Georgios Tzanis1, Stavros Dimopoulos2, Eleftherios Karatzanos3, Athanasios Tasoulis4, John Terrovitis5, Dimitra Rontogianni6, Serafim Nanas7*
1MD, 1st Critical Care Medicine Department, Cardiopulmonary Exercise Testing and Rehabilitation Laboratory, “Evgenidio Hospital”, National & Kapodistrian University of Athens, Greece
2MD, PhD, 1st Critical Care Medicine Department, Cardiopulmonary Exercise Testing and Rehabilitation Laboratory, “Evgenidio Hospital”, National & Kapodistrian University of Athens, Greece
3MSc, PhD, 1st Critical Care Medicine Department, Cardiopulmonary Exercise Testing and Rehabilitation Laboratory, “Evgenidio Hospital”, National & Kapodistrian University of Athens, Greece
4MD, PhD, 3rd Cardiology Department, “Laiko” Hospital, National & Kapodistrian University of Athens, Greece
5MD, PhD, Assistant Professor, 3rd Cardiology Department, “Laiko” Hospital, National & Kapodistrian University of Athens, Greece
6MD, PhD, Director of Department of Pathology, Evangelismos Hospital, Athens, Greece
7MD, PhD, Professor, 1st Critical Care Medicine Department, Cardiopulmonary Exercise Testing and Rehabilitation Laboratory, “Evgenidio Hospital”, National & Kapodistrian University of Athens, Greece
- *Corresponding Author:
- Serafim Nanas
MD, Professor of Medicine1st Critical Care Medicine Department
Cardiopulmonary Exercise Testing and Rehabilitation Laboratory
“Evgenidio Hospital”, National & Kapodistrian University of Athens
Athens, 11528, Greece
E-mail: a-icu@med.uoa.gr
Telephone: +30-210-7236743, +30-6973036448
Fax: +30-210-7242785
Key words
Myopathy, fiber type, exercise capacity, exercise training, interval training, strength training
Introduction
Heart failure (HF) is associated with severe exercise intolerance consisting of fatigue and dyspnea at low exercise workloads. These symptoms were thought to result from central hemodynamic derangements. However, significant skeletal muscular pathology was found in HF, presenting with a reshift to anaerobic fibers, reduced capillarization and volume density of mitochondria that contribute to the decreased exercise capacity of these patients. [1,2]
Exercise training induces peripheral muscle adaptations in HF patients and appears to be a suitable intervention to enhance their functional capacity. Exercise by continuous aerobic training has been shown to improve the skeletal muscle myopathy, the endothelium, the autonomous nervous system derangement, the functional capacity and survival of HF patients. [3-8]
Interval exercise training is closer to patients’ daily activities and by applying higher exercise intensity seems to induce pronounced exercise stimuli on peripheral muscles with minimal cardiac strain.9 Recent studies have shown that high intensity interval aerobic training improves the aerobic capacity [9,10] , the endothelium and the myocardial contractility [10] in HF patients. The addition of strength training improves the microcirculation [11] , the respiratory drive [12] and it seems to induce greater beneficial effect on muscle strength [13] and vascular reactivity than interval exercise training alone [14] . A recent meta-analysis suggests that combined interval training with strength training elicits larger benefits in aerobic capacity and that interval exercise was superior to continuous exercise training [15] , indicating that interval exercise could be a valid alternative modality of training in HF patients.
However, the effects of interval aerobic training in the skeletal muscle characteristics of patients with HF and the prescription of the ideal form of exercise (type, intensity and duration), remain a challenging field for investigation.
We hypothesized that different protocols of interval exercise training including high intensity interval with or without the addition of strength training would have beneficial effects on skeletal muscle histology of patients with HF.
The aim of this case study was to investigate the effect of different interval exercise training regimens on skeletal muscle fiber-type distribution and metabolic profile in HF patients.
