Keywords
Kidney rejection; Transplant rejection; Kidney transplantation; Kidney allograft loss; Post kidney transplantation
Introduction
Kidney Transplantation is a well-established and treatment of choice for selected End Stage Renal Disease (ESRD) patients, extending their survival and improving their quality of life, while benefiting from the reduction in the mortality associated with long-term dialysis [1-3]. Kidney transplantation is a surgical procedure where a healthy, functioning kidney is removed from a living or brain-dead donor and implanted into a patient with non-functioning kidneys [4,5]. According to Assounga et al. [4] kidney, transplantation is performed on patients with ESRD. ESRD is a progressive, debilitating chronic illness whereby kidneys are no longer capable of adequately removing fluids and wastes from the body, or maintaining the proper level of certain kidney-regulated chemicals in the bloodstream [4].
Studies have revealed statistics that show progressive increase in the number of kidney transplants performed each year in United States, Iran, Mexico, Japan and Brazil [5,6]. In the Brazilian state of Rio Grande do Sul, 548 kidney transplants were performed in 2012, versus 468 in 2011 [5]. Furthermore, about 650 transplants are performed annually in North Africa from live donors, the majority in Egypt [7]. Marsicano et al. [8] indicated that kidney transplant is the most widely performed transplantation procedure worldwide. This confirms the growing increase of transplantation worldwide, contrary, to South Africa where 263 transplants were done in 2009 and only 222 in 2013 [9].
Thomas [10] identified important key aspects in transplant management, namely, pain management, fluid and electrolyte balance, urine output-catheter care, wound management, infection control and post-operative medications. Furthermore, kidney recipients must undergo routine, lifetime physician and laboratory visits, manage a complex immunosuppressant medication regimen, track graft function vigilantly monitor their vital signs, manage symptoms, exercise, and maintain a low-cholesterol [11]. The aim of postoperative management is to provide the appropriate care to support primary transplant function and to aid optimal recovery [10]. Pain relief medication is given and the regimen of immunosuppressive medication is prescribed to prevent the body’s immune system from rejecting the new kidney [4]. Furthermore, the recipient is required to take immunosuppressant drugs for the lifespan of the new kidney [4]. Ineffective management of post kidney transplantation patients results in graft loss.
Graft loss is defined as the absence of kidney function, occurring any time after transplantation due to either patient death or irreversible graft injury requiring chronic dialysis and/or retransplantation [12,13]. In a study, done by El-Zoghby et al. [12] graft losses can be due to primary non-functional, defined as permanent absence of kidney function starting immediately post-transplant [12], in addition, graft losses was due to patients death, acute rejection, glomerular disease and medical/surgical conditions. Again, graft losses were also associated with fibrosis/atrophy [12]. Chronic allograft injury (CAI) remains one of the main causes for allograft failure [14-16]. Kidney allograft rejection may ultimately cause allograft loss. Kidney allograft rejection occurs via cellular, humoral or combined mechanisms of which in many cases, the endothelium is the main target of the recipient immune system [14]. Thomas [10] identifies three types of renal transplant rejection namely, hyperacute, acute and chronic rejection or chronic allograft nephropathy (CAN). Hyperacute rejection occurs rapidly, within minutes to hours of revascularisation of the transplant, acute rejection occurs between 4 days and 2 months after transplantation and chronic rejection or chronic allograft nephropathy usually occurs over months or years [10].
According to Samaan et al. [17] the majority of kidney transplant patients present with chronic renal failure already at 1-year post transplantation. The frequency of late allograft loss remains excessive with approximately 7% of kidney transplants failing each year, with approximately half of the losses being due to patient’s death and the remainder being due to loss of functioning grafts [18]. However, prevalence of graft failure is not well documented in the African continent.
Gago et al. [19] confirm that good progress has been made in the multifactorial field of kidney transplantation over the last two decades, however, the survival of kidney allografts continue to be short for most recipients. Studies have indicated that immunosuppressive protocols and the medical care of transplant recipients have improved the early outcomes of kidney transplantation, however, long-term survival has not shown significant improvement; grafts continue to fail [20-24]. Thus, identification of factors and consequences related to kidney allograft loss will assist in identifying gaps in transplant management and consequently improve on long-term graft survival.
The purpose of the review is to highlight the contributory factors and associated consequences to kidney allograft loss among post transplantation patients.
