Abstract
Introduction: Medicinal plants play a central role in the healthcare system of the large proportions of the world’s population. Plant derived compounds are an important source of several clinically useful anticancer agents. Cytotoxicity screening models are the preliminary methods for the selection of active extracts against cancer.
Materials and methods: A comprehensive study was carried out to determine the Cytotoxic activity of active extracts of the plant-P. vulgaris, T. buccatta against human lung cancer (A549) cell line at eight different concentration to determine the IC50 (50% growth inhibition) by MTT assay including morphological study by fluorescence microscopy, Apoptosis by Annexin V, caspase 3-7 assay and levels of expression of genes. Each sample was assayed in triplicate and control samples include cells without plant extracts.
Results: Results demonstrate that the percentage of growth inhibition increases with increasing concentrations of test compounds. The P. vulgaris extracts induced significant cytotoxic effects on the A549 cancer cell line and these effects were stronger than the other selected plant extracts reflecting a promising way of chemotherapy to counter the spread of non- small cell lung cancer.
Conclusion: Extract which exhibit substantial antipoliferative and apoptic activity may represent a source for novel natural anticancer entities.
Keywords
A549; P. vulgaris; T. buccatta
Introduction
Over the decades, herbal medicine has become a topic of
global importance, making an impact on both world health and
international trade. Medicinal plants continue to play a central
role in the healthcare system of large proportions of the world’s
population. This is particularly true in developing countries,
where herbal medicine has a long and uninterrupted history of
use. Recognition and development of the medicinal and economic
benefits of these plants are on the increase in both developing and
industrialized nations. Continuous usage of herbal medicine by
a large proportion of the population in the developing countries
is largely due to the high cost of western pharmaceuticals and
healthcare.
Among the human diseases treated with the medicinal plants
is cancer. Globally, cancer represents a substantial burden of
disease in the community and appears to be a prime cause of
concern. Cancer is a third leading cause of mortality and it
strikes more than the one third of world’s population and it is
the cause of more than 20% of all deaths [1]. Cancer is depicted as a disease when cell proliferation and differentiation becomes
uncoupled and damage to numerous regulatory genes resulting
in the development of invasive and metastatic cancer which is
the culmination of the chronic disease process, carcinogenesis
[2]. According to the International Agency for Research on Cancer
(2002), cancer killed >6.7 million people around the world and
another 10.9 million new cases were diagnosed [3]. If the results
are extrapolated, at the same rate, an estimated 15 million people
will have cancer, annually, by 2020. According to an estimate given
by American Cancer Society (2009), about 1,500,000 new cases
and over 500,000 deaths are expected in the US by 2009. Every
year over 200,000 people are diagnosed with cancer in the United
Kingdom only, and approximately 120,000 die as an aftermath of
the disease (Department of Health, 2000). The National Cancer
Registry of South Africa has spotted the cancers of bladder,
colon, breast, cervix, lungs and melanoma commonly among
inhabitants [4]. In India around 55000 people died of cancer in 2010, according to eternals in the Lancet today (March 2012) as
stated by the International Agency for Research on cancer (IARC).
Parts of India have the world’s highest incidence of cancers of
gall bladder, mouth and lower pharynx, India’s first cancer atlas
shows. Among the states of India, J&K also shows the highest
incidence of cancer [5-8 ].
Among this dreadful disease Lung cancer is the leading cause
of cancer deaths and its incidence continues to rise worldwide.
Small-cell lung cancer makes up to about 25% of lung cancer
cases and is characterised by a rapid and aggressive clinical
course. Any practical solution in combating this dreadful disease
is of paramount importance.
Taxanes are the most recently solicited chemotherapeutic drugs
of our era. During the past decades, these unique hydrophobic
mitotic inhibitors have been thoroughly investigated through
numerous experimental and clinical trials which have brought
hope in breast, ovarian, lung, prostate [9 ,10], pancreas, gastric
[11,12] and head and neck [13] cancer treatments.
In brief, the taxanes mainly group Paclitaxel (Taxol) and Docetaxel
(Taxotere) as well as taxanes homologs, which are derived
from natural sources; taxol [14] is derived originally from Tagus
Brevifolia (bark of Pacific yew/Western yew conifers) while
Docetaxel is a semisynthetic analogue of the latter; an esterified
derivative of 10-deacetylbaccatin-III (10-DAB) extracted from
Taxus Baccata [15 ] (needles of European yew tree). During course
of time due to poor oral bioavailability, solubility and numerous
side effects; views for the development of new similar antimitotic
have been encouraged and brought to light. Moreover,
since there have been numerous multidrug resistance (MDR) [16]
in patients, combination therapies are preferred over single drug
therapy and Texans also known to be having a radio sensitizing
[13] effect have proved to be helpful in the palliative treatment
of patients if not in the curative one. After an initial response
to chemotherapy, most small cell lung cancers relapse in a drug
resistance form, leading to a 2 year survival of only 5% [1]. The
emergence of resistance to chemotherapeutic agents remains
a major problem in the treatment of patients with small-cell
lung cancer, despite the fact that these patients usually have a
good initial response to chemotherapy. Although a number of
genetic alterations associated with drug resistance in small-cell
lung cancer are known, including the deregulated expression of
proteins involved in drug transport and activity [17], and in cell
cycle checkpoint control [18], few advances have been made in
improving the therapeutic options.