Methodology
Population study
The population consisted of 10 stable (males, age: 56±13 yrs, BMI: 27±4 kg/m2) patients with HF at optimal medical treatment. . Patients were referred to the Cardiopulmonary Rehabilitation Center from the Heart Failure Clinic of our Institution. All patients underwent serial evaluation with cardiopulmonary exercise test (CPET) and skeletal muscle biopsy before and after a 3 month rehabilitation program. The baseline characteristics of the patients are listed in Table 1. Informed consent was obtained from all patients, and the study was approved by the Human Study Committee of our Institution (No.4522/20.11.07). Τhe study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
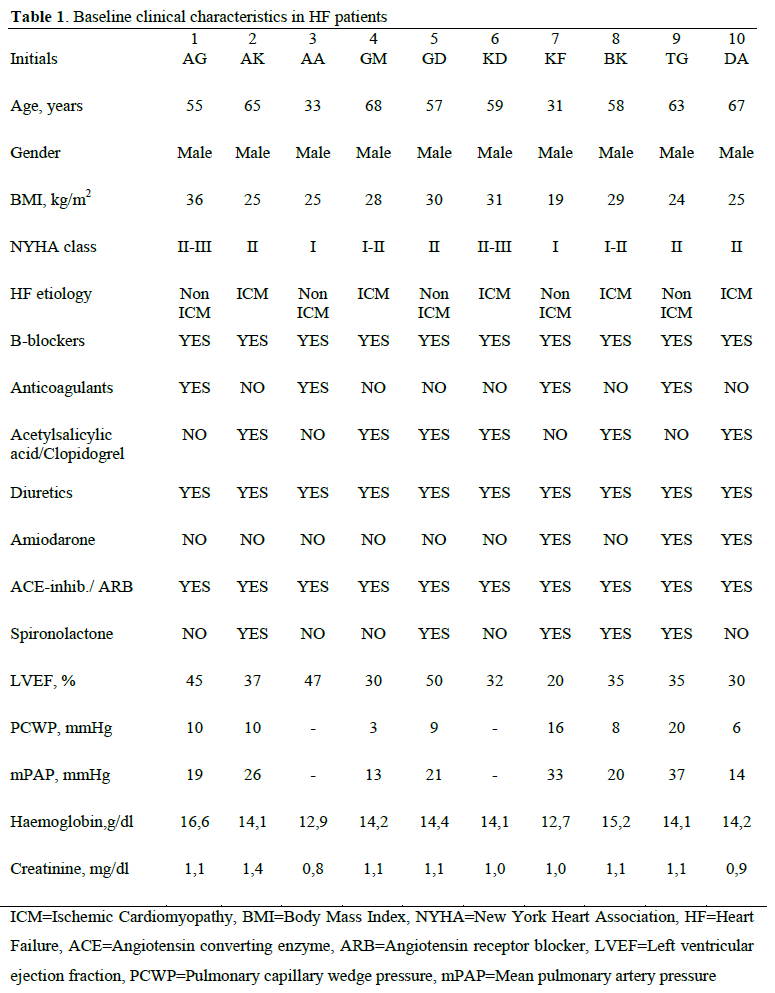
Design of the study
All patients underwent exercise endurance training on electro-magnetically braked cycle ergometers (Cateye Ergociser, EC1600; Tokyo, Japan) 3 times per week, for a total of 36 sessions. Patients participated in three different protocols:
1) Three patients were assigned to a high intensity interval training, exercised for 3 min at 50% of oxygen uptake at peak exercise (VO2p) followed by 4 cycles alternating 4 min of exercise at 80% of VO2p with 3 min at 50% of VO2p for a total duration of 31 min (Modified Wisloff protocol [10] ).
2) Four patients were assigned to a high intensity interval training combined with strength training, exercised for 3 min at 50% of VO2p followed be 2 cycles alternating 4 min of exercise at 80% of VO2p with 3 min at 50% of VO2p for a total duration of 17 min combined with 14 min of strength training consisting of 4 sets of 10-12 repetitions at 60-70% of one repetition maximum for the hamstrings and quadriceps.
3) Three patients were assigned to interval exercise training, exercised at the 100% of WRp, alternating 30 sec of exercise with 30 sec of rest, for 40 min.
Cardiopulmonary Exercise Testing
After 2 minutes of rest followed by 3 minutes of unloaded pedaling, subjects performed a ramp-incremental exercise test, with individual work rate increments calculated using the equation published by Hansen et al [16] , to aim for a test of 8 to 12 minutes duration. WRp was defined as the highest work rate reached and maintained at a pedaling frequency of no less than 50 rounds per minute for 30 seconds. Gas exchange was studied with the patient breathing through a low resistance valve, with a model Cosmed (Cosmed, Quark PFT, Italy) calibrated with a known gas mixture before each test. Oxygen uptake (VO2), carbon dioxide output (VCO2) and ventilation (VE) were measured breath-by-breath at rest, throughout exercise, and for the first 5 minutes of recovery. Baseline VO2 was the average of measurements for 2 minutes before exercise, with the patient seated. Peripheral oxygen saturation was monitored continuously by pulse oxymetry. Heart rate and rhythm were monitored and blood pressure was measured every 2 minutes with a mercury sphygmomanometer. All patients were verbally encouraged to exercise to exhaustion, as defined by intolerable leg fatigue or dyspnea. Respiratory exchange ratio was also calculated.