Methodology
Articles from 2001 -2017 were identified from online data bases namely, Medline, PubMed, Ebsco Host, CINAHL, google scholar and grey literature. A comprehensive search was done to identify studies that identify contributory factors and associated consequences to kidney allograft loss post transplantation. The following words were used for this search: Kidney transplantation, Kidney rejection, and Kidney allograft loss, Transplant rejection, contributing factors to graft loss, Post kidney transplantation patients and associated consequences of graft loss. The review included English only articles, Qualitative and Quantitive studies were included. Editorials and commentary were excluded. In addition, articles with no outcomes of interest were excluded from the review. Another expert in the nephrology area agreed with the selected articles for review. Figure 1 shows the search strategy.
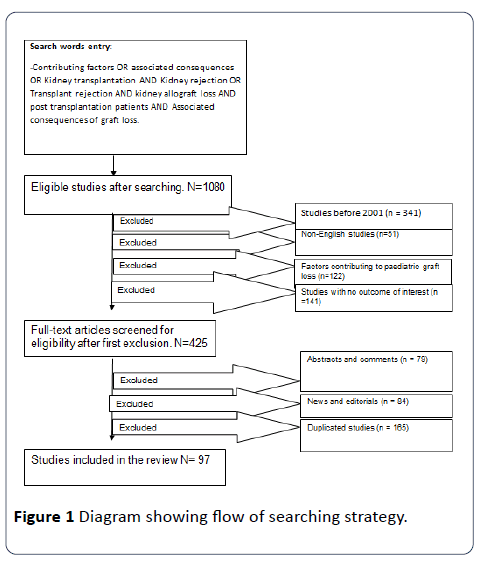
Figure 1: Diagram showing flow of searching strategy.
Results
There were 97 articles identified from the search. Some articles were utilised more than once. The background/ introduction utilised 24 articles. Five categories of factors contributing to kidney allograft loss post transplantation included Patient related factors 35 articles, Donor related factors 13 articles, Physiological factors 8 articles, Socioeconomic factors 9 articles, and Therapy related factors 27 articles (Table 1). Associated consequences which utilised 7 articles mainly include expense of graft loss, meaning going back to dialysis and Disease burden due to high mortality rate mainly due to complications of End Stage Renal Disease (Table 2).
Table 1 Identified contributing factors to kidney allograft loss.
| Factors contributing to Kidney allograft loss |
Sub factors for each category |
| Patient Related Factors |
Patient Adherence |
| Age |
| Gender |
| Ethnicity |
| Health Habits-Smoking |
| Obesity |
| Hypoalbuminemia |
| Hypertension |
| Donor Related Factors |
Age |
| Gender |
| Live/Deceased |
| Expanded Criteria Donors |
| Donor Kidney Weight/Recipient Kidney Weight |
| Physiological Factors |
Sensitization prior to transplantation |
| Prolonged Ischaemic time |
| Pre-emptive transplantation |
| Socioeconomic Factors |
Income status |
| Environmental status |
| Social Support |
| Family/Peer support-Caregiver |
| Therapy related Factors |
Malignant Diseases |
| Nephrotoxicity |
| Infection |
| Under suppression |
| Complex regimen |
| Post-Transplant Diabetes mellitus |
| Hypertension |
Table 2 Identified consequences of kidney allograft loss.
| S. No |
Consequences of kidney allograft loss |
| 1 |
Disease Burden |
| 2 |
High Mortality Rate |
| 3 |
Complications of ESRD |
| 4 |
Economic Burden |
| 5 |
Back on Dialysis |
| 6 |
Higher Health Care Expenditure |
| 7 |
Re-Transplantation |
Discussion
Patient-related factors
Patient adherence: Adherence is a universal problem and significant barrier to effective management of patients post transplantation. Among recipients of kidney transplants, nonadherence with prescribed immunosuppressive medications commonly occurs and frequently precedes allograft loss [25]. According to Ponticelli and Graziani [26] poor adherence to therapeutic prescriptions is frequent in patients requiring longterm therapies and represents a serious public health issue with only 50% of patients who suffer from chronic diseases adhering to treatment recommendations. Kidney transplantation is a chronic disease since treatment is for life. Adherence to medication is defined as the extent to which patients take medications as prescribed by their health care provider [26]. The same authors indicated that the term adherence is usually preferred to compliance because compliance means that the patient is passively following doctor’s orders.