Despite, their side effects related mostly to their vehicles
[19], they remain one of the most acceptable treatments for
metastatic breast [20], ovarian, prostate and lung carcinomas.
The list for laboratory experiments and clinical oncological
trials is very long concerning taxanes; but their outcome is of
tremendous eagerness in the cancer field. An alternative solution
to western medicine embodied with severe side effects is the use
of medicinal plant preparation to arrest the insidious nature of
the disease. Attempts are underway to work out the therapeutic
and anti-neoplastic properties of medicinal plants [21-26]. A
recent review of Butler lists seventy natural products or natural
product derivatives currently undergoing trials in United States,
Europe, Japan and Korea [27 ]. Out of seventy compounds twenty
eight were plant derived.
There are about 45,000 plant species in India, with concentrated
hotspots in the region of Eastern Himalayas, Western Ghats and
Andaman & Nicobar Island. The officially documented plants
with medicinal potential are 3000 but traditional practitioners
use more than 6000. India is the largest producer of medicinal
herbs and is appropriately called the botanical garden of the
world [28]. There are currently about 250 000 registered medical
practitioners of the Ayurvedic system (total for all traditional
systems: approximately 291 000), as compared to about 700,000
of the modern medicine system. In rural India, 70 per cent of the
population is dependent on the traditional system of medicine,
the Ayurveda [29]. In India, the J&K region is endowed with a wide
range of physiography, climate, soil and biological wealth. The
region is one of the richest reservoirs of biological diversity in the
world and is considered as a store house of the valuable medicinal
plant species [30,31]. Cytotoxicity screening models are the
preliminary methods for selection of active plant extracts against
cancer [32]. Consequently; herbal medicines have received much
attention as substitute anticancer drugs. The current research
proposal will address screening, characterization and therapeutic
evaluation of Indigenous Kashmiri medicinal plant extracts as
anti-cancer agents against different human cancer cell lines in an
in vitro model system. Once these extracts found to have anticancer
activity they will be further evaluated to characterize
active compounds, subsequently effective formulations will be
developed in future.
Scanty reports are available asserting the use of Kashmiri
medicinal plants as anticancer agents. Keeping in view the same,
the present study aims to investigate the efficacy of indigenous
Kashmiri medicinal plants (Primula vulgaris, Hippophae, Rubus
armeniacus, Arctium lappa) in ascertaining the anti-cancer
activity of aqueous and organic solvent extracts on various
human cancer cell lines.
Methodology
Preparation of extracts
Aqueous extraction: Whole plants of P. vulgaris var. lilacina,
Taxol buccatta, Articum Lappa were collected from the forests of
the Ramban & Bohrihallan, Karewa, and area of J&K, identified
at J&K Medicinal Plant Introduction Centre. The plant material
of P.vulgaris var. lilacina, Taxus buccatta, Articum Lappa was cut
to increase the surface area, and then dried in an oven at 40°C
overnight. The dried plant material was blended to a powder
of 80-mesh particle size and stored at -70°C. 10 g was weighed
out and mixed with 500 ml distilled water. This mixture was
then stirred for 16 hr. The supernatant containing the extracted
secondary metabolites was then removed and extracted through
percolation & was repeated twice on the same plant material.
Each fraction was filtered through Whatman filter paper No.
2 (Advantec, Tokyo, Japan). Subsequently, the filtrates were
combined and evaporated under a vacuum and then lyophilized
with a freeze dryer (Ilshine Lab, Suwon, Korea) at -70°C under
reduced pressure (< 20 Pa). The dry residue was stored at
-20°C. For further analysis, we reconstituted the dry extract and fractions with DMSO to produce a 40 mg/ml stock solution that
was stored at-20°C.
Cells and cell culture
A549 (Human lung cancer cell lines) were procured from NCCS
Pune. Cell lines were grown in 25 cm² & 75 cm²tissue culture
flasks (Corning, Sigma Aldrich) containing 10% feotal bovine
serum (Sigma Aldrich), penicillin (100 IU/ml) and streptomycin
(100 μg/ml) in Standard incubator at 37°C, 5%CO2 and 90%
humidity in RPMI -1640 medium (Sigma Aldrich USA), The
cells were grown confluent, which could be observed under an
inverted microscope and sub – cultured at three to four days
interval. Each extract (initially dissolved in DMSO), was diluted
with the medium and passed through a 0.2 μm filter. 10 μg/ml
of each extract was tested initially, and, from the results, the
active extracts were considered to be those which gave less
than 50% survival at exposure time 72 hours. The active extracts
were further diluted in medium to produce eight concentrations
(control, 1 μg/ml, 2 μg/ml, 5 μg/ml, 10 μg/ml,) of each extract.
