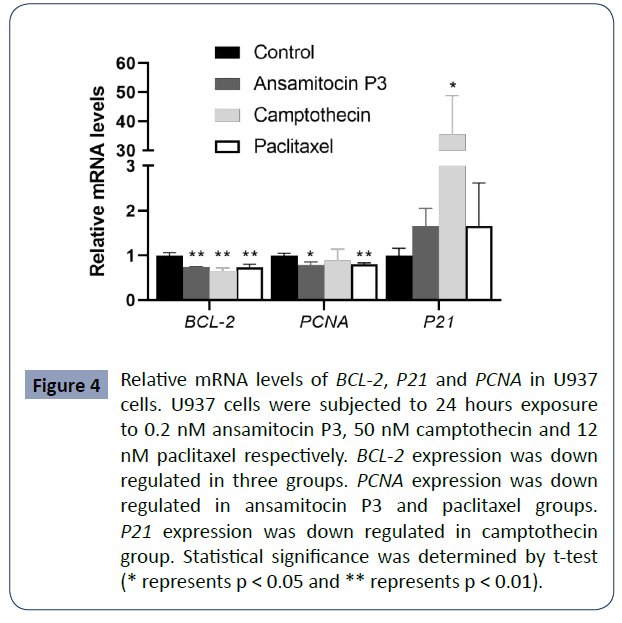
Altered expression of BCL-2, P21 and PCNA in
cells treated with ansamitocin P3, camptothecin
and paclitaxel
To study the putative mechanism of cell apoptosis induced by the
three compounds, U937 cells were treated for 24 hours with 0.2
nM ansamitocin P3, 50 nM camptothecin, and 12 nM paclitaxel
respectively. Then, the mRNA levels of BCL-2, P21, P63 and PCNA
(proliferating cell nuclear antigen) were analyzed by qPCR. As
shown in (Figure 4), BCL-2 was down regulated in all the cells
treated with either of the three compounds, which is consistent
with the results of high percentages of apoptotic cells. Besides,
decreased expression of PCNA mRNA was detected in ansamitocin
P3 and paclitaxel groups (P < 0.05), but no significant difference
was observed in camptothecin treated group compared with the
control group.
Figure 4 Relative mRNA levels of BCL-2, P21 and PCNA in U937
cells. U937 cells were subjected to 24 hours exposure
to 0.2 nM ansamitocin P3, 50 nM camptothecin and 12
nM paclitaxel respectively. BCL-2 expression was down
regulated in three groups. PCNA expression was down
regulated in ansamitocin P3 and paclitaxel groups.
P21 expression was down regulated in camptothecin
group. Statistical significance was determined by t-test
(* represents p < 0.05 and ** represents p < 0.01).
To understand the effect of cell cycle arrest, we examined
the expression of P21. After treated with camptothecin, P21
expression was obviously upregulated. However, for ansamitocin
P3 and paclitaxel group, P21 mRNA was slightly upregulated
without significant difference compared with that of the control
groups.
In addition, we examined P63 expression in the cells, and it was
barely detectable in all the cells with or without treatment with
the three compounds (data not shown).