Cardiopulmonary Measurements
The gas exchange measurements served to calculate VO2 at peak exercise (VO2p, mL/kg/min), and at anaerobic threshold (AT, mL/kg/min). The ventilatory response to exercise was calculated by the method of the least squares linear regression analysis, as the slope of VE vs. VCO2 (VE/VCO2 slope) from the beginning of exercise to anaerobic threshold, when the relationship is linear, using an appropriate computerized statistical program. [17] The peak values for VO2, VCO2 and VE were calculated as the average of measurements during the 20-second period before exercise was terminated. AT was determined using the V-slope technique [18] and the result was confirmed graphically from a plot of ventilatory equivalent for oxygen (VE/VO2) and carbon dioxide (VE/VCO2) against time as well as pressure of end tidal O2 (PetO2) and pressure of end tidal CO2 (PetCO2) . In order to examine oxygen uptake kinetics during the early recovery phase, the first-degree slope of oxygen uptake for the first minute of recovery (VO2/t-slope) was calculated by a linear regression model using an appropriate computerized statistical program. [17]
Skeletal muscle biopsies
Percutaneous needle biopsies of the vastus lateralis muscle obtained within a week, before and after the completion of the program, but were separated from any type of exercise by at least 24 hours. The biopsies were performed at mid-thigh under local anesthesia with a method described by Bengstrom. [19] Samples were placed in embedding compound and immediately frozen in isopentane precooled to its freezing point. All samples were kept at -80°C until the day of analysis.
Fiber type distribution and size. Cryostat transverse sections were cut at -20°C and were stained for myofibrillar adenosine triphosphatase after pre-incubation at pH values of 4.3, 4.7, and 10.4. Muscle fibers were determined as type I, slow twitch and type II, fast twitch. The specimens were examined using an Olympys/BX41 microscope and were photographed and analyzed with a program (analysis-getIT, 5.1). Determination of each muscle fiber type and the number of muscle fibers of each type was counted visually, as described previously [3] , by a single blinded expert clinician. The muscle fiber cross section area (CSA) was determined with a computerized digitizing program (ImageJ, 1.45s, Wayne Rasband, NIH) by drawing along the edges of the fibers. Regions of a section were excluded from analysis if they contained oblique (which may overestimate CSA) or histologically abnormal fibers.
Statistical Analysis
All continuous variables are presented as mean±SD. Before analysis, all continuous variables were tested by Kolmogorov-Smirnov test for normal distribution. Individuals’ changes were compared by paired sample t-test and Wilcoxon signed rank test when there was non-normal distribution of data. The level of significance was set at P<.05. Correlations between variables were tested by Pearson’s correlation coefficient.
All statistical computations were made by the SPSS statistical package, version 20.0.0 (IBM SPSS Statistics, New York City, NY).