100 μl/well of each concentration was added to the plates in six
replicates. The final dilution used for treating the cells contained
not more than 1% of the initial solvent, this concentration being
used in the solvent control wells. The plates were incubated for
24 hours [33].
MTT cytotoxicity assay
All cell lines were trypsin zed and counted using haemocytometer
then were seeded in 96-well micro plate at 5×105 cells/ml and
then incubated at 37°C in 5%CO2 to allow cells attachment.
The medium was removed and replaced with fresh medium
containing various concentrations of plant extracts starting with
the highest concentration of 1 mg/ml (eight folded dilution). Cells
were incubated at 37°C, 5%CO2 for 48 hours. Each concentration
was assayed in triplicates (n=3). Twenty-four hours later, 20 μl of
MTT (5 mg/ml) solution was added to each well and then the
plate was further incubated for 4 h. All remaining supernatant
were removed and 200 μl of DMSO was added to dissolve the
formed crystal Formosan. MTT assay reading was performed
using ELISA plate reader (Tecan 200, USA) [34].
The MTT cell proliferation assay
To confirm anti-proliferative effects of plant extracts on A549
cells, MTT cell proliferation assay was carried out. In this assay,
two different concentrations of compound with cells were
prepared together with control. The concentration chosen were
IC25 and IC50 concentrations (1 and 1.5 μg/ml). Each sample was
assayed in triplicate, and control samples include cells without
plant extracts. The cells were treated by different extracts for 24
hours. At the end of incubation periods, 20 μl of MTT solution (5
mg/ml MTT dissolved in PBS) were added to each well containing
cells and the plate was incubated at 37°C in an atmosphere of
5%CO2 for 4 hours. After that, most of the medium was removed,
then a volume 200 μl of DMSO (dimethyl sulfoxide) was added
into the wells to soluble the crystals. Finally the absorbance was
measured by ELISA reader at a wavelength of 570 nm. Graphs
(OD of samples against time) were plotted to determine the
growth rates of cells in a given values.
Cell multiplication study
Exponentially growing A549 cells were seeded at 1.3×104 cells/
ml in RPMI in six well culture plates. After 24 hours, the cells were
treated with control, 1 μg/ml, 2 μg/ml, 5 μg/ml, 10 μg/ml) of each
extract. To be sure that nutrient depletion would not be a factor
in cell growth inhibition, the medium and drug were removed
and replaced with fresh RPMI and drug on each day of the study.
Cell number was determined after 24, 48, 72, and 96 hours using
the Trypan Blue exclusion method. Control and treated cells were
photographed directly in the culture plate using an inverted light
microscope equipped with a Nikon camera [35].
Alcidine orange (AO) propodium iodide (PI)
double staining using fluorescent microscopy
A549 cells were quantified using propodium iodide (PI) and
alcidine orange (AO) double staining according to standard
procedures and examine under fluorescence microscope (Lieca
attached with Q-Floro Software). Cell suspension was mixed with
an equal volume of staining solution (1:1) containing 10 μg/ml
propidium iodide and 10 μg/ml alcidine orange (dissolved in PBS)
and observed under fluorescence microscope within 30 minutes.
The viable (green intact cells), apoptotic (green shrinking cells
with condensed of fragmented nucleus), and necrotic (red cells)
were the morphological changes that were examined under
fluorescence microscope.
A549 cells were seeded in six –well plate and incubated at 37°C
in 5% CO2 atmosphere. Twenty –four hours later, the medium in
each well was removed and replaced with the selected desired
drug at IC50 concentration dissolved in medium and incubated
at 37°C in 5% CO2 atmosphere for 24, 48 and 72 h. After
incubation period, Cells suspension was mixed with an equal
volume of staining solution (1:1) containing 10 ug/ml acridine
orange and 10 ug/ml propidium iodide (dissolved in PBS) and
observed under fluorescence microscope within 30 minutes.
The viable (green intact cells), apoptotic (green shrinking cells
with condensed of fragmented nucleus), and necrotic (red cells)
were the morphological changes that were examined under
fluorescence microscope (Leica Germany) [36]. Each experiment
was assayed three times (n=3) to provide a useful quantitative
evaluation. Viable, apoptotic, and necrotic cells was quantified
in a population of 200 cells. The results were expressed as a
proportion of the total number of the cells examined.