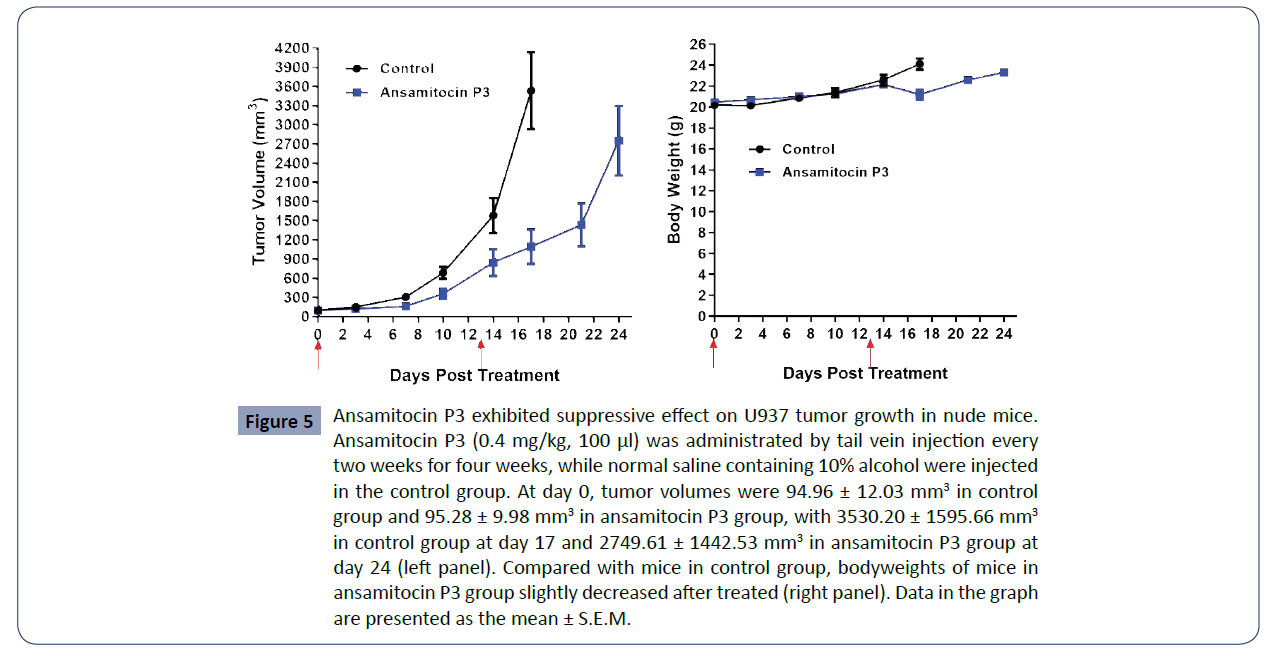
Ansamitocin P3 inhibited tumor growth of U937
in vivo
To further investigate the antitumor efficacy of ansamitocin
P3, U937 xenograft mouse tumor models were established.
When the tumors reached size at 70-120 mm3, the mice were
separated into two groups (n=8 each): the control (PBS) group
and ansamitocin P3 treated group, which was administered by
tail vein injection at the concentration of 0.4 mg/kg for 100 μl.
and (Table 4), in the control group, tumor volumes increased
from 94.96 ± 12.03 mm3 (Day 0) to 3530.20 ± 1595.66 mm3
(Day 17). According to the requirement of the ethics of animal
experiments, the mice in the control group were sacrificed at
day 17. In ansamitocin P3 treated group, tumor volumes of mice
rose from 95.28 ± 9.98 mm3 (Day 0) to 2749.61 ± 1442.53 mm3
(Day 24). When giving ansamitocin P3 at a low dose of 0.4 mg/
kg every two weeks, the relative tumor proliferation rate (T/C, %)
was 29.98 at day 17, while bodyweights slightly decrease after
treated (Figure 5 and Table 5). The detailed information of each
mouse analyzed was shown in (Table 6). The results support that
ansamitocin P3 inhibited tumor growth of the U937 xenograft
tumor model.
| Days Post Treatment |
0 |
3 |
7 |
10 |
14 |
17 |
21 |
24 |
| Control |
Tumor Volume (mm3) |
Mean |
94.96 |
145.94 |
302.14 |
682.34 |
1579.61 |
3530.2 |
/ |
/ |
| group |
SD |
12.03 |
45.23 |
147.91 |
252.95 |
725.9 |
1595.66 |
/ |
/ |
| |
SEM |
4.55 |
17.09 |
55.9 |
95.61 |
274.36 |
603.1 |
/ |
/ |
| |
Relative Tumor Volume |
Mean |
1 |
1.55 |
3.18 |
7.16 |
16.62 |
36.92 |
/ |
/ |
| |
SD |
0 |
0.5 |
1.62 |
2.57 |
7.89 |
16.4 |
/ |
/ |
| |
SEM |
0 |
0.19 |
0.61 |
0.97 |
2.98 |
6.2 |
/ |
/ |
| Ansamitocin |
Tumor Volume (mm3) |
Mean |
95.28 |
117.1 |
156.71 |
353.92 |
843.29 |
1088.91 |
1431.42 |
2749.61 |
| P3 group |
SD |
9.98 |
13.6 |
38.91 |
199.16 |
556.15 |
710.3 |
893.1 |
1442.53 |
| |
SEM |
3.77 |
5.14 |
14.71 |
75.28 |
210.2 |
268.47 |
337.56 |
545.23 |
| |
Relative Tumor Volume |
Mean |
1 |
1.23 |
1.64 |
3.64 |
8.55 |
11.07 |
14.62 |
28.28 |
| |
SD |
0 |
0.14 |
0.33 |
1.88 |
5.21 |
6.67 |
8.56 |
14.16 |
| |
SEM |
0 |
0.05 |
0.13 |
0.71 |
1.97 |
2.52 |
3.23 |
5.35 |
| |
% T/C |
100 |
79.35 |
51.57 |
50.84 |
51.44 |
29.98 |
/ |
/ |
Table 4. Comparation of tumor volumes between control group and treated group.
