Keywords
Molluscum; Atopic dermatitis; Bird breeding
Introduction
Molluscum contagiosum (MC) are common cutaneous lesions caused by the Molluscum contagiosum virus (MCV); of which birds are known to be a carrier for certain subtypes. Atopic dermatitis (AD) is a chronic eczematous skin disease, predisposing the patient to bacterial and viral infections attributable to abnormalities in the innate and acquired immune system. It has been reported that patients with AD are more susceptible to MC infections, and these MC lesions tend to be more extensive with a protracted clinical course [1,2]. MC has been identified microscopically in birds including fowls, turkeys and pigeons; however further literature is scant. We describe an ornamental bird breeder rearing a wide variety of passerine East Asia bird species, who presented with disseminated MC on a background of Atopic Dermatitis. Further work may be helpful in delineating relative risks for specific viral infections such as MC with respect to a patient’s level of AD control, as well as in evaluation of the possible risk predisposition from an occupational perspective.
Case Presentation
A 57-year-old Chinese male, who worked as an ornamental bird breeder, presented with a 1-year history of disseminated, umbilicated, pearly-white papular eruptions on the face, trunk, buttocks and all 4 limbs (Figure 1). A caseous material was expressible. They were asymptomatic and gradually increasing in size. There was no fever or associated coryzal symptoms. The patient had childhood asthma and poorly-controlled longstanding AD for over 40 years which mainly affected his limbs and trunk. This included recurrent extensive excoriated papules and plaques with lichenification over the flexural areas in affected sites. He was treated with topical steroids (0.0125% betamethasone valerate and 0.1% betamethasone propionate twice daily for the face and trunk respectively) and emollients, having repeatedly refused systemic immunomodulators and phototherapy. He also had ischaemic heart disease, for which he was taking Clopidogrel 75 mg daily.
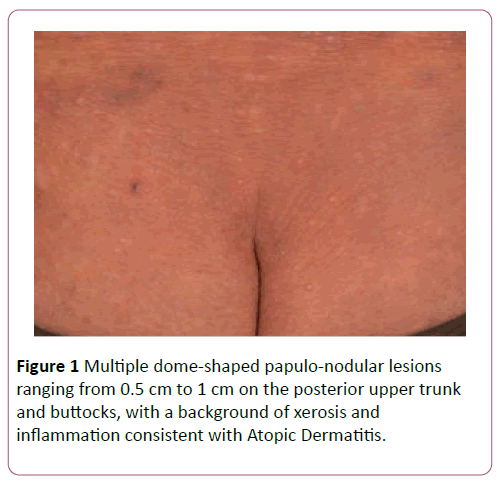
Figure 1: Multiple dome-shaped papulo-nodular lesions ranging from 0.5 cm to 1 cm on the posterior upper trunk and buttocks, with a background of xerosis and inflammation consistent with Atopic Dermatitis.
A diagnosis of MC was confirmed by histopathology of two different sites showing characteristic Henderson-Patterson bodies of molluscum contagiosum (Figure 2). Antibodies to human immunodeficiency virus were not detected on two separate occasions. Complete blood count showed a normal total white cell count of 7.34 × 109/L with eosinophilia (19%) and reduced percentage of lymphocytes (10.4%). Laboratory data including liver and renal function were normal. There was no family history of immunodeficiency, recurrent skin abscesses or sinopulmonary infections since infancy to suggest hyperimmunoglobulin E syndrome.
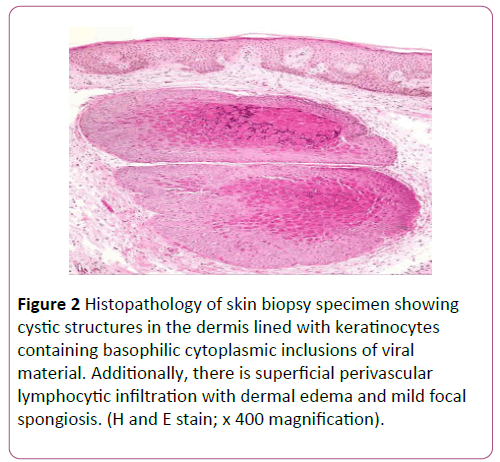
Figure 2: Histopathology of skin biopsy specimen showing cystic structures in the dermis lined with keratinocytes containing basophilic cytoplasmic inclusions of viral material. Additionally, there is superficial perivascular lymphocytic infiltration with dermal edema and mild focal spongiosis. (H and E stain; x 400 magnification).
