Keywords
Microbiological analysis; Skin microbiome; Chlorhexidine gluconate; Without rinsing; Washing without water
Introduction
The human microbiome is a symbiosis of billions of Microorganisms (MO) that inhabit the human body. The microbiome builds up the gut microbiota, upper respiratory tract, skin and vagina. This bacterial population is the normal microbial flora of the human body with which commensal or symbiotic relationships are formed. It plays an important physiological role in maintaining the health of the body, provides vital functions that the human genome has not developed, plays a key role in protecting it from external pathogens and is an integral part of it.
The skin is the largest organ of the human body. Its main role is to serve as a physical barrier to protect our bodies from bacteria or toxins. It is in constant contact with the environment and as such is colonized by a wide range of MO - bacteria, fungi, viruses [1]. The composition and amount of the dermal microflora varies depending on the conditions of the microenvironment in terms of pH, temperature, moisture and sebum [2].
The skin surface forms areas with diverse environments and a wide range of vital parameters for the development of various MO. Most of them live in the superficial layers of the stratum corneum layer of the epidermis and in the upper parts of the hair follicles. Some bacteria live deep within the hair follicles and are beyond the scope of ordinary disinfection procedures. These bacteria are a reservoir for recovery after removal of bacteria from the surface [1]. The number of bacteria on an individual's skin is relatively constant, depending on habitat characteristics. The bacterial species that are the main representatives of normal skin flora are:
Staphylococcus epidermidis - can reach up to 90% of the inhabited aerobic flora in places with high humidity - axillary vault, inguinal and gluteal folds, popliteal fossa, antecubital fossa. This skin commensal can cause infections when it enters unusual or normally sterile areas in patients with impaired immune responses or hospitalized patients. It is a common cause of Health care-associated infections (HAIs) when placing implants, catheters or heart valves, etc. Entering the body, virulent strains can form biofilms on catheters or other medical devices, protecting them from the body's immune system and the action of antibiotics. Multiple antibiotic pressure increases the resistance of staphylococci. Of particular importance is resistance to methicillin and oxacillin, which respectively excludes all betalactam antibiotics from the treatment regimen and treatment of infections caused by S. epidermidis and the condition is complicated. Increased antibiotic resistance is encoded into resistance genes that S. epidermidis transmits to the more virulent and pathogenic representative of the genus S. aureus.
Other members of the Coagulasenegative staphylococci (CNS) group are also included in the skin flora [3].
• Diphtheroid/coryneforms/– to this group belong many species included in the composition to g. Corynebacterium.
• Anaerobic bacteria – Propionibacterium spp. – are most common in areas rich in sebaceous glands. Micrococcus, Streptococcus - α-hemolytic, Acinetobacter spp. and others [1].
• Gram negative bacteria are not common in the skin. They are normal inhabitants of the gastrointestinal tract. Isolation of E. coli, Proteus spp, Klebsiella spp, Enterobacter spp., as well as Enterococcus spp. of skin samples is a sign of poor hygiene and in patients in intensive care units - of poor or absent hygiene care. In such cases, the skin can also be colonized by existing multidrug-resistant bacterial strains in the hospital environment: Methicillin-resistant staphylococcus aureus (MRS?), Vancomycin-resistant enterococci (VRE), Klebsiela pneumony carbapenemasis (KPC), Enterococci resistant glycopeptide. This, in turn, leads to an increased risk of developing a HAsI when performing invasive diagnostic and therapeutic procedures. All people colonized with the cited pathogens can potentially cause infections to others by exuding them, leading to widespread environmental pollution. Critically ill patients in Intensive Care Unit (ICU) are at high risk of infection as a result of underlying immunodeficiency, comorbidity and placement of invasive devices - endotracheal tubes, intravascular catheters [4].