| Days Post Treatment |
|
0 |
3 |
7 |
10 |
14 |
17 |
21 |
24 |
| Control |
Body Weight (g) |
Mean |
20.19 |
20.14 |
20.87 |
21.39 |
22.59 |
24.11 |
/ |
/ |
| group |
SD |
0.89 |
0.87 |
0.87 |
1.14 |
1.29 |
1.46 |
/ |
/ |
| |
SEM |
0.34 |
0.33 |
0.33 |
0.43 |
0.49 |
0.55 |
/ |
/ |
| |
Change (g) |
0 |
-0.05 |
0.68 |
1.2 |
2.4 |
3.92 |
/ |
/ |
| Ansamitocin |
Body Weight (g) |
Mean |
20.47 |
20.68 |
21 |
21.25 |
22.15 |
21.19 |
22.58 |
23.3 |
| P3 group |
SD |
0.49 |
0.72 |
0.83 |
0.91 |
0.66 |
1.26 |
0.76 |
0.89 |
| |
SEM |
0.19 |
0.27 |
0.32 |
0.35 |
0.25 |
0.48 |
0.29 |
0.34 |
| |
Change(g) |
0 |
0.21 |
0.53 |
0.78 |
1.68 |
0.72 |
2.11 |
2.83 |
Table 5. Body weights and changes in control group and ansamitocin P3 group.
| |
Animal # |
|
|
Days Post Treatment |
| |
|
0 |
3 |
7 |
10 |
14 |
17 |
21 |
24 |
| Control Group |
1 |
Tumor Size |
Length (mm) |
6.36 |
7.05 |
10.16 |
13.88 |
16.92 |
21.95 |
/ |
/ |
| Width (mm) |
5.98 |
6.45 |
8.9 |
11.58 |
15.38 |
20.45 |
/ |
/ |
| Body Weight (g) |
21.88 |
21.42 |
22.39 |
23.5 |
24.02 |
24.75 |
/ |
/ |
| 2 |
Tumor Size |
Length (mm) |
6.01 |
7.15 |
10.92 |
14.74 |
16.86 |
23.72 |
/ |
/ |
| Width (mm) |
5.84 |
5.91 |
6.7 |
9.74 |
13.18 |
19.41 |
/ |
/ |
| Body Weight (g) |
19.61 |
19.55 |
20.14 |
21.06 |
22.73 |
24.51 |
/ |
/ |
| 3 |
Tumor Size |
Length (mm) |
6.24 |
7.8 |
9.88 |
11.56 |
15.04 |
19.43 |
/ |
/ |
| Width (mm) |
5.58 |
5.68 |
6.6 |
9.91 |
14.48 |
19.34 |
/ |
/ |
| Body Weight (g) |
20.68 |
20.09 |
20.83 |
21.26 |
22.68 |
24.21 |
/ |
/ |
| 4 |
Tumor Size |
Length (mm) |
6.24 |
8.31 |
9.5 |
12.09 |
13.7 |
17.05 |
/ |
/ |
| Width (mm) |
5.55 |
7.11 |
7.4 |
9.53 |
11.48 |
14.3 |
/ |
/ |
| Body Weight (g) |
20.38 |
21.22 |
21.4 |
21.26 |
21.85 |
23.52 |
/ |
/ |
| 5 |
Tumor Size |
Length (mm) |
5.88 |
6.25 |
9.08 |
11.08 |
14.31 |
18.95 |
/ |
/ |
| Width (mm) |
5.59 |
5.71 |
7.41 |
9.68 |
13.89 |
15.97 |
/ |
/ |
| Body Weight (g) |
20.04 |
20.11 |
20.84 |
20.82 |
22.03 |
23.09 |
/ |
/ |
| 6 |
Tumor Size |
Length (mm) |
5.81 |
8.9 |
13.87 |
15.06 |
20.59 |
29.49 |
/ |
/ |
| Width (mm) |
5.52 |
6.82 |
9.23 |
12.15 |
16.9 |
20.16 |
/ |
/ |
