Abstract
However, propofol and midazolam are widely used for intravenous sedation in very ill patients. Midazolam and propofol are used under light sedation in order to evaluate the effects of different sedation strategies on the clinical outcomes for sepsis patients. In a retrospective cross-sectional study between June 2021 and June 2023, an ICU in Baghdad Medical City examined sepsis patients who required mechanical ventilation and compared the sedation of those who got midazolam and propofol to those who did not. The outcomes of the sequential organ failure assessment (SOFA), day one acute physiology and chronic health evaluation II (APACHE II), and emergency surgery were all recorded for the patients. The midazolam and propofol groups each had 73 and 78 patients, with a mean age of 50 years (SD, 15.4 years) for the midazolam group and 56 years (SD, 16 years) for the propofol group, respectively. Of these participants, 52 (71.2%) and 56 (71.8%) were men. Midazolam and propofol groups' median APACHE II scores were 21 (17-25) and 20(16-25), respectively. The median SOFA scores for midazolam and propofol were 6(4-8) and 6(4-9) respectively. In comparison to propofol groups, midazolam groups had longer median weaning periods, recovery times, and lengths of stays. Both the midazolam and propofol groups had higher rates of infection in the belly, lungs, and other body regions; the length of artificial breathing in both the midazolam and propofol groups exceeded 5 days. To support this finding, additional prospective studies are required.
Keywords
Intensive care unit; Midazolam; Propofol; SOFA; Sepsis
Introduction
Worldwide, sepsis is thought to affect 48.9 million people
annually, of whom 11 million (22.5%) pass away [1]. Sepsis is a
severe problem globally despite regional diversity and a declining
fatality rate [2]. About 40-80% of sepsis patients in the intensive
care unit (ICU) require invasive mechanical ventilation (MV),
highlighting the significance of respiratory treatment [3,4].
When treating sepsis patients who needed ventilator support,
maintaining modest sedation was linked to better clinical results
[5]. According to the clinical recommendations for sedation
techniques in intensive care, mild sedation management should
start early on with the use of a suitable sedation regimen [6].
According to a recent study, managing light sedation during the
acute phase of the first 48 hours of intensive care was linked to
improved survival, decreased delirium, and successful ventilator
weaning [7 ]. Therefore, throughout the first 48-72 hours of
intensive care, we concentrated on sedation control. A handy
medication with no impact on hemodynamic, midazolam has
been shown to build up in the body, extending the duration of MV
and raising the likelihood of delirium in patients in intensive care
units [8,9 ]. The best of our knowledge, there have been a limited
number of studies comparing the effects of midazolam and
propofol on clinical outcomes in sepsis patients that concentrate
on light sedation during the study. In order to assess the effects of
various sedation techniques employing midazolam and propofol
under light sedation on clinical outcomes for patients with sepsis
Methods
Between June 2021 and June 2023, an ICU in Baghdad Medical
City conducted a retrospective cross-sectional study among
sepsis patients who needed mechanical ventilation, comparing
sedation between the patients who received midazolam and
propofol and those who did not. For sedation, only midazolam
and propofol were utilized. This research did not include patients
who were discharged from the intensive care unit (ICU) by day 2
and who did not receive either midazolam or propofol, or who
received both medications during the first two days of enrolment.
Dexmedetomidine, fentanyl, and sedatives given in a bolus were
not included in the grouping.
The Iraqi Ministry of Health provided ethical approval before we
began collecting our data.
Data on the patients' age, sex, emergency surgery, day one acute
physiology and chronic health evaluation II (APACHE II), and
sequential organ failure assessment (SOFA) results were gathered.
We also gathered information on mortality, ventilator-free days
(VFD), infection site (abdomen, lung, or other), and length of stay
in the ICU. Each variable of the data was entered into an excel
spread sheet and examined using SPSS version 20.Continuous
variables were expressed as median (interquartile range [IQR])
or mean (SD), whereas categorical variables were expressed as a
number (%).