The nurse is aware of various strategies for preventing HAIs - hand hygiene, use of PPE, application of aseptic techniques, hygiene care with antiseptic agents and more. Chlorhexidine gluconate (CHG) is an antiseptic agent with high antimicrobial activity, rapid action and unaffected by the presence of body fluids (blood). According to the literature data of previous studies, the results are varied. According to some, daily full body CHG bathing can significantly reduce the risk of HAIs – Catheter-related infection, Surgical site infection and colonization with MDROs, and according to others, the effect is comparable to that of routine care [5-9]. Another study examines the colonization of patients' arms with MDROs and their release into the environment. They were found to be carriers of VRE - 13% and MRSA - 10,9% [4].
The MO inhabited by inpatient hospitals may affect the recovery, treatment outcomes and length of hospital stay of patients [10]. In order to reduce this impact, it is necessary to know and strictly apply infection control measures, the criteria for identifying patients at risk for the occurrence of HAIs, the requirements for hand hygiene, microbiological control of the hospital environment and the hands of staff. Efforts should be made to transform the hospital into a safe environment without the risk of nosocomial infections for patients, leading to a reduction in morbidity, mortality, increased length of stay and costs [11,12].
The microflora examination is carried out by swab, scarification and biopsy methods. For this study, the swab method was chosen as easy to perform, fast and non-invasive [2].
The skin is one of the largest reservoirs of MO in the human body, some of which are potential agents of HAIs. Methods based on bacterial culture using swabs were used to perform microbiological analysis of the skin surface. These are one of the most commonly used methods for isolating and identifying aerobic and optional anaerobic MO that are part of the skin flora. Most of them live in the superficial layers of the stratum corneum and in the upper hair follicles, and are within the scope of routine hygiene procedures.
The purpose of this study is to demonstrate the bacterial contamination of the skin after a second day of hospital stay and the need to use disinfectant with CHG for hygien bathing of patients by the method of "dry bathing".
Methods
Following the written permission of the director of University Multidisciplinary Hospital for Active Treatment (UMHAT) "Kanev" in the period from May to November 2019, an experimental application of the method of "dry bathing" was carried out on 120 patients from the Orthopedics and Traumatology Unit, Department of Vascular Neurology, Department of ICU of Kanev Hospital. The method is innovative for the Republic of Bulgaria and has not been imposed and has not yet been implemented as a routine practice in hospitals in Bulgaria. Selection criteria for patients:
• hospitalization duration of more than 48 hours;
• a condition requiring compensatory care;
• written informed consent to be included in the study.
All patients enrolled in the experiment gave informed consent in writing after detailed knowledge of the procedures ahead. The bathing of the patients was done according to the prepared technical sheets for bathing the patient in bed and changing the underwear for the seriously ill by the method of "dry bathing". Patients hospitalized in surgical wards were administered a Zivasept-containing hygiene procedure containing 0.6% HCG, and Wash Cream TENA and Wash Mousse TENA containing no disinfectant were used in patients with therapeutic conditions. Disposable dry TENA Wash Glove gloves or TENA Cellduk towels were used to apply the cream and foam.
The skin sampling before and after the patient's hygiene procedure was performed by a nurse and the microbiological examination was performed by a microbiologist. The microbiological samples are analysed at the Microbiology Laboratory at UMHAT Kanev in the period from May to November 2019.
The location of the microbiological monitoring was determined to be the inner part of the elbow, the cubital well. The choice was made according to accessibility criteria - to be easily accessible and to be the place where medical procedures are carried out, which usually carry the risk of occurrence of HAIs (blood sampling for hemocultures and laboratory tests, inclusion of Peripheral venous source).