| Body Weight (g) |
19.42 |
19.32 |
20.8 |
22.02 |
24.3 |
26.64 |
/ |
/ |
| 7 |
Tumor Size |
Length (mm) |
5.56 |
6.67 |
8.48 |
12.14 |
13.8 |
19.58 |
/ |
/ |
| Width (mm) |
5.19 |
5.62 |
5.99 |
8.11 |
10.71 |
13.81 |
/ |
/ |
| Body Weight (g) |
19.35 |
19.28 |
19.69 |
19.81 |
20.54 |
22.02 |
/ |
/ |
| Ansamitocin P3 Group |
8 |
Tumor Size |
Length (mm) |
6.53 |
6.79 |
6.84 |
8.87 |
12.96 |
13.96 |
15.43 |
20.01 |
| Width (mm) |
5.84 |
6.11 |
6.3 |
8.64 |
12.88 |
13.31 |
14.45 |
18.49 |
| Body Weight (g) |
19.55 |
19.39 |
19.51 |
19.44 |
21.21 |
18.73 |
21.38 |
22.09 |
| 9 |
Tumor Size |
Length (mm) |
6.63 |
6.7 |
10.4 |
15.9 |
19.84 |
19.99 |
20.2 |
24.27 |
| Width (mm) |
5.62 |
6.15 |
6.53 |
9.12 |
13.43 |
15.36 |
16.68 |
18.57 |
| Body Weight (g) |
20.21 |
20.16 |
20.24 |
20.88 |
21.96 |
21.3 |
22.44 |
23.43 |
| 10 |
Tumor Size |
Length (mm) |
6.1 |
6.31 |
7.47 |
12.42 |
16.81 |
16.9 |
18.2 |
22.04 |
| Width (mm) |
5.64 |
5.84 |
7.28 |
9.62 |
11.32 |
12.9 |
15.04 |
18.65 |
| Body Weight (g) |
20.24 |
20.54 |
21.8 |
22.16 |
22.63 |
22.68 |
23.75 |
24.84 |
| 11 |
Tumor Size |
Length (mm) |
6.13 |
6.41 |
6.97 |
7.76 |
9.3 |
10.33 |
9.72 |
13.04 |
| Width (mm) |
5.49 |
5.72 |
6.48 |
6.55 |
8.08 |
9.64 |
8.58 |
10.17 |
| Body Weight (g) |
20.88 |
21.44 |
21.58 |
21.71 |
22.31 |
20.58 |
21.93 |
22.75 |
| 12 |
Tumor Size |
Length (mm) |
6.03 |
6.7 |
7.2 |
11.64 |
16.96 |
17.01 |
17.03 |
21.25 |
| Width (mm) |
5.5 |
6.44 |
6.47 |
7.01 |
8.55 |
9.57 |
11.73 |
16.9 |
| Body Weight (g) |
20.82 |
21.36 |
21.58 |
21.62 |
22.91 |
21.45 |
22.8 |
23.62 |
| 13 |
Tumor Size |
Length (mm) |
5.95 |
6.4 |
7.57 |
10.88 |
15.5 |
15.89 |
15.93 |
20.63 |
| Width (mm) |
5.44 |
5.88 |
5.91 |
7.87 |
10.82 |
12.18 |
14.47 |
18.05 |
| Body Weight (g) |
20.83 |
21.02 |
21.26 |
21.87 |
22.66 |
21.95 |
22.88 |
23.69 |
| 14 |
Tumor Size |
Length (mm) |
5.9 |
6.99 |
7.06 |
7.45 |
7.55 |
8.1 |
9.62 |
12.47 |
| Width (mm) |
5.28 |
5.46 |
5.64 |
5.7 |
5.89 |
6.74 |
8.45 |
10.89 |
| Body Weight (g) |
20.74 |
20.86 |
21.02 |
21.06 |
21.4 |
21.67 |
22.9 |
22.7 |
Table 6. Tumor sizes and body weights of mice in two group.