Annexin V-FITC apoptosis detection
Invitrogen (USA) for apoptosis analysis. After being cultured for
2 days, the cells were treated with the P-60 in 125, 250 and 500
μg crude drug/mL and then maintained at 5% CO2 and 37°C for
48 h. These cells were detached with 0.25% trypsin-0.01% EDTA
solution and centrifuged at 2000 × g for 5 min. After removing
supernatant, the cells were washed twice with phosphate
buffered solution (PBS, pH =7.4) and centrifuged at 2000 × g
for 5 min to collect 5 × 105 cells. Cells were stained with 5 μL
annexin V-FITC and 5 μL propidium iodide according to the
manufacturer’s instructions of V-FITC apoptosis detection kit.
Then the cells samples were detected by using a flow cytometer
(Beeton-Diekinson USA) with fluorescence excitation wavelength at 488 nm and emission wavelength at 530 nm [37].
Cell cycle analysis
SPC-A-1 cells (4×104 cells/mL) were seeded in 25 cm2 ask for cell
cycle distribution analysis. The cells were treated with various
concentrations of P-60 (125, 250 and 500 μg crude drug/mL)
for 48 h and then detached by using 0.25% trypsin-0.01% EDTA
solution. Cell suspension was mixed with 70% ethanol (v/v) for 2
h and washed in PBS, then added with 100 μL RNase A (1 mg/mL)
and heated in a warm bath at 37°C for 30 min. The cells were then
stained with 400 μL propidium iodide (50 μg/mL) and incubated
in the dark at room temperature for 30 min. The samples
were detected by flow cytometry with fluorescence excitation
wavelength at 488 nm and emission wavelength at 530 nm. Data
from 10,000 cells were collected for each data [38].
Caspases Glo 3/7 assay
The evaluation of caspase-3/7 was performed according to the
manufacturer’s instructions. Briefly, 100 signal recorded with the
Glo Max-Multi Detection System (Promega, USA) after incubation
for 1 hour. ��L of 5 × 1 cells/mL was seeded walled in white walled
96-well micro plates and incubated for 24 h. The cells were
treated with the eight-fold dilution of the IC 50 values of the
selected plant extracts for 24 h. After treatment, an equal volume
of Caspase-Glo 3/7 reagent was added and agitated for 30 sec
and the luminescence [39 ].
Real-time reverse transcription polymerase
chain reaction analysis (RT-PCR)
To determine the expression levels of p53, Bax and Bcl-2 RT-PCR
was performed using a Qiagen Rotor –Gene Q real time thermal
cycler (Valencia, CA, USA) in accordance with the manufacturer’s
instructions. The cells were treated with P. vulgaris, T. buccatta,
A. lappa extracts and cultured for 24 h, 48 h. Thereafter, cDNA
was synthesized from the total RNA isolated from cells. The
PCR reaction was performed using 2x SYBR Green mix (Qiagen,
Valencia, CA, USA). All results were normalized to beta actin
expression. The following primer sequence were used for the
real-time PCR: GAPDH, 5’- CGG AGT CAA CGG ATT TGG TAT-3’
(forward), 5’ AGC CTT CTC CAT GGT GGT GAA GAC -3’(reverse),
p53, 5’- GCT CGT ACT GTA CCA CCA TCC-3’ (forward), 5’- CTC
TCG GAA CAT GGT GGT GAA GAC-3’(reverse), Bax, 5’- ATG GAC
GGGTCC GGG GAG-3’(forward),5’TCA GCC CAT CTT CTT CCA-
3’(reverse), Bcl-2, 5’-CAG CTG CAC CTG ACG-3, (forward), 5’-ATG
CAC CTA CCC AGC-3’(Reverse) .
Statiscal analysis
Experimental results are expressed as mean ± SEM. All
measurements were replicated three times. The data were
analyzed by an analysis of variance (P < 0.05). The IC50 values
were calculated from non-linear regression analysis.
Results
Cytotoxic activity of three plants extracts were carried out against
A549 cell line at different concentrations to determine the
IC50 (50% growth inhibition) by MTT assay. Results of different
concentrations of plant extracts including (control, 1 μg/ml, 2 μg/
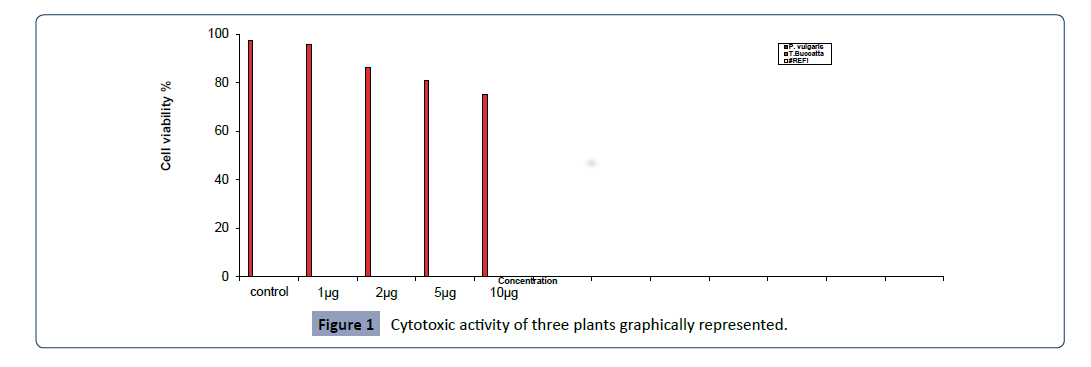
ml, 5 μg/ml, 10 μg/ml) are tabulated in Figure 1, and graphically
represented in Figure 1.