Methods for collecting, processing and analysing the results of the study
Microbiological analysis of the skin surface - the sampling area was limited by polypropylene/plastic/square with a total free area of 5 cm2. The squares are cleaned with 70% alcohol before being reused. The skin surface is wiped with a sterile cotton swab soaked in 0.9% sterile saline, then the material is placed in a tube with 0.5 ml sterile 0.9% NaCl. The washings were poured into blood agar plates and the entire surface of the culture medium was inoculated with the swab. The crop is cultivated at 36°C for 24 h. Tests for catalase, plasm coagulase, Gram staining, esculin test, and polymicrotests for biochemical identification of Gram/- bacteria were used to identify the bacteria. The isolated strains were tested for unusual resistance by Disc-diffusion method:
Staphylococcus spp. with Cefoxitin to determine methicillin resistance;
Enterococcus spp. – with Vancomycin for the detection of VRE;
Gram/-/bacteria – with Meropenem and Imipenem to detect carbapenemase producers.
Statistical methods
• Variational analysis for one-dimensional distributions and multidimensional distributions - derivation of basic numerical characteristics of studied traits
• Parametric methods - Levene's test for sample homogeneity and Student's T-test for comparison of relative proportions (test of working hypothesis H_0) and medium values at significance level α=0.05.
• The processing, analysis and graphical presentation of data and results have been carried out with the help of the statistical program SPSS 20.0 - powerful modern software used in various scientific fields [13-15].
Framework of methodological approach of the conducted research
In Figure 1 a scheme of methodical approach is presented which is applied to prove the effectiveness of the method used for hygienic bathing with CHG and cleaning agent. The links are numbered 1 through 4.
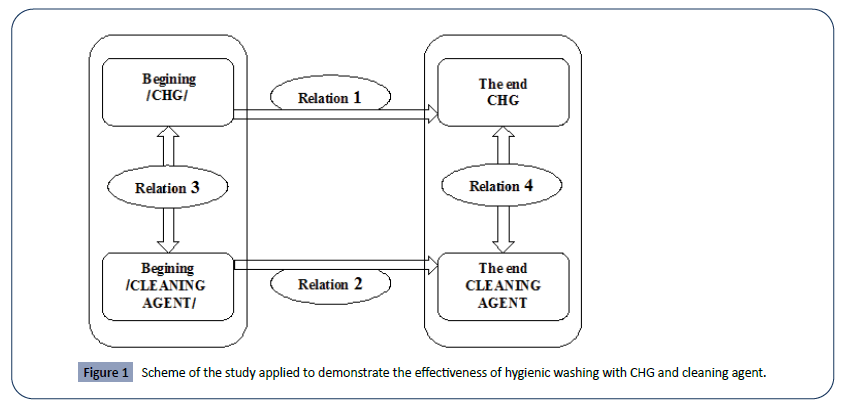
Figure 1: Scheme of the study applied to demonstrate the effectiveness of hygienic washing with CHG and cleaning agent.
The following main tasks are solved in the process of statistical analysis:
• Determining the effect of applying the alternative method of hygiene washing before and after with CHG (relation 1 in Figure 1).
• Determining the effect of applying the alternative method of hygiene washing before and after cleaning agent (relation 2 in Figure 1).
• Checking the statistical significance of differences in the ratings of the experimental group to be treated with CHG and the experimental group to be treated with cleaning agent at the beginning of the study; e. both groups should have an "equal start" (relation 3 in Figure 1).
• Investigation of the statistical significance of the differences in the ratings of the CHG-treated experimental group and the cleaning agent - treated experimental group at the end of the study (relation 4 in Figure 1).
Results and Discussion
Samples were obtained from 80 patients undergoing a hygiene toilet with Zivasept containing CHG 0.6% and 40 patients using Wash Cream and Wash Mousse containing no disinfectant. Two skin samples were taken from all patients included in the project - before and after the skin treatment with the disinfectant or detergent.
The following bacteria were isolated in the microbiological examination of the skin surface: Coagulase-negative methicillinsensitive staphylococci (CNMSS), Coagulase-negative methicillinresistant staphylococci (CNMRS), Enterococcus, Methicillinsensitive S. aureus (MSSA), Bacillus spp, Gram/- bacteria, Streptococcus α-hem, Corynebacterium (Table 1).