Figure 5 Ansamitocin P3 exhibited suppressive effect on U937 tumor growth in nude mice.
Ansamitocin P3 (0.4 mg/kg, 100 μl) was administrated by tail vein injection every
two weeks for four weeks, while normal saline containing 10% alcohol were injected
in the control group. At day 0, tumor volumes were 94.96 ± 12.03 mm3 in control
group and 95.28 ± 9.98 mm3 in ansamitocin P3 group, with 3530.20 ± 1595.66 mm3
in control group at day 17 and 2749.61 ± 1442.53 mm3 in ansamitocin P3 group at
day 24 (left panel). Compared with mice in control group, bodyweights of mice in
ansamitocin P3 group slightly decreased after treated (right panel). Data in the graph
are presented as the mean ± S.E.M.
Discussion and Conclusion
Ansamitocin P3, paclitaxel and camptothecin are antitumor
biomolecules with great effects. In this study, we first
compared the cytotoxic effect of ansamitocin P3, paclitaxel and
camptothecin on U937 lymphoma cells in vitro. No more than
10% cells survived after treatment with these three compounds,
and ansamitocin P3 showed the strongest anti-tumor effect with
lowest IC50.
In cell cycle analysis, a lot of cells were arrested in G2/M phase
when cells were treated with increased concentration of
ansamitocin P3 and paclitaxel. As tubulin inhibitors, ansamitocin
P3 and paclitaxel have previously been shown to arrest U937
cells in mitosis [8, 12]. In our study, cells were arrested in G2/M
phase when treated with low concentration of camptothecin
but S phase at high concentration, which may be related to
Topo I activity as previously reported [31]. Low concentration of
camptothecin might cause low levels of DNA damage, and thus,
the cells accumulate at G2 check point [32]. When treated with
high concentration of camptothecin, cells might suffer severe
DNA damage and thus arrested in S phase.
Apoptosis analysis results showed that after treatment with three
compounds for 48 hours, percentages of early and late apoptotic
cells were all increased, which was positively correlated with
drug concentration. High concentration of compounds induced more than half of the cells to undergo apoptosis, suggesting the
great antitumor effect.
To further explore the difference of mechanisms by which the
compounds exhibit their inhibition effects, expressions of several
genes were analyzed by qPCR. BCL-2 was known as a typical antiapoptotic
protein [33-35], and was down regulated in all the
cells treated with either of the three compounds. Proliferating
cell nuclear antigen (PCNA), which was known to function
in DNA replication and repair, is also involved in apoptosis signaling pathway [36]. In previous study, PCNA was reported
to be inhibited by paclitaxel in MG-63 cells [37]. In this study,
PCNA was down regulated after treatment with ansamitocin P3
and paclitaxel, suggesting their role of proliferation inhibition.
However, no difference was observed between camptothecintreated
and the control group. P21 is a known cyclin-dependent
kinase (CDK) inhibitor and apoptosis inhibitor [38, 39]. Compared
to the control groups, P21 was obviously upregulated in
camptothecin group as previously reported [40], while slightly
increased in ansamitocin P3 and paclitaxel treated groups but
without statistically significant difference. These results indicated
that while differ from camptothecin, ansamitocin P3 and
paclitaxel induced cell apoptosis via the same signaling pathway.
In the xenograft animal model, tumor volumes of mice under
treatment of ansamitocin P3 were obviously smaller than the
control group. At day 17, the relative tumor proliferation rate
(T/C, %) was about 30, indicating the great antitumor effect of
ansamitocin P3. Meanwhile, treatment for every two weeks
only resulted a slight decrease for the bodyweight, suggesting
insignificant side effect.
To summarize, we compared the effect of ansamitocin P3,
paclitaxel and camptothecin in inhibition of U937 lymphoma
cells in vitro and confirmed that ansamitocin P3 has the same
mechanism in inducing apoptosis with paclitaxel and with stronger
antitumor activity. The in vivo anti-tumor assay also showed that
ansamitocin P3 inhibited tumor growth obviously with slight
side effect. With the development of biomolecule conjugation
technology and tumor targeting therapy, the biomolecules with
high cytotoxicity are able to selectively kill tumor cells while
reduce the side effect. Our results suggest that ansamitocin P3
shows great potential in the treatment of lymphoma and thus a
suitable candidate biomolecule for tumor targeting therapy.