Figure 1: Cytotoxic activity of three plants graphically represented.
MTT assay of primula vulgaris L. shows significant effect on
A549 cell in concentration range between 1 μg/ml to 5 μg/ml,
compared with control. MTT assay of primula vulgaris L. shows
significant effect on A549 cell in concentration range between 1
μg/ml to 5 μg/ml, compared with control. The highest cytotoxicity
of this extract against A549 cell was found in 1 μg/ml and 1.5 μg/
ml concentrations with 51.45 and 56.49 percent of cell growth
inhibition. It was found that the percentage of growth inhibition
to be increasing with increasing concentration of test compounds
and IC50 value of this assay was 1.01 μg/ml, however the IC50 of
Taxus bucatta extract was about 0.1 μg/ml & the Paclitaxel with
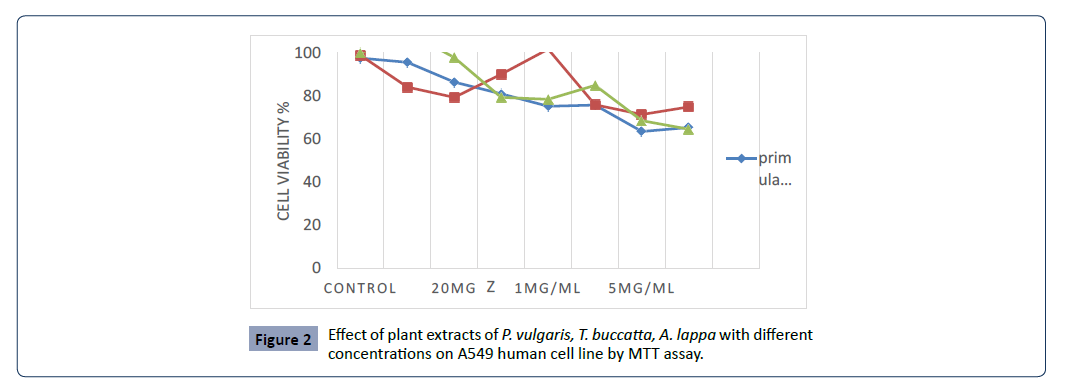
IC 50 0.01 μg/ml , a comparative analysis as shown in Figure 2.
Figure 2:Effect of plant extracts of P. vulgaris, T. buccatta, A. lappa with different
concentrations on A549 human cell line by MTT assay.
Cytotoxic activity of three plants extracts were carried out against
A549 cell line with IC50 (50% growth inhibition) to determine
the morphological, apoptotic changes, mRNA expression by the
following methods- acridine orange (AO)/propodium iodide (PI)
double staining fluorescence microscope, caspase 3-7 activity
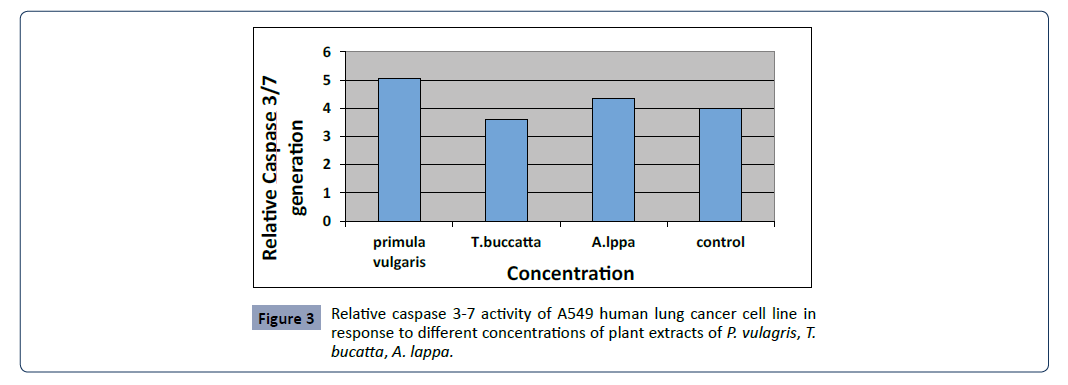
assay, real time RT-PCR as given in Figures 3 and 4.
Figure 3:Relative caspase 3-7 activity of A549 human lung cancer cell line in
response to different concentrations of plant extracts of P. vulagris, T.
bucatta, A. lappa.