Although immunosuppressive therapy after organ transplantation is paramount for long-term graft survival, nonadherence rates are higher than expected varying from 15% to 50% of patients depending on numerous factors such as definition of adherence, patient characteristics, and country [27]. Furthermore, non-adherence to long-term therapies increases sharply after 6 months, which might partly explain the discrepancy between improved short-term allograft and unchanged long-term allograft survival with the modern immunosuppressive regimens [25,28-30]. Similarly, nonadherence is often subtle and unintentional occurring early and/or late after transplantation and tends to increase with time [31]. Individuals with ambivalence about treatment and prior history of non-adherence, substance abuse, poor social support and poor organisational skills are more prone to treatment non-adherence [30] contributing to poor graft survival. In addition, history of adverse health effects, lack of knowledge about treatment and complex medical regimens may cause graft failure [28-30].
In 2003, the World Health Organization determined that patient adherence is “the extent to which a person’s behaviour (taking medications, following a recommended diet and/or executing lifestyle changes) corresponds with the agreed recommendations of a health care provider” [32]. Similarly, non-adherence in kidney transplantation is more than being not compliant to immunosuppressant drugs. In contrast, transplant experts defined non-adherence in transplantation as “deviation from the prescribed medication regimen sufficient to influence adversely the regimen’s intended effect” [32]. Muduma et al. [33] elaborated that adherence was also explained in terms of the sense of obligation to care for the donated organ, and equally out of consideration to avert the burden of the patient’s care on relatives.
Non-adherence with health care recommendations and health promotion behaviours is one of the top three reasons for graft loss [34]. Treatment adherence generally includes regular intake of medications, monitoring vitals, undergoing diagnostic tests, following dietary and exercise protocols, abstinence from substance abuse, and regular follow up [35]. Non-adherence to the oral immunosuppressant medication regimen is prevalent and significantly compromises the longterm graft survival and life span of adolescents with kidney transplants [36].
According to Gordon et al. [37] medication adherence can be difficult to attain. Immunosuppressants have to be taken once or twice daily, at consistent times to ensure sufficient and steady blood drug levels to maximise their anti-rejection effects. Dosages are often changed in the first few weeks to obtain optimal immunosuppression while minimizing side effects [37]. According to Morales et al. [31] adherence to treatment is influenced by several factors related to patients’ lifestyle, socio-demographic and psychosocial characteristics or to the treatment regimen itself, which can act as either barriers or facilitators and constitute the main predictors of medication adherence.
According to Dew at el. [38] across all types of transplantation, average non-adherence rates ranged from 1 to 4 cases per 100 patients per year (PPY) for substance use (tobacco, alcohol, illicit drugs), to 19 to 25 cases per 100 PPY for non-adherence to immunosuppressants, diet, exercise, and other healthcare requirements. Furthermore, immunosuppressant non-adherence was highest in kidney recipients (36 cases per 100 PPY vs. 7 to 15 cases in other recipients) [38]. Non-adherence has serious consequences, including infection, rejection episodes, and graft loss with consequent resumption of dialysis [37].
Smoking: According to Ponticelli and Graziani [26] patients who continue to smoke after transplantation also run an increased risk of graft failure, a history of smoking before kidney transplantation can also contribute significantly to allograft loss though the pathogenesis of smoking-related renal damage is largely unknown. These authors indicate that, the intermittent increase in blood pressure during smoking might play a major role in causing renal damage. Moreover, after kidney transplantation, only 28% of patients stop smoking [26,39]. Cigarette smoking before kidney transplantation contributes significantly to allograft loss [40]. However, the same authors reported that smoking cessation before renal transplantation has beneficial effects on graft survival. Sung et al. [40] reported that these effects be emphasized to patients with end-stage renal disease who are considering renal transplantation [40].