Cimetidine treatment was considered, but withheld as the patient was receiving concomitant Clopidogrel, so as to avoid adverse drug interactions. Imiquimod was not administered in view of its high cost and the risk of exacerbating his atopic dermatitis. Lesions were enucleated with a sterile needle and iodine was applied. Cryotherapy with liquid nitrogen was administered to recurrent lesions on his left thigh. Response to therapy was acceptable, with local clearance within the treated areas.
Discussion
Although the precise pathophysiology of MC in persons with AD is not well understood, contributing factors include downregulation of antimicrobial peptides and reduced cellmediated immunity [3,4]. However, some studies did not establish any significant association of MC infection in patients with associated AD [5,6]. Additionally, our patient worked as an ornamental bird breeder. This may have been a risk factor for disseminated MC which has been reported in a pigeon fancier, as birds are a known carrier for certain pox viruses [7,8].
Shattock has identified Molluscum microscopically in the North American bunting-sparrow, from growths above the beak, as well as in the pheasant [9]. In addition, it is in domesticated birds that the disease has been the most frequently observed; being fairly common among fowls, turkeys and pigeons. This identification was first established by Bollinger, who confirmed this on histology and also proved its contagiousness in fowls by experimental inoculation [10]. {Hanson, 2003 #6}{NOTE:Virchow’s Archiv, 1873, Bd. lviii} Interestingly, in poultry the disease is located primarily on the comb, wattle, margin of the nose, eyelids and auditory meatus, although it may be found in other areas including the abdomen and leg. Our patient reported a 40-year duration of ornamental bird rearing, during which he reared a wide variety of passerine East Asia bird species consisting primarily of the Oriental white-eye (Zosterops palpebrosus), Chinese Hwamei (Garrulax canorus), Parakeets and Sparrows.
From the literature, treatment modalities available for disseminated MC infection include physical destruction or manual extrusion of lesions, cryotherapy, curettage, imiquimod and retinoids. In one study by Reitamo et al. topical tacrolimus, a macrolide immunosuppressant that inhibits T-cell function and cell-mediated immunity, has been shown to be effective with significant improvement in eczema control demonstrated in more than 80% of the patients [11]. Despite being a potent immunosuppressant, it is not linked with an increased risk of cutaneous infection and is a viable option in resistant cases [12,13].
Conclusion
we present a case of disseminated MC in an ornamental bird breeder with longstanding poorly-controlled AD. Cellular immunity models have been suggested as possible explanations for the association; however there have been conflicting studies in the literature. Further work may be helpful in delineating relative risks for specific viral infections such as MC and eczema herpeticum, with respect to a patient’s level of AD control, as well as in evaluation of the possible risk predisposition from an occupational perspective.
18766
References
- Lee R, Schwartz RA (2010) Pediatric molluscum contagiosum: Reflections on the last challenging poxvirus infection Cutis 86: 230-236.
- Baker BS (2006) The role of microorganisms in atopic dermatitis. Clin Exp Immunol 144: 1-9.
- Bao L, Shi VY, Chan LS (2013) IL-4 up-regulates epidermal chemotactic, angiogenic, and pro-inflammatory genes and down-regulates antimicrobial genes in vivo and in vitro: Relevant in the pathogenesis of atopic dermatitis. Cytokine 61: 419-425.
- Pauly CR, Artis WM, Jones HE (1978) Atopic dermatitis, impaired cellular immunity, and Molluscum contagiosum. Arch Dermatol 114: 391-393.
- Seize MB, Ianhez M, Cestari Sda C (2011) A study of the correlation between Molluscum contagiosum and atopic dermatitis in children. An Bras Dermatol 86: 663-668.
- Hayashida S, Furusho N, Uchi H, Miyazaki S, Eiraku K, et al. (2010) Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci 60: 173-178.
- Little EG (1910) Two cases of Molluscum contagiosum. Br J Dermatol 22: 181-191.
- Thiel T, Whiteman NK, Tirape A, Baquero MI, Cedeno V, et al. (2005) Characterization of canarypox-like viruses infecting endemic birds in the Galapagos Islands. J Wildl Dis 41: 342-353.
- Shattock SG (1898) Molluscum contagiosum in two mated bunting-sparrows. J Pathol Bacteriol 5: 309-313.
- Reitamo S, Wollenberg A, Schopf E, Perrot JL, Marks R, et al. (2000) Safety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. European Tacrolimus Ointment Study Group. Arch Dermatol 136: 999-1006.
- Paller A, Eichenfield LF, Leung DY, Stewart D, Appell M (2001) A 12-week study of tacrolimus ointment for the treatment of atopic dermatitis in pediatric patients. J Am Acad Dermatol 44: 47-57.
- Soter NA, Fleischer AB, Webster GF, Monroe E, Lawrence I (2001) Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: Part II, safety. J Am Acad Dermatol 44: 39-46.