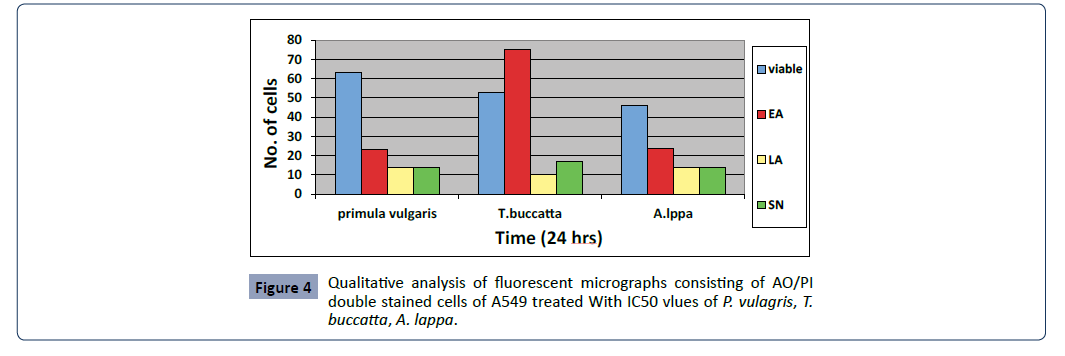
Figure 4: Qualitative analysis of fluorescent micrographs consisting of AO/PI
double stained cells of A549 treated With IC50 vlues of P. vulagris, T.
buccatta, A. lappa.
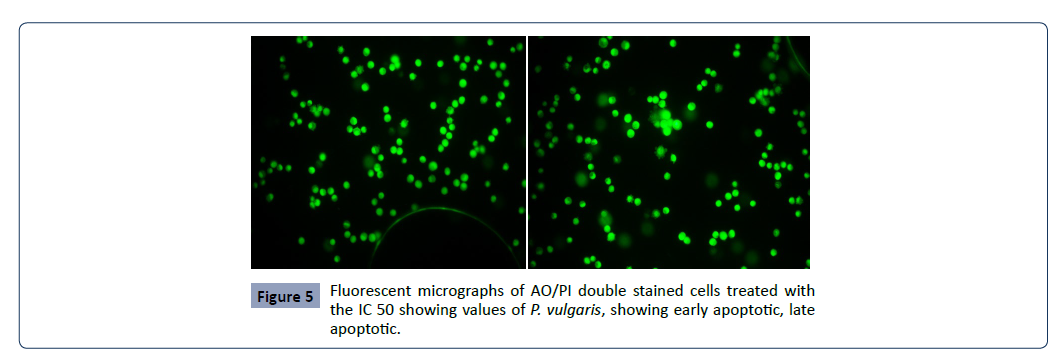
Fluorescent microscopy was conducted to study morphological
changes of cell death mode induced by P. vulgaris, A. lappa, T.
buccatta extracts, Paclitaxel after 12, 24, 48 and 72 h. Viable cells
displayed green fluorescence with the appearance of circular
cell; intact nucleus. The early apoptotic cells have fragmented DNA, which gives several green coloured nuclei and cell blebbing.
Late apoptotic and necrotic cell’s DNA would be fragmented and
stained orange red. No necrosis was obtained in P.Vulagris but the
apoptotic effect was significantly observed however necrosis was
attained by Taxus buccatta, paclitaxel. The percentage of apoptotic
cells in untreated cells slightly increased from 0.06% after 24 h to
5.33% and 7% after 48 and 72 h, respectively. The percentage of
apoptotic cells increased rapidly from 37% after 24 h to 53% and
63% after 48 h and 72 h respectively as given in Figure 5.
Figure 5: Fluorescent micrographs of AO/PI double stained cells treated with
the IC 50 showing values of P. vulgaris, showing early apoptotic, late
apoptotic.
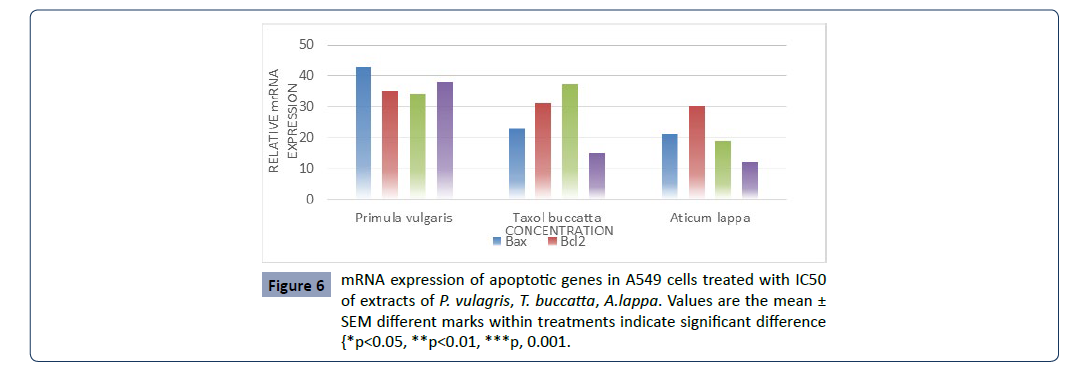
The expression of genes associated with apoptotic cell death,
including the tumor suppressor p53, pro-apoptotic Bax, the antiapoptotic
Bcl-2 and Fas gene in A549 cells was determined by
RT-PCR. Results show that P. vulgaris, T. buccatta and A. lappa
extracts, significantly increased the expression of p53, Bax, Fas
compared to control. However the expression of Bcl-2 was not
decreased compared to that of the control (Figure 6).