REFERENCES
- Armitage JO, Gascoyne RD, Lunning MA, Cavalli F (2017) Non-hodgkin lymphoma. Lancet 390: 298-310.
Indexed at, Google Scholar, Crossref
- Freedman AS, LaCasce AS (2016) Non-H odgkin's Lymphoma. Holland‐Frei Can Med 1-19.
Google Scholar
- Wang L, Qin W, Huo Y-J, Li X, Shi Q (2020) Advances in targeted therapy for malignant lymphoma. Signal Transduct Tar 5:1-46.
Indexed at, Google Scholar, Crossref
- Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J (2020) Antibody–drug conjugates: a comprehensive review. Mol Cancer Res 18: 3-19.
Indexed at, Google Scholar, Crossref
- Yaghoubi S, Karimi MH, Lotfinia M, Gharibi T, Mahi‐Birjand M (2020) Potential drugs used in the antibody-drug conjugate (ADC) architecture for cancer therapy. J Cell Physiol 235:31-64.
Indexed at, Google Scholar, Crossref
- Gezici S, Şekeroğlu N (2019) Current perspectives in the application of medicinal plants against cancer: novel therapeutic agents. Anti-Cancer Agent Me19:101-111.
Google Scholar, Crossref
- Chen L, Zhang Q-Y, Jia M, Ming Q-L, Yue W (2016) Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Crit Rev Microbiol 42:454-473.
Indexed at, Google Scholar, Crossref
- Steinmetz MO, Prota AE (2018) Microtubule-targeting agents: strategies to hijack the cytoskeleton. Trends Cell Biol 28:776-792.
Indexed at, Google Scholar, Crossref
- Mukhtar E, Adhami VM, Mukhtar H (2014) Targeting microtubules by natural agents for cancer therapy. Mol Cancer Ther 13:275-284.
Indexed at, Google Scholar, Crossref
- Lemjabbar-Alaoui H, Peto CJ, Yang Y-W, Jablons DM (2020) AMXI-5001 a novel dual parp1/2 and microtubule polymerization inhibitor for the treatment of human cancers. Am J Cancer Res 10: 2649.
Indexed at, Google Scholar
- Higashide E, Asai M, Ootsu K, Tanida S, Kozai Y (1977) Ansamitocin, a group of novel maytansinoid antibiotics with antitumour properties from Nocardia. Nature 270:721-722.
Indexed at, Google Scholar, Crossref
- Venghateri JB, Gupta TK, Verma PJ, Kunwar A, Panda D (2013) Ansamitocin P3 depolymerizes microtubules and induces apoptosis by binding to tubulin at the vinblastine site. PloS one 8: e75182.
Indexed at, Google Scholar, Crossref
- Cassady JM, Chan KK, Floss HG, Leistner E (2004) Recent developments in the maytansinoid antitumor agents. Chem Pharm Bull 52:1-26.
Google Scholar, Crossref
- McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS (1989) Armstrong DK and Donehower RC. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med 111:273-279.
Indexed at, Google Scholar, Crossref
- Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM (2007) Paclitaxel a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf 6:609-621.
Indexed at, Google Scholar, Crossref
- Markman M, Mekhail TM (2002) Paclitaxel in cancer therapy. Expert Opin Pharmaco 3:755-766.
Indexed at, Google Scholar, Crossref
- Wang TH, Wang HS, Soong YK (2000) Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer: Interdisciplinary Inte Am J Canc Society 88:2619-2628.
Indexed at, Google Scholar, Crossref
- Brito DA, Yang Z, Rieder CL (2008) Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J Cell Biol 182:623-629.
Indexed at, Google Scholar, Crossref
- Alves RC, Fernandes RP, Eloy JO, Salgado HRN, Chorilli M (2018) Characteristics, properties and analytical methods of paclitaxel: a review. Crit Rev Anal Chem 48:110-118.
Indexed at, Google Scholar, Crossref
- Alqahtani FY, Aleanizy FS, El Tahir E, Alkahtani HM, AlQuadeib BT (2019) Paclitaxel In: editors. Profiles of drug substances, excipients and related methodology. Elsevier 205-238.
Google Scholar
- Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA (2017) Paclitaxel: what has been done and the challenges remain ahead. Int J Pharmaceut 526:474-495.