Table 1 Basic numerical characteristics of the number of MO before and after bathing regardless of the agent.
| |
nsum_ Before |
nsum_ After |
| N |
Valid |
120 |
120 |
| Missing |
0 |
0 |
| Mean |
1,8333 |
1,1250 |
| Median |
2,0000 |
1,0000 |
| Mode |
2,00 |
1,00 |
| Std. Deviation |
,62622 |
,72833 |
| Minimum |
1,00 |
,00 |
| Maximum |
4,00 |
2,00 |
The average number of isolates before bathing is 1.83, and after bathing there is a decrease of 1.25. Tendency shows that the most common number of germs in the group before bathing is 2, in the group after is 1. The medians in the two groups are 2 and 1, respectively. The standard deviation of the number of MO in the group before bathing is 0.626 and after bathing is 0.0728. In the pre-bath group, the smallest number of MO possessing a respondent is 1 and the largest is 4. In the group after bathing these are 0 and 2, respectively (Table 2).
Table 2 Basic numerical characteristics of the CHG treatment group.
| |
Statistics – CHG |
Statistics – CLEANING AGENT |
| nsum_ Before |
nsum_ After |
nsum_ Before |
nsum_ After |
| N |
Valid |
80 |
80 |
40 |
40 |
| Missing |
0 |
0 |
0 |
0 |
| Mean |
1,8750 |
,9500 |
1,7500 |
1,4750 |
| Median |
2,0000 |
1,0000 |
2,0000 |
1,0000 |
| Mode |
2,00 |
1,00 |
2,00 |
1,00 |
| Std. Deviation |
,62389 |
,76141 |
,63043 |
,50574 |
| Minimum |
1,00 |
,00 |
1,00 |
1,00 |
| Maximum |
3,00 |
2,00 |
4,00 |
2,00 |
The average number of isolate species before bathing is 1.875, and after bathing there is a decrease of 0.95. Tendency shows that the most common number of germs in the group before bathing is 2, in the group after is 1. The medians in the two groups are 2 and 1, respectively. The standard deviation of the number of MO in the group before bathing is 0.624 and after bathing is 0.0761. In the pre-bath group, the smallest number of isolates possessing a respondent is 1 and the largest is 3. In the group after bathing these are 0 and 2, respectively.
The average number of isolates species before bathing is 1.75, and after bathing there is a decrease of 1,475. Tendency shows that the most common number of germs in the group before bathing is 2, in the group after is 1. The medians in the two groups are 2 and 1, respectively. The standard deviation of the number of MO in the group before bathing is 0.63 and after bathing is 0.051. In the pre-bath group, the smallest number of isolates possessing a respondent is 1 and the largest is 4. In the group after bathing these are 1 and 2, respectively (Table 3).
Table 3 "Equal Start" - "Different End" in the experiment.
| |
Levene's Test for Equality of Variances |
t-test for Equality of Means |
| |
. |
F |
Sig |
t |
df |
Sig. (2-tailed) |
| nsum_Before |
Equal variances assumed |
,125 |
,724 |
1,031 |
118 |
,305 |
| Equal variances not assumed |
|
|
1,027 |
77,387 |
,307 |
| nsum_After |
Equal variances assumed |
1,612 |
,207 |
-3,943 |
118 |
,000 |
| Equal variances not assumed |
|
|
-4,495 |
108,619 |
,000 |
In order to prove the statistically equal start of the average number of MO at the beginning of the period from the groups that will be treated with two different cleaning agents, it is necessary to know whether there is equality in the statistical dispersion in the two samples. From the Table 3 in the Levene’s test for equality of variances, it follows that the variation (dispersion) in the two samples at the outset is statistically insignificant/ Sig.=0,724/. The Student's test was then performed with equal variations for the statistical significance of the difference in the average values. There is no statistically significant difference/Sig. (2-tailed=0.305)/, i.e. there is a so-called even start.