Figure 6:mRNA expression of apoptotic genes in A549 cells treated with IC50
of extracts of P. vulagris, T. buccatta, A.lappa. Values are the mean ±
SEM different marks within treatments indicate significant difference
{*p<0.05, **p<0.01, ***p, 0.001.
Taken together, the P. vulgaris extracts were more effective in inducing apoptosis through the regulation of p53, Bax and Fas
expression.
Review of Literature
Taxanes currently known to suppress and inhibit cell growth,
differentiation and proliferation in indefinitely known cancer cell
Either it be in experimental or clinical trials, their mechanisms
related to decrease cell growth has been thoroughly appreciated
by everyone including patients in the oncological field [40].
Inspire of their excellent anti- tumor/anti-cancer activity an
emergence of resistance to chemotherapeutic agents remains
a major problem in the treatment of patients especially lung
cancer, despite the fact that these patients usually have a
good initial response to chemotherapy, as number of genetic
alterations associate d with drug resistance in lung cancer are
known, including the deregulated expression of proteins involved
in drug transport and activity [17], and in cell cycle checkpoint
control [18]. An alternate and more appropriate therapy needs
to be carried out to delineate & prevent chemo resistance. In
the present study, results indicate that docetaxel, paclitaxel and
prunella vulgaris induce cytotoxicity in cancer cells in a timeand
dose‐dependent manner, which is consistent with results
of previous studies [33-36] In addition several studies [37, 38]
Possible mechanisms suggested the inhibition of the c-Jun N-terminal kinase (JNK) pathway and the Akt pathway [39,40]. P.
vulgaris inhibits the proliferation of human cancer cell lines [41]
including human oesophageal cancer cell line Eca 109 liver cancer
cell line HepG2, cervical cancer Hela cell, and stomach cancer
MKN 45 cell line Ethanol extract of P. vulgaris was found to inhibit
colon cancer cell line HT-29 by arresting the cell cycle at the G1/S
checkpoint and reducing the expression of pro-proliferative cyclin
D1 and cyclin-dependent kinase 4 (CDK4) at the transcriptional
and translational level P. vulgaris has also been shown to have
combinatorial effects with other agents. P. vulgaris extracts
enhanced the effects of paclitaxel (TAX) and adriamycin (ADM) on
inhibiting cell growth of cancer cells A combination of P. vulgaris
and Cremastra appendiculata exhibited an enhanced effects in
inhibiting the growth of thyroid cancer cell line along with downregulation
of the c-myc expression.
In addition to P. vulgaris extracts, some of its ingredients have
also been examined for anti-cancer activities. Triterpenoicacids,
a component of P. vulgaris exhibited strong cytotoxic activity
against human lung cancer cell line A459 Triterpenoic acid isolated
from P. vulgaris has been shown to inhibit cell growths of various
human cancer cell lines, namely, A549 cell lines, SK-OV-3 (ovarian
cancer cell), SK-MEL-2 (skin melanoma), and HCT 15 (colon
cancer cell). Ursolic acid one of the most abundant triterpenoic
acids in P. vulgaris, showed inhibitory effect on colon cancer cell
lines HCT-15 and DLD-1. Ursolic acid also reduced proliferation in many other tumor cell lines, like human leukemic cell line HLmouse
melanoma cell line B16 human breast MCF7 Oleanic acid,
an active component of P. vulgaris inhibited the proliferation of
HT-29 cells in dose-dependent manner through the mechanism
of G0/G1 checkpoint arrest. Oleanolic acid also exhibited strong
anti-proliferation activity against human lung SPC-A-1 cells.