Age: Non-adherence to treatment regimens is prevalent among adolescents, is related to increased healthcare use and constitutes a major cause of graft loss in kidney transplant recipients within this age category [24,25,41,42]. Furthermore, among all age groups, adolescents and young adults have the highest rate of kidney allograft loss beyond the first year posttransplant and the same age groups have the highest risk of graft loss, independent of any potential confounders [41,43]. A large-scale analysis cited in Akchurin et al. [41] demonstrated that in the US patients with a functioning graft at age 17 year, would be expected to lose the graft by age 24, making it critical to develop targeted interventions to improve adherence in adolescence, the age group with the highest risk of graft loss [41]. Kidney transplantation should be encouraged for the most suitable recipients in the elderly. Hatamizadeh et al. [44] reported that advanced age is associated with relatively better kidney allograft outcomes, additionally, most comorbidities are not associated with poorer outcomes in the oldest kidney transplant recipients (>75 year old).
Similarly, patients aged 65 years and older were more likely to be adherent versus non-adherent compared with patients aged 19 to 64 years. Furthermore, patients aged 35 to 49 years and 50 to 64 years, compared to those aged 19 to 34 years were least likely to be adherent [45]. Similarly, Foster et al. [46] reported that graft failure were highest in the 19 year olds. In addition, the death-censored graft failure rates were higher in 17 to 24 year olds (hazard ratio, 1.20) irrespective of age at transplant [46].
Gender: Female renal transplant recipients have an increased relative risk for acute rejection compared with male renal transplant recipients [47]. According to the same author, in contrast, women have a decreased relative risk for the development of chronic allograft failure. It is interesting to note that the increased risk of acute rejection in female renal transplant recipients is of equal magnitude as their overall decreased risk for chronic allograft failure [47].
Ethnicity: It has been highlighted that non-adherence behaviours persist in a relevant proportion of renal recipients undergoing IT (immunosuppressant therapy) [48]. Ethnicity has been associated with adherence with blacks showing less or non-adherence [33,41,49,50]. Moreover, it has been well documented that black adolescents have an increased risk of graft failure [45], mostly due to non-adherence. However, the black race, patients’ personal schedules and routines were associated with non-adherence [25]. According to Ortega at el. [48], Gordon et al. [37] the total number of doses and the convenience of treatments appear to be relevant determinants of adherence.
Obesity: Among kidney transplant recipients, obesity is associated with higher risk of graft failure, allograft loss and death [51-53]. The same authors defined obesity as one with a Body Mass Index (BMI) of ≥ 30 kg/m2. After transplantation, many patients have spontaneously improved appetite, which is also stimulated by use of glucocorticoids [26,54]. However, as a result, many patients show weight gains, and some of them become obese, which may cause metabolic syndrome and can increase the risk of cardiovascular morbidity and mortality and limit graft survival [24,26,42,54].
Nath et al. [55] elaborated that, an obese recipient is at twice the risk of losing their transplanted kidney, if the graft they received was of complex vascular anatomy compared to one of normal anatomy. According to Nath et al. [55] the reasons for this are not clear and probably multifactorial. According to these authors the hypercoagulable, pro- inflammatory state of obesity predisposes to both arterial and venous thrombosis, delayed graft function and wound complications which is compounded by associated decline in mobility [56]. Furthermore, technical difficulties, prolonged implant time, and local pressure effects of the abdominal overhang on small vessel anastomoses also may be contributing factors [55]. Meier-Kriesche et al. [47] elaborated on increased risk of adverse transplant outcomes, further, reported that according to a study done, the cox regression modelling showed that obesity also increased graft failure. Due to the associated comorbidities and increased risk of adverse outcomes following transplantation, some centers have excluded patients with a high BMI (e.g., ≥ 35 kg/m2) from transplantation [50].
Hypoalbuminemia: Tancredi and Butani [57] state that in the adult renal transplant population, post transplantation hypoalbuminemia is a well-documented risk factor for patient and graft loss. The same authors revealed that adult patients with hypoalbuminemia receiving hemodialysis in the pretransplantation period had worse graft and patient survival and a higher incidence of delayed graft function (DGF) [57]. Previous studies have also confirmed that pretransplantation C-reactive protein level is a predictor of post-transplantation Acute Rejection (AR) and in a primate model, it was also associated with vasculopathy, both of which could lead to poorer graft long-term survival [56].
Hypertension: According to Pourmand et al. [58] hypertension can be due native kidneys and increased BMI. Furthermore, it is mostly found in the elderly and female recipients. In addition, Kubo [59] elaborated, that hypertension in the kidney recipient was linked to transplant renal artery stenosis and deteriorating renal function secondary to both acute and chronic rejection [59].