Results
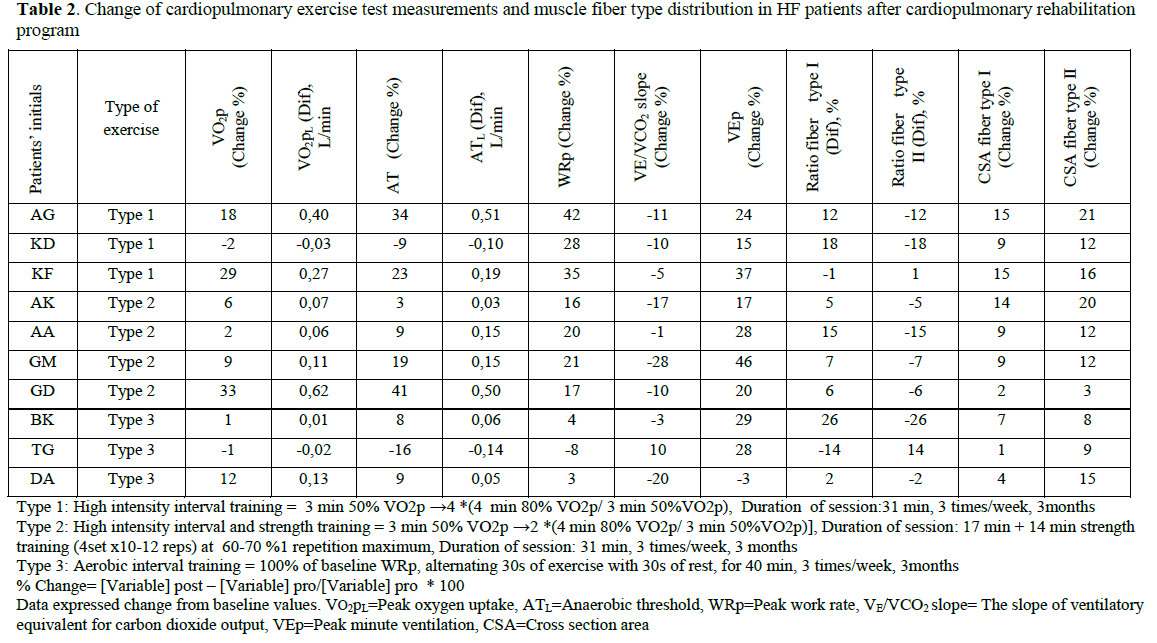
All patients finished the exercise training protocol without presenting with any adverse event. After 3 months of exercise training CSA of muscle fiber type I increased from 4509±1184 to 4862±1154 μm2 (p<0.001) and fiber type II from 4408±1166 to 4937±1160 μm2 (p<0.001). Aerobic type I muscle fibers increased significantly from 43±11 to 51±6 (p=0.05).
The percentage change of CSA of type I fibers was significantly correlated with the percentage change of WRp (r=0.814, p=0.004, Fig.1).
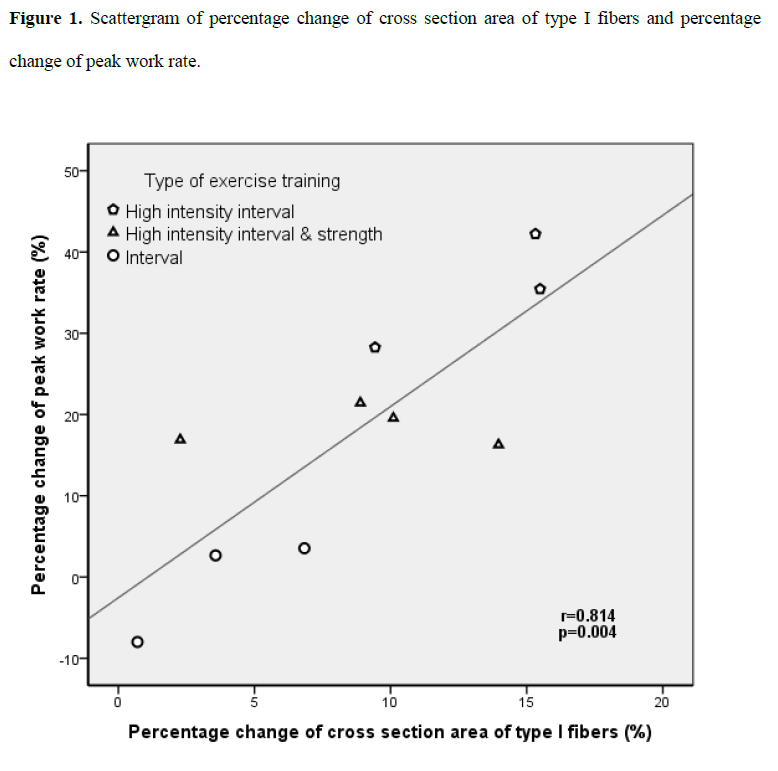
Figure 1: Scattergram of percentage change of cross section area of type I fibers and percentage change of peak work rate.
WRp, VO2p, AT, VEp and VO2/t-slope increased significantly (from 108±36 to 129±49 watts, p=0.008; from 17.7±4.2 to 19.7±4.6 ml/kg/min, p=0.024; from 12.0±3.3 to 13.5±4.1 ml/kg/min, p=0.05; from 67±20 to 83±25 l/min, p<0.001; from 0.61±0.29 to 0.76±0.43 L/min2, p=0.038; respectively). VE/VCO2 slope decreased significantly (from 35.1±8.0 to 31.3±5.7, p=0.045).