Regulation of cell cycle progression and cell cycle arrest
Taxanes achieve favourable apoptotic outcomes by ability of
binding to microtubules-stabilize them, inhibit depolymerisation,
interfere with the G2/M phases (64) which are achieved by
blocking the cell cycle during mitosis in the transition from
prometaphase to metaphase and hence, induce apoptosisprogrammed
cell death confirmed through cytometry studies
which is a crucial checkpoint in cancer treatment. Moreover,
they also initiate a whole cascade of cell death pathways P.
vulgaris has also been shown to induce cell cycle arrest at various
checkpoints in cancer cells. After thyroid carcinoma cell line
SW579 was treated with P. vulgaris, the proportion of cells in the
S phase was observed to be reduced, while those in the G0/G1
phase was significantly increased when compared to the control
group. In another study, the ethanol extract of P. vulgaris arrested
cells at the G1/S checkpoint in human colon carcinoma cells and
inhibited the expression of both cyclin D1 and CDK4. Rutin, one
of the flavonoids from P. vulgaris, showed anti-tumor effect
against human neuroblastoma LAN-5 cells by inducing G2/M cell
cycle arrest and apoptosis. Ursolic acid, another component of
P. vulgaris, was shown to block B16 mouse melanoma cell line in
G1 phase which is in accordance with the present study. These
reports suggest that P. vulgaris is also capable of inducing cell
cycle arrest in various cancer cell lines.
Induction of apoptosis
Taxanes induce apoptosis by regulating c-Raf-1 kinase an
important mediator of programmed cell death which is somehow
concentration dependent; increase stabilization of protein by
induction of wild-type p53 and p21WAF1 and down regulate the
proto- oncogene c-myc thus, promoting apoptosis. Amongst the
whole myriad of genes enhancing taxanes induced apoptosis,
there is the Bcl-2 family where it is speculated that taxanes can
interact and induce cytotoxicity via phosphorylation of Bcl-xl
(B-cell lymphoma-extra-large) and Bcl-2(B-cell lymphoma 2)/BAX
(Bcl-2–associated X protein) which are members of the apoptosis
regulator proteins they are also known to cause resistance in
tumor cells but, nevertheless, play a pivotal role in both breast
and prostate cancer treated regimens. However, recently Bcl-
2 has been found to enhance taxanes chemo sensitivity in
some solid tumors therefore, changing it from a protector to
a killer which proves to be a completely novel strategy and a
plus in cancer battle. P. vulgaris and its components have also
been shown to induce apoptosis in a variety of cancer cell lines
(including Raji cells, SGC- 7901, SW 579, Eca 109, EL-4, Jurket cells,
PANC-1, T24, HepG2, HT29, A549, MKN-45, and Hela cells Several
phytochemicals from P. vulgaris including oleanic acid ursolic
acid rosmarinic acid and caffeic acid have also been shown to
either induce or promote apoptosis in cancer cells. Mechanisms
suggested by several studies are both the up-regulation of the
expression of p53,Bax Fas Bad caspase 3 and caspase 9 as well
as down-regulation of the expression of c-myc Bcl-2 Mcl and Bclxl
Other mechanisms have been suggested are the inhibition of
mitogen-activated protein kinase/extracellular signal-regulated
kinase (MAPK/ERK) pathway the mitochondrial pathway the
nuclear transcription factor NF-κB pathway, and the intracellular
generation of reactive oxygen species (ROS) The present study
highlighted that P. vulgaris var. lilacina affected the expression of
genes associated with apoptotic cell death, including the tumor
suppressor p53,pro-apoptotic Bax, the anti-apoptotic Bcl-2 and
Fas genes in A549 cells.
P53-mediated apoptosis primarily occurs through the intrinsic
apoptotic program It was reported that p53 induces apoptosis
by either increasing transcriptional activity of proapoptotic genes
such as Bax or suppressing the activity of the anti-apoptotic
genes of the Bcl-2 family Our data show that P. vulgaris var.
lilacina significantly increased the expression of p53, Bax and Fas
compared to the control. However, the expression of Bcl-2 was not
decreased compared to that of the control (Figure 6). Therefore,
the treatments altered the expression of Bax/Bcl-2, resulting in a
shift in their ratio favouring apoptosis. Several other groups have
shown in various cancer cell lines that P. vulgaris var. lilacina can
lead to cell death by inducing apoptosis through regulation of p53
and Bax/Bcl-2 expression In our study, the resulting elevation in
p53 and Bax protein expression in lung cancer cells is consistent
with our earlier proposed involvement of p53 and Bax-related
response systems. Taken together, we suggest that P. vulgaris var.
lilacina induce apoptosis through the regulation of p53, Bax, and
Fas expression.
Conclusion
P. vulgaris has been extensively used in China both independently
and as a part of a multi-modal approach to treat cancer patients
with standard chemotherapy. P. vulgaris appears to target
multiple signalling pathways and has a complex mechanism of
action. The complexity of the herb may be a key element of its
therapeutic or preventive effectiveness. However, the pleiotropic
effects that it causes make determining definitive targets for
future pharmaceutical development more challenging. Based
on its strong efficacy in both pre-clinical model systems and in a
number of clinical trials with limited toxicity or adverse effects,
further studies should focus on characterizing P. vulgaris as a
promising cancer chemo preventive agent.