Donor Related Factors
Kidneys from elderly deceased donors have substantially increased organ supply, although Karatzas et al. [60] state that it is associated with worse graft function and survival rates. According to Schweer et al. [54] the male donor gender has a risk factor for Post-Transplant Diabetes Mellitus (PTDM), concluding that, post-transplant diabetes was limiting for the patient and graft survival. Kidneys from live donors result in overall better graft and recipient survival than those from deceased donors [61]. Kostakis et al. [42] addressed that fiveand 10-year graft survival rates of recipients receiving grafts from female donors are worse than those receiving grafts from male donors. Furthermore, they concluded that the worst combination between donor and recipient was female donor– female recipient and the best one was male donor–female recipient [42].
However, a study done by Vavallo et al. [62] concluded that, there was no significant impact of gender on the short- and long-term graft and patient’s survival. Nevertheless, lower Creatinine level in the male donors to female recipients was reported [62]. Several aetiologies have been proposed as potential explanations of a gender effect on renal transplantation, including anatomical and immunological mechanisms [62]. Moreover, it was noted that women have smaller kidneys and fewer nephrons than men do, so the discrepancy between the mass of the grafted kidney and that of the recipient, by inducing nephron overload, could be responsible for the poor long-term outcome of grafts from female donors to male recipients [62]. Nemati and Taghipour [24], Barba et al. [62] support that expanded criteria donors may be risk factors to graft failure. Reese et al. [63] defined expanded criteria as donors who are greater than 50 years with at least hypertension history, serum creatinine >1.5 mg/dl or cause of death from cerebrovascular accident.
There are changes associated with age in the number and size of glomeruli, a progressive decrease in glomerular filtration rate (kidney function) as well as increased immunogenicity of the aging kidney [42,65]. Shin et al. [66] study revealed that increased Donor-recipient age gradient (DRAG) is associated with development of graft rejection, increased post-transplant serum Creatinine levels, and reduced overall and death censored graft survival. Furthermore, Mazaris et al. [42] suggested that the age of a donor of over 55 years negatively affects the one- and fiveyear graft survival rate displaying tissue inflammation at the time of procurement that may increase immune recognition. There are changes associated with age in the number and size of glomeruli, a progressive decrease in glomerular filtration rate (kidney function) as well as increased immunogenicity of the aging kidney [42,65].
A study done by Codas et al. [67] on influence of allograft weight to recipient bodyweight ratio on outcome of cadaveric renal transplantation, concluded that it was better to avoid transplanting deceased or living donor kidneys with Donor kidney weight (DKW)/Recipient kidney (RBW) < 2.5g/kg as this influenced the graft function. Furthermore, Laging et al. [68] confirmed that there is an advantage for patients receiving a young living donor kidney (below age 40), even transplantation with an older living donor kidney provides comparable or better graft survival outcomes than with a deceased donor kidney. Similarly, Glorie et al. [69] indicated that grafts taken from living donors generally function twice as long as grafts taken from deceased donors. Thus, donor screening becomes an initial and imperative step in kidney transplantation.
Physiological Factors
According to Thomas [10]; Crespo et al. [70] hyperacute rejection is caused by the presence of preformed cytotoxic antibodies in the recipient’s blood. This type of rejection results from previous failed transplants, blood transfusions or pregnancies reacting against the donor’s histocompatibility antigens and also ABO incompatibility between the donor recipient [57]. Acute rejection is usually a combination of cellular and anti-body-mediated rejection and the exact mechanism involved in chronic rejection is still unclear, there may be immunological or non-immunological factors involved [10]. Recurrent kidney rejections can eventually lead to graft loss.
One of the major predictive factors for outcome is cold ischemia time (CIT). CIT is part of organ preservation, all living cells require oxygen and once blood supply to organ ceases, lack of oxygen results in cellular ischaemia [10]. According to Thomas [10] ischaemia which occurs before the organ is cooled is termed warm ischaemia and ischaemia from the beginning of cooling to reperfusion and rewarming at the time of transplantation is termed cold ischaemia time. However, if blood supply is interrupted without cooling, tubular cells suffer from warm ischaemia resulting in acute tubular necrosis (ATN). Furthermore, if not attended to for upto an hour glomeruli may suffer irreversible damage and kidney may not regain function [10]. In addition, the same author suggested that the ice should maintain the kidney at approximately 4 Degrees Celsius to minimise ischaemic damage.