The changes of CPET measurements and skeletal muscle fiber distribution and CSA in HF patients according to the exercise training program are shown in Table 2.
Discussion
This case study has shown that exercise training improves skeletal muscle fiber-type distribution and exercise capacity in patients with HF. Specifically, CSA of both types of muscle fibers increased and type I fibers’ proportion increased in patients that participated in interval exercise training protocols. There was also a significant correlation between the increase of type I fibers’ CSA and increase of WRp.
The increase of CSA has been previously shown even with low intensity exercise training programs. [5] This finding is important for patients suffering from HF, since it implies that exercise can possibly counteract the excessive muscle atrophy presenting in this group of patients. The increase of CSA after exercise training indicates that exercise might have anabolic effects possibly via insulin-like growth factor 1 specific actions, as it is already known. Exercise training improves local insulin-like growth factor 1 expression without significant changes of systemic parameters of the growth hormone/insulin-like growth factor 1 axis. [20]
In our study exercise training induced a reshift to aerobic type I fibers of HF patients. A previous study by Hambrecht et al [3] has shown that continuous exercise training reversed the fiber type distribution of HF patients. The interval exercise training has been shown to increase percentage of type I fibers in an animal study by Luginbuhl et al. [21] However, this is the first study to our knowledge to demonstrate that interval exercise training might change fiber type distribution in skeletal muscle of HF patients.
Muscle fibers are characterized as type I or II according to their oxidative capacity and therefore the increase of type I fibers probably means increased oxidative capacity of skeletal muscle after exercise training. This finding could be primarily explained by the increased oxidative capacity of mitochondria [3-5] and by the improved mitochondrial function as indicated by the study of Wisloff et al [10] , which showed that the protein levels of PGC-1a (factor coordinating mitochondrial biogenesis) and the maximal rate of Ca2 reuptake into sarcoplasmatic reticulum by sarco/endoplasmic reticulum Ca2+-ATPase were increased after high intensity aerobic interval training. This increase was correlated with the improvement in VO2p. Our finding could also be explained via the improved endothelial function [14] and microcirculation [11] of skeletal muscle after exercise training.
The training-induced increase in fibers’ CSA area has been also found in relation to patients’ aerobic capacity after resistant exercise training. [22] In a previous study held in our Institute [13] we demonstrated that the addition of strength training to an aerobic interval training protocol induced greater muscle strength without lean muscle mass changes. By the findings of the present study, it seems that muscle strength improvement could be due to skeletal muscle fiber CSA changes.
The correlation between the increase of type I fibers’ area and the increase of WRp after aerobic exercise training might be partially explained by the fact that the improvement of functional capacity is mainly induced by the improvement of intrinsic muscular oxidative capacity. The increased muscle fiber area could contribute to the increased oxidative content of skeletal muscle. It has been previously shown that changes in skeletal muscle oxidative capacity are correlated with changes in exercise capacity. [1] Additionally, aerobic capacity is correlated to type I fibers’ percentage [2] and improvement of functional capacity after exercise training has been associated with changes in skeletal muscle oxidative capacity as assessed by cytochrome c oxidase-positive mitochondria [4] , by ultrastructure [3] and volume density of mitochondria [5] and by factors associated with the biogenesis of mitochondria. [10] The increased type I fibers’ CSA that was found in our study might contribute to increased oxidative capacity of muscle fiber and therefore increased functional capacity as assessed with WRp.
In our study type II fibers’ area increase was not correlated to the increase of WRp after exercise training. In a previous study was noted an inverse correlation between type IIb fibers and VO2p, while type I fibers were correlated with VO2p2 and thus aerobic fibers seemed to mainly affect aerobic capacity of HF patients.
This study indicates also that exercise training improves the aerobic capacity of HF patients. This finding is in agreement with earlier studies demonstrating the beneficial effects of exercise training on functional capacity, regardless of the different types of exercise that were investigated. [3-15,22]
The main limitation of this study was the small number of patients which does not allow comparison between the three different types of exercise training. Further, larger, randomized studies are needed to confirm the preliminary findings of this case study. Another limitation of the study is that all participants were males limiting conclusions for female population.
In conclusion, this study implies that interval exercise training improves the exercise capacity and could partially reverse skeletal muscle fiber-type distribution of HF patients. High intensity interval training constitutes a training modality for HF patients, which possibly induces pronounced beneficial skeletal muscle changes.