Results
Of the 151 patients, 73 and 78 patients were in the midazolam
and propofol groups, respectively (Table 1). Table 1 displays the
characteristics of the two groups. With a mean age of 50 years
(SD, 15.4 years) for the midazolam group and 56 years (SD, 16
years) for the propofol group, respectively, 52 (71.2%) and 56
(71.8%) of these participants were men. In the midazolam and
propofol groups, the number of patients with BMI was 22.9 (SD,
3.5 kg/m2) and 24.2 (SD, 4 kg/m2), respectively. The median
APACHE II score for the midazolam and propofol groups was 21
(17-25) and 20 (16-25), respectively. Midazolam and propofol
each had a median SOFA score of 6(4-8) and 6(4-9) respectively.
Additionally, the median RASS score for midazolam and propofol
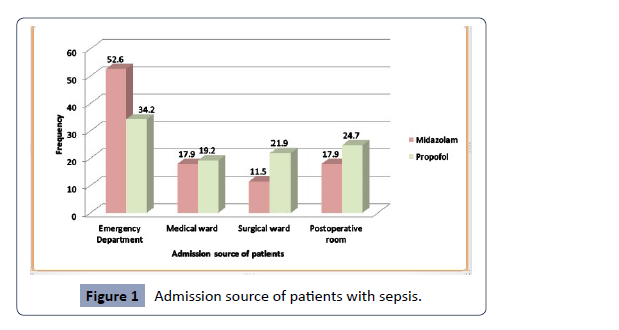
was -3, respectively. According to Figure 1, the emergency room,
medical ward, postoperative room, and surgical ward were
the places where patients were most frequently admitted. The
primary outcome measure reveals that the median weaning time
for midazolam was 49 (26.5-73.0) hours and for propofol was 32
(23-51) hours. The median recovery time for the midazolam and
propofol groups, respectively, was 7(2-28) hours and 2(1-4.5)
hours. Midazolam and propofol groups' median lengths of stay
in the ICU were 18 days and 14 days, respectively. The median
hospital stay was 24 days for the midazolam group and 19 days for
the propofol group, respectively. In the midazolam and propofol
groups, the ICU fatality rates were 5 and 7, respectively (Table 2).
| Propofol (n=78) |
Midazolam(n=73) |
Characteristic |
| 51.0±16.0 |
50.8±15.4 |
Mean/SD (year) |
| 56(71.8) |
52(71.2) |
Male |
| 22(28.2) |
21(28.8) |
Female |
| 24.2±4.0 |
22.9±3.5 |
Mean/SD (kg/m2) |
| 20(16-25) |
21(17-25) |
Median (IQR) |
| 6(4-9) |
6(4-8) |
Median (IQR) |
| -3(-4 to 3) |
-3(-4 to 2) |
Median (IQR) |
| BMI, Body Mass Index; APACHE II, Acute Physiology and Chronic Health Evaluation II; IQR, Interquartile Range; SOFA, Sequential Organ Failure Assessment; RASS, Richmond Agitation and Sedation Scale |
Table 1. Baseline characteristic of patients with sepsis (n= 151)
Figure 1: Admission source of patients with sepsis.
Propofol(n=78) |
Midazolam(n=73) |
Outcome measure |
| 32(23-51) |
49(26.5-73.0) |
Weaning time , median (IQR), h |
| 2(1-4.5) |
7(2-28) |
Recovery time , median (IQR), h |
| 3.5(1.5-5.5) |
7(2-30.2) |
Extubation time , median (IQR), h |
| 14.2(9.8-18.9) |
18.3(11.7-28.7) |
ICU duration, median (IQR), day |
| 19.4(14.9-34.5) |
23.8(17.3-40.3) |
Length of hospital stay, median (IQR), day |
| 7(9.0) |
5(6.8) |
ICU mortality, No. (%) |
Table 2. Raw data of main outcome
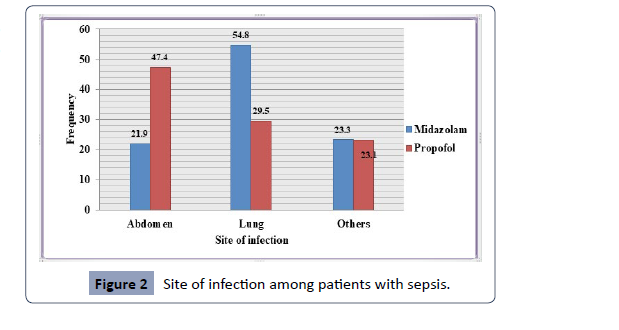
According to the Figure 2, the most common sites of infection
were the lung (54.8%), others (23.3%), and abdomen (21.9%) in
the midazolam groups and the abdomen (47.4%), lung (29.5%),
and others (23.1%), all in the propofol groups.