Emmanouilidis et al. [71]; Kienzl-Wagner et al. [72]; Solini et al. [15] established that with increasing ischaemia time, graft survival rates and graft function worsen, particularly with kidney transplants from deceased donors performed beyond 18 hour of cold ischaemia decreasing the graft survival significantly. Contrary, Thomas [10] suggested that most kidneys can be stored for 24-48 hours and that recent studies state that prolonged cold ischaemia does not reduce the function of the transplant. Accumulating evidence supports that prolonged pre-transplant dialysis time may be a risk factor to long-term graft survival [20]. According to Bozkurt et al. [73]; Thomas [10] pre-emptive transplants (transplant before any dialysis) result in better graft function and survival. Furthermore, pre-emptive kidney transplantation increases health-related quality of life reducing treatment costs by avoiding dialysis [73].
Therapy related factors
Infections
The recipient’s immune characteristics as well as posttransplant immunosuppressive drugs such as Tacrolimus and mycophenolate mofetil are considered as the greatest risk factors for BK Viral Nephropathy (BKVN) onset [74,75] that causes graft loss in renal transplant. Patients post kidney transplantation is prone to severe infections. According to Mitterhofer et al. [75] factors such as local injury/regeneration with variable tissue permissiveness as well as recipient and donor characteristics may be capable of favouring BKV replication. According to Smedbraten et al. [76] previous studies have shown that cytomegalovirus (CMV) infection is associated with shorter renal graft survival and increased risk of post-transplant CMV Diabetes Mellitus. According to these authors CMV disease is defined by a positive CMV pp65 Ag blood test and /or presence of CMV in tissue biopsies accompanied by either CMV syndrome or organ involvement such as hepatitis, gastrointestinal ulceration, pneumonitis or retinitis [76].
Cytomegalovirus (CMV) and Epstein Barr Virus (EBV) disease and asymptomatic infection have been associated with poor outcomes in kidney transplantation [77-79]. Recipients who acquire primary infection through transplantation from a seropositive donor may be at particular risk of complications [77,78,80]. According to these authors these herpes viruses, both CMV and EBV achieve a latent state within a host after primary infection and may reactivate in the setting of immunosuppression. Chronic allograft damage and dysfunction has also been linked to both CMV and EBV and may occur in the absence of symptomatic infection [77,78,80]. Recurrent glomerulonephritis is a known cause of allograft loss [81]. Furthermore, recurrence has been reported in 6.0 to 19.4 percent of renal-allograft recipients, and the prevalence increases with the duration of follow-up [81].
Bloodstream infection is common in kidney transplant recipients (KTRs) and could be lethal, Urinary Tract Infections (UTIs) represent the majority of bacteraemia source among the Kidney transplant recipients [82], and this could be a threat to the transplanted kidney. Similarly, Wong et al. [83] identified urinary tract infection as the most common infectious complication post kidney transplantation with an incidence of 26% to 76%. Urinary tract infection (UTI) effects on renal parenchyma have shown how infections of the urinary system which may result in prolonged inflammation and potential renal scarring, can lead to impaired renal function [84]. Moreover, kidney transplant patients are at higher risk for complicated UTI such as pyelonephritis [84]. Again, poor hygienic measures can cause infection, which can in turn cause rejection [10,26]. According to Naik [53], all the first-year infections were associated with increased risk of death and allograft loss within the first year post-transplant.
Nephrotoxicity
According to Liu et al [77]; El-Agroudy et al. [85]; Shihab et al. [20]; Thomas [10] calcineurin inhibitors (CNIs) used to prevent kidney rejection, may initially protect the renal transplant against immunologic injury but may subsequently cause damage as a result of long-term nephrotoxicity. According to these authors, the low early acute rejection rates achieved using CNIs are not accompanied by improvements in long-term outcomes. Savikko et al. [86] indicate that although cyclosporine (CsA) has markedly improved the short-term results, CsA-induced nephrotoxicity is an important nonimmunologic factor contributing to graft dysfunction and loss.