2762
References
- Drexler H, Riede U, Münzel T, König H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 1992; 85(5): 1751-1759.
- Mancini DM, Coyle E, Coggan A, Beltz J, Ferraro N, Montain S, et al. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation 1989; 80(5):1338-1346.
- Hambrecht R, Fiehn E, Yu J, Niebauer J, Weigl C, Hilbrich L, et al. Effects of Endurance Training on Mitochondrial Ultrastructure and Fiber Type Distribution in Skeletal Muscle of Patients With Stable Chronic Heart Failure. J Am Coll Cardiol 1997; 29(5): 1067-1073.
- Hambrecht R, Niebauer J, Fiehn E, K?lberer B, Offner B, Hauer K, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol 1995; 25(6): 1239-1249.
- Belardinelli R, Georgiou D, Scocco V, Barstow T, Purcaro A. Low Intensity Exercise Training in Patients With Chronic Heart Failure. J Am Coll Cardiol 1995; 26(4): 975-982.
- Dimopoulos S, Anastasiou-Nana M, Sakellariou D, Drakos S, Kapsimalakou S, Maroulidis G, et al. Effects of exercise rehabilitation program on heart rate recovery in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 2006; 13(1): 67-73.
- Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA 2000; 283(23): 3095-101.
- Piepoli MF, Davos C, Francis DP, Coats AJ; ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004; 328(7433): 189.
- Meyer K, Samek L, Schwaibold M, Westbrook S, Hajric R, Beneke R, et al. Interval training in patients with severe chronic heart failure: analysis and recommendations for exercise procedures. Med Sci Sports Exerc 1997; 29(3): 306-312.
- Wisl?ff U, St?ylen A, Loennechen JP, Bruvold M, Rognmo ?, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007; 115(24): 3086-3094.
- Gerovasili V, Drakos S, Kravari M, Malliaras K, Karatzanos E, Dimopoulos S, et al. Physical exercise improves the peripheral microcirculation of patients with chronic heart failure. J Cardiopulm Rehabil Prev 2009; 29(6): 385-391.
- Tasoulis A, Papazachou O, Dimopoulos S, Gerovasili V, Karatzanos E, Kyprianou T, et al. Effects of interval exercise training on respiratory drive in patients with chronic heart failure. Respir Med 2010; 104(10): 1557-1565.
- Bouchla A, Karatzanos E, Dimopoulos S, Tasoulis A, Agapitou V, Diakos N, et al. The addition of strength training to aerobic interval training: effects on muscle strength and body composition in CHF patients. J Cardiopulm Rehabil Prev 2011; 31(1): 47-51.
- Anagnostakou V, Chatzimichail K, Dimopoulos S, Karatzanos E, Papazachou O, Tasoulis A, et al. Effects of interval cycle training with or without strength training on vascular reactivity in heart failure patients. J Card Fail 2011; 17(7): 585-591.
- Smart NA, Dieberg G, Giallauria F. Intermittent versus continuous exercise training in chronic heart failure: A meta-analysis. Int J Cardiol 2011; In press.
- Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Resp Dis 1984; 129(2 pt 2): S49-55.
- Nanas SN, Terrovitis JV, Charitos C, Papazachou O, Margari Z, Tsagalou EP, et al. Ventilatory response to exercise and kinetics of oxygen recovery are similar in cardiac transplant recipients and patients with mild chronic heart failure. J Heart Lung Transplant 2004; 23(10): 1154-1159.
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986; 60(6): 2020-2027.
- Bengstrom J. Muscle electrolytes in man: determination by neutron activation analysis on needle biopsy specimens; a study on normal subjects, kidney patients and patients with chronic diarrhea. Scand J Clin Lab Invest 1962; 14: 1-110.
- Hambrecht R, Schulze PC, Gielen S, Linke A, M?bius-Winkler S, Erbs S, et al. Effects of exercise training on insulin-like growth factor-I expression in the skeletal muscle of non cachectic patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 2005; 12(4): 401-406.
- Luginbuhl AJ, Dudley GA, Staron RS. Fiber type changes in rat skeletal muscle after intense interval training. Histochemistry 1984; 81(1): 55-58.
- Tyni-Lenn? R, Jansson E, Sylv?n C. Female related skeletal muscle phenotype in patients with moderate chronic heart failure before and after dynamic exercise training. Cardiovasc Res 1999; 42(1): 99-103.