Figure 2: Site of infection among patients with sepsis.
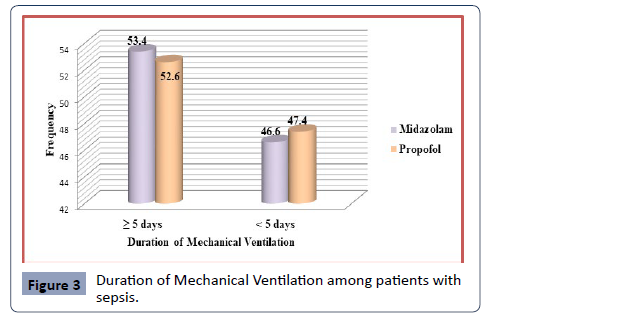
According to the length of mechanical ventilation, the midazolam
and propofol groups exhibit 53.4% and 52.6% in the ≥ 5 days, and
46.6% and 47.4% in the ˂ 5 days (Figure 3).
Figure 3: Duration of Mechanical Ventilation among patients with
sepsis.
This study sought to assess the effectiveness of midazolam and
propofol among sepsis patients in the intensive care unit. In
the sedative treatment of ICU patients with sepsis, propofol, midazolam, and dexmedetomidine are efficient and secure [10].
In this investigation, it was discovered that the midazolam and
propofol groups, respectively, contained 73 and 78 individuals.
In contrast to another study conducted in China by Yu et al.
(2015), the patients in this study were split into two groups:
those receiving midazolam (n = 21) and those receiving propofol
(n = 22) [11]. In a 2019 study in China, 146 individuals received
midazolam, whereas 152 received propofol [12 ]. The midazolam
group's mean age in the current study was 50 years (SD, 15.4
years), while the propofol group's mean age was 56 years (SD,
16 years). While a 2019 study in China found that the median
ages for the midazolam and propofol groups were 54 years
(SD, 11.6 years) and 55 years (SD, 11 years), respectively [12 ]. A
prospective randomized trial with 20 patients in each midazolam
and propofol group was carried out in Egypt. The results showed
that the mean ages of the midazolam group were 55.7 ± 9.6 and
the propofol group was 53.9 ± 7.7.52 (71.2%) and 56 (71.8%) of
the participants in the current study were men [13].
The authors noted that in 2019 men in China were 101 (69.2) for
the midazolam group and 110 (72.4) for the propofol group [12 ].
There were 22.9 (SD, 3.5 kg/m2) and 24.2 (SD, 4 kg/m2) patients
with BMI in the midazolam and propofol groups, respectively. In
2019, Ding found that the BMI for the midazolam group was 21.3
± 5.1 and for the propofol group it was 21.9 ± 5.4 [10 ].
Midazolam and propofol groups' median APACHE II scores were
21 (17-25) and 20(16-25), respectively. According to the authors,
the mean and SD of the APACHE II test in China were 16 ± 6 for
midazolam groups and 15 ± 6 for propofol groups [12]. Clinical
traits and background medical therapy did not differ statistically
significantly. Ding discovered that the mean of the APACHE II test
in 2019 was 19.02 ± 4.63 for midazolam groups and 18.7 ± 4.2
for propofol groups [10]. Additionally, the median SOFA scores
for midazolam and propofol in this trial were 6 (4-8) and 6 (4-9)
respectively. The mean and SD of the SOFA scores 9 ± 3 for the
midazolam and propofol groups in China were described by the
authors [12].