In order to prove the statistically significant difference in the average number of bacteria at the end of the period from the groups treated with two different cleaning agents, it is necessary to know whether there is again a statistical dispersion in the two samples. From Table 3 in the Levene’s test for equality of variances it follows that the variation (variance) in the two samples at the beginning is statistically insignificant/Sig. =0,207/. After that, a Student’s test with equal variations was performed for the statistical significance of the difference in the average values. There is a statistically significant difference/Sig. (2-tailed=0.000)/.
The average relative decrease ratio of cleaning agent – treated isolates was 7.14% respectively, using the CHG decrease rate was significantly higher - 50.33% (Table 4). The difference in the efficacy of the two cleaning agents is statistically significant at significance level α = 0.05. Following the use of CHG, isolates without growth of MO were observed in: S. aureus, MRSA and Streptococcus a hem. The insignificant difference in results after a hygienic bath with a washable cream or bath without rinsing is expected, since the composition of this group of hygiene products lacks an antiseptic agent. Their advantage in a hygienic bath is in achieved patient’s comfort, hygienic cleaning and moisturizing of the skin.
Table 4 Average number of isolates and a relative decrease in percentage before and after bathing in both groups of respondents.
| Bacterial flora |
CLEANING AGENT |
CHG |
| Before bathing – isolates (n) |
After bathing – isolates (n) |
Relative reduction (%) |
Before bathing – isolates (n) |
After bathing – isolates (n) |
Relative reduction (%) |
| CNMSS |
12 |
11 |
8,3 |
46 |
24 |
47,8 |
| CNMRS |
28 |
28 |
0 |
40 |
23 |
42,5 |
| Enterococcus |
3 |
2 |
33,3 |
20 |
12 |
40 |
| S. aureus |
0 |
0 |
- |
3 |
0 |
100 |
| MRSA |
0 |
0 |
- |
3 |
0 |
100 |
| Bacillus spp |
17 |
17 |
0 |
15 |
10 |
33,3 |
| Gram /-/ bacteria |
3 |
0 |
100 |
8 |
2 |
75 |
| Streptococcus a hem |
5 |
5 |
0 |
6 |
0 |
100 |
| Corynebacterium |
2 |
2 |
0 |
12 |
5 |
58,3 |
| All |
70 |
65 |
7,1 |
153 |
76 |
50,3 |
Conclusions
The following conclusions can be drawn from the analysis of the microbiological results obtained:
• In skin samples in the area of the cubital well, 44 Gram/-/ bacterial and enterococcal isolates were found that are not within the normal skin flora. These bacteria are characteristic of the intestinal bacterial flora and are regarded as an indicator of fecal contamination and poor hygiene care. In the samples after the hygiene procedure with CHG were found 14 isolates, while 2 were found in the samples used cleaning agent, which represent a risk for the occurrence of HAIs.
• CNS-126 (n) (CNMRS-68 n and CNMSS-58 n) were the largest share isolated from the representatives of normal skin flora, followed by Corynebacterium spp. 14 (n). CNMRS are discovered in the hospital environment as a result of increased antibiotic pressure and are one of the most common causes of HAIs. The 42.5% decrease in CNMRS after skin treatment with CHG was significantly greater compared to that using a cleaning agent where there is with no difference. This MO group is not affected by washing cream or foam, which are without rinsing.
• VRE, Gram/-/bacteria producing carbapenemase and MRSA were not identified from the studies performed in the experimental study;
• A total of 153 bacterial strains, as described in above table, were isolated from patients who had a hygienic bath with CHG (80 persons) before the procedure. The average relative decrease ratio of cleaning agent – treated isolates was 7.14%, while with the use of CHG the reduction rate was significantly higher – respectively 50.33%.