Indexed at, Google Scholar, Crossref
- Adrianzen Herrera D, Ashai N, Perez-Soler R, Cheng H (2019) Nanoparticle albumin bound-paclitaxel for treatment of advanced non-small cell lung cancer: an evaluation of the clinical evidence. Expert Opin Pharmaco 20: 95-102.
Indexed at, Google Scholar, Crossref
- Martino E, Della Volpe S, Terribile E, Benetti E, Sakaj M et al. (2017) The long story of camptothecin: From traditional medicine to drugs. Bioorg Med Chem Lett 27:701-707.
Indexed at, Google Scholar, Crossref
- Venditto VJ and Simanek EE (2010) Cancer therapies utilizing the camptothecins: a review of the in vivo literature. Mol Pharmaceut 7:307-349.
Indexed at, Google Scholar
- Li F, Jiang T, Li Q, Ling X (2017) Camptothecin (CPT) and its derivatives are known to target topoisomerase I (Top1) as their mechanism of action: did we miss something in CPT analogue molecular targets for treating human disease such as cancer? Am J Cancer Res 7:2350.
Indexed at, Google Scholar
- Liu LF, Desai SD, LI TK, Mao Y, Sun M (2000) Mechanism of action of camptothecin. Ann Ny Acad Sci 922:1-10.
Google Scholar, Crossref
- Bailly C (2019) Irinotecan 25 years of cancer treatment. Pharmacol Res 148:104398.
Indexed at, Google Scholar, Crossref
- Kciuk M, Marciniak B, Kontek R (2020) Irinotecan Still an important player in cancer chemotherapy: A comprehensive overview. Int J Mol Sci 21:4919.
Indexed at, Google Scholar, Crossref
- Moertel CG, Schutt AJ, Reitemeier R, Hahn R (1972) Phase IT study of camptothecin (NSC-1 00880) in the treatment of advanced gastrointestinal cancer. Cancer Chemother Rep 56:95-101.
Indexed at, Google Scholar, Crossref
- García-Alonso S, Ocaña A, Pandiella A (2020) Trastuzumab emtansine: mechanisms of action and resistance, clinical progress, and beyond. Trends Cancer 6:130-146.
Indexed at, Crossref
- Jones CB, Clements MK, Wasi S, Daoud SS (1997) Sensitivity to camptothecin of human breast carcinoma and normal endothelial cells. Cancer Chemoth Pharm 40:475-483.
Indexed at, Google Scholar, Crossref
- Goldwasser F, Shimizu T, Jackman J, Hoki Y et al. (1996) Correlations between S and G2 arrest and the cytotoxicity of camptothecin in human colon carcinoma cells. Cancer Res 56:4430-4437.
Indexed at, Google Scholar
- Singh R, Letai A, Sarosiek K (2019) Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Bio 20:175-193.
Indexed at, Google Scholar, Crossref
- Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ (2017) From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov 16: 273-284.
Indexed at, Google Scholar, Crossref
- Marquez RT, Xu L (2012) Bcl-2: Beclin complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res 2:214.
Indexed at, Google Scholar
- González-Magaña A, Blanco FJ (2020) Human PCNA structure, function and interactions. Biomolecules 10:570.
Indexed at, Google Scholar, Crossref
- Liu S-Y, Song S-X, Lin L, Liu X (2010) Molecular mechanism of cell apoptosis by paclitaxel and pirarubicin in a human osteosarcoma cell line. Chemo 56:101-107.
Indexed at, Google Scholar, Crossref
- Shamloo B, Usluer S (2019) in cancer research. Cancers 11:1178.
Indexed at, Google Scholar, Crossref
- Karimian A, Ahmadi Y, Yousefi B (2016) Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA repair 42:63-71.
Indexed at, Google Scholar, Crossref
- Chiu C-F, Lin Y-Q, Park JM, Chen Y-C, Hung S-W (2020) The novel camptothecin derivative, CPT211, induces cell cycle arrest and apoptosis in models of human breast cancer. Biomed Pharma 128:110309.
Indexed at, Google Scholar, Crossref
Citation: Zheng Q, Sun L, Ye J, Yang M, Wang S, et al. (2022) Differential Inhibitory Effects of Cytotoxic Agents on Lymphoma Cell Proliferation and Tumor Growth. Health Sci J. Vol. 16 No. 4: 936.