According to Moeen et al. in 2022, the median SOFA score for
midazolam groups was 10 [6-13] while for propofol groups
it was 9(6-13) [14]. The mean SOFA score was 6.25 (4.03) for
midazolam groups and 5.10 (3.35) for propofol groups, according
to Sun et al in 2022 [15 ]. Additionally, midazolam and propofol
had median RASS scores of -3 and -3, respectively. In a doubleblind,
randomized, controlled research Hughes et al. conducted
in 2021 at 13 medical sites in the United States with 208 patients
receiving propofol, the authors noted the median RASS score
while taking the medicine was- 1.95 (ranging from-3.03 to 0.98)
[16]. Medical ward, postoperative room, and surgical ward were
the areas where patients were frequently hospitalized with regard
to the emergency department. According to Zhang et al. in 2019,
patients with an EDLOS of 6 hours or less had a crude mortality
rate of 21.4%, which was considerably lower than patients with
an EDLOS of 12 to 24 hours (31.9%) and patients with an EDLOS
of more than 24 hours (31.8%). EDLOS remained independently
linked with increased risk of hospital mortality after controlling
for PaO2/FiO2, serum creatinine, age, Sequential Organ Failure
Assessment, body mass index, lactate, comorbidities, and
infection site [17]. The major outcome measure indicates that
the median weaning time for propofol was 32 (23-51) hours and
for midazolam was 49 (26.5-73.0) hours. In Germany, Muellejans
et al. conducted a prospective, open-label, randomised, singlecenter
study in 2006 with 33 patients receiving midazolam and 39
patients receiving propofol. The authors found that the mean and
Sd of weaning time were 5.7 ± 6.6 for the midazolam groups and
2.2 ± 4.3 for the propofol groups [18]. As was said, the midazolam
and propofol groups' respective median recovery times were
7(2-28) and 2(1-4.5) hours. The recovery period was 58 hours for
midazolam groups and 1.5 hours for propofol groups, according
to Zhou et al. (2014). The median lengths of stay in the ICU were
18 days for the midazolam group and 14 days for the propofol
group, respectively [19]. According to a 2019 study by Ding, the
average length of stay in China was 7.93 ± 3.5 for midazolam
groups and 7.8 ± 3.3 for propofol groups [10]. For the midazolam
group and the propofol group, the median length of stay in the
hospital was 24 days and 19 days, respectively. The median
length of stay was 72 hours for midazolam groups and 69.8 hours
for propofol groups, according to a multicenter, randomized,
open label trial conducted in Canada by Hall et al in 2001 [20].
Patients using midazolam had a median hospital length of stay
of 9.5 days, according to research by Tekwani et al. from 2010
(interquartile range [IQR]: 4.6 to 16 days) [21]. The ICU fatality
rates were 5 and 7 for the midazolam and propofol groups,
respectively. Additionally, Tekwani et al. (2010) explained that
hospital mortality was 21 of 59 (36%; 95% confidence interval
[CI] 24% to 49%) for patients taking midazolam [21]. According to
Zhou et al. (2014), ICU mortality was 7(16.3) for groups receiving
midazolam and 6(14.3) for those receiving propofol [19].
Infections most frequently occurred in the abdomen (47.4%),
lung (29.5%), and others (23.1%) in the propofol groups and
the lung (54.8%), others (23.3%), and abdomen (21.9%) in the
midazolam groups. In Japan, a nested cohort analysis was carried
out by Miyagawa et al. in 2022 among 51 patients for midazolam
and 66 patients for propofol groups. The authors found that the
abdomen for the propofol groups and the lung for the midazolam
groups were the sites of infection, respectively [22]. The
midazolam and propofol groups show 53.4% and 52.6% in the
first 5 days, and 46.6% and 47.4% in the next 5 days, respectively,
depending on the duration of mechanical breathing. According
to Miyagawa et al. 2022, sedation with midazolam based on
a mild sedation strategy may be related with inappropriate
sedation during the acute phase, with greater coma and delirium
as compared to propofol, in patients with sepsis who required
mechanical ventilation [22]. A retrospective analysis with 2,174
cases (51.91%) in the propofol group and 2,014 cases (48.09%)
in the midazolam group was carried out in China in 2022 by
sun et al. The rate of patients getting mechanical ventilation
was demonstrably greater in the propofol group than in the
midazolam group (99.60% vs. 76.90%, P0.001), according to the
authors [15].
Conclusion
We come to the conclusion that there were significantly more
male patients than female cases in both the midazolam and
propofol groups, with the emergency room serving as the main
entry point. Midazolam groups had longer median weaning times, recovery times, and lengths of stays than propofol groups.
The belly, lung, and other body parts were the most often
infected ones among both the midazolam and propofol groups;
the duration of mechanical breathing in both the midazolam and
propofol groups surpassed 5 days. Additional prospective studies
are needed to confirm this finding.
Conflict of interest:
None