• Bacillus spp. Was detected in the skin specimens of both groups of patients before and after the hygiene procedures with CHG/cleaning agent, as it is noticeable that its effect was negligible in both agents. The diminished effect is due to the fact that the agents used do not have a sporicidal effect. In microbiological studies of hemocultures, punctures and wound secretions which are not covered by this study, were isolated Bacillus spp. which are considered contaminants. This is due to improper disinfection of the skin surface prior to invasive manipulation. In this regard, the use of disinfectants and sporicidals is recommended.
• In the process of statistical analysis, an “equal start” and a “different end” were proved (relation 3 and relation 4 of above figure), which implies that one approach outperforms the other, in this case the hygiene bath with CHG shows statistically better results.
Recommendations
• From the analysis of the results obtained and the ongoing drive to increase patient safety, we consider that it is advisable for nurses to use CHG for hygiene procedures in the "dry bath" method in patients at risk of HAI.
• The presence of bacteria with high resistance in the skin samples of patients is a risk for their spread into the environment and for their transmission through patient-nurse-patient contact. From an epidemiological point of view, microbiological monitoring of the hospital environment and apparatus as well as the hands of nurse is recommended. Another important recommendation is strict adherence to the rules for the use of personal protective equipment and the rules for hygiene washing and disinfection of hands.
Acknowledgment
This work was supported by project: No 2019-RU-07 "Development and research of a comprehensive concept for changing traditional methods with alternative methods and means for the hygiene of patients who are unable to self-serve", funded by the fund "Science ressearch" of Ruse University "Angel Kanchev".
Conflicts of interest
None.
26663
References
- Baron S (1996) Medical Microbiology (4thedn). Galveston (TX): University of Texas Medical Branch at Galveston.
- Grice EA, Kong HH, Renaud G, Young AC, NISC Comparative Sequencing Program, et al. (2008) A diversity profile of the human skin microbiota. Genome Res 18: 1043-1050.
- Grice EA, Segre JA (2011) The skin microbiome. Nat Rev Microbiol 9: 244-253.
- Teska P, Gauthier J, Calabrese C (2018) Patient Colonization: Implications and Possible Solutions for Contamination of the Healthcare Environment. Infection control today.
- Coffey PS, Metzler M, Islam Z, Koehlmoos TP (2013) Chlorhexidine for Umbilical Cord Care: Selected Bibliography. BMC Int Health Hum Rights 13: 44.
- Jordan JR, Bloom HL (2010) Prevention of Bacterial Infections in Patients with Cardiac Implantable Devices: Old Traditions Meet New Technology. The Journal of Innovations in Cardiac Rhythm Management 1: 63-71
- Weinstein RA, Milstone AM, Passaretti CL, Perl TM (2008) Chlorhexidine: expanding the armamentarium for infection control and prevention. Clinical Infectious Diseases 46: 274-281.
- Georgieva D (2018) Alternative methods and means for the realization of quality and safe compensatory hygienic care. Proceedings of University of Ruse 57: 61-67.
- Webster J, Osborne S (2015) Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev pp: CD004985.
- Lax S, Sangwan N, Smith D, Larsen P, Handley KM, et al. (2017) Bacterial colonization and succession in a newly opened hospital. Sci Transl Med 9.
- Hristova I (2018) The basic hygienic health care as a factor for the rise of infections due to medical service (IDMS). Proceedings of University of Ruse 57: 53-60.
- Mehta Y, Gupta A, Todi S, Myatra S, Samaddar DP, et al. (2014) Guidelines for prevention of hospital acquired infections. Indian J Crit Care Med 18: 149-163.
- Ganeva Z (2016) Discovering statistics using IBM SPSS statistics. Sofia: Print Elestra Ltd.
- Pencheva V, Tsekov A, Georgiev I, Kostadinov S (2018) Analysis and assessment of the regularity of mass urban passenger transport in the conditions of the city of Ruse. Transport Problems 13: 109-118.