Malignant diseases
Cancer is one of the major causes of mortality and morbidity in kidney transplant recipients [87,88]. Furthermore, the synergistic effects of immunodeficiency and latent viral infections have been considered as likely sources of oncogenesis in transplant recipients [83]. It is indicated that organ transplant recipients have a threefold excess risk of cancer relative to the age and sex-matched general population and screening is of paramount importance [26]. Similarly, elevated cancer risk after transplantation is thought to result from the interplay of several factors, which include chronic uraemic state [89]. Furthermore, carcinogenesis, and cumulative exposure to immunosuppression disrupts both anti-tumor immunosurveillance and anti-viral activity, and may potentiate the carcinogenic effects of other agents such as sunlight [89]. However, some drugs promote carcinogenesis by mechanisms independent of their immunosuppressive effects [89]. According to Euvrard et al. [87] multiple skin cancers develop in 60 to 80% of kidney-transplant recipients within 3 years. Transplant recipients share common risk factors with the nonimmunosuppressed population, but the specific tumor burden of such patients is linked to the immunosuppressive medications used [87].
Hypertension
According to Kubo [59] and Pourmand et al. [58] uncontrolled hypertension results in reduced graft and patient survival. The same authors indicate that the prevalence of hypertension remains high at 80-90%, despite improvement in glomerular filtration rate and fluid status. The pathophysiology of post-transplant hypertension has been linked to immunosuppressant use [62]. Furthermore, control of hypertension in renal transplant recipients has been shown to be inadequate, with only 16.5% of local RTRs achieving blood pressure target levels of <130/80 mmHg. Kubo [62] stated that in the same population low rates of controlled hypertension at 21.2% were found.
Post-transplant diabetes mellitus
According to Schweer et al. [54] post-transplant diabetes (PTDM) has become an increasing problem in kidney transplant recipients (KTRs), with an incidence of 10–20%. Post-transplant diabetes mellitus (PTDM) is that follow renal transplantation. In addition, new-onset diabetes mellitus after kidney transplantation is an important co morbid condition that is associated with inferior graft and patient survival [88]. The risk of PTDM increases continuously with time posttransplant [90].
Furthermore, characteristics of the medicines and their dosage and schedules, practical issues related to access to medications and pharmacy refills, and medication costs contribute to therapy related risks [25]. However, reduction in acute rejection with these drugs has not directly translated to improvements in allograft survival, and suggests that CNIbased immunosuppression may not improve long-term graft survival [20]. Furthermore, the same authors state that kidney recipients take eight to ten related medications daily that includes anti-ulcer medication, prophylactic medication, medication for comorbidities and risk factors that contribute to ESRD such as Diabetes Mellitus [37].
Socioeconomic Status
Kidney transplantation is associated with higher quality of life, lower mortality, and lower health care expenditure than haemodialysis or peritoneal dialysis [91]. Despite these findings, there are important differences in rates of transplantation between Whites and Blacks in the United States, while Blacks comprise approximately 32% of those receiving dialysis, they receive approximately 25% of deceased donor kidneys [91]. Graft failure is associated with racial and ethnic differences that are related to the level of health services, [24,42]. Saunders et al. [91]; Waterman et al. [92] describe three complementary mechanisms that may account for the associations, firstly, Blacks are more likely to live in poor and racially isolated neighbourhoods that are resourcepoor, which may in turn lead to reduced access to transplant waitlists.
Secondly, residents of poor, minority areas may have lower levels of social capital and weaker social networks, which may lead to a lack of information about transplantation, unreliable transportation to appointments, or fewer caretakers who could assist them in navigating the transplant process [91]. Finally, Blacks are geographically concentrated within urban areas in the Midwest, Northeast and South, and the rural areas in the South. Regional variations in incidence of end-stage renal disease, deceased donor donation and waitlist patterns contribute to differences in the magnitude of the transplant waitlist disparity within neighbourhood category and overall [91]. The kidney transplant recipients living in non-metro rural areas rather than those in metro areas were significantly less likely to be adherent versus non-adherent [45].
Similarly, Schold et al. [93] indicate that kidney transplant candidate processes and outcomes are independently associated with risk factors in patients’ community. Schold et al. [93] characterize patient communities based on access and quality of care, prevalence of comorbid conditions, environmental hazards and behavioral attributes of individuals within counties. These authors confirm that factors associated with patients’ communities are indicative of health conditions, access to care, socioeconomic status and environmental conditions and have a prominent role in delivery of care and outcomes for kidney transplant patients [93]. Kidney transplantation offers patients with chronic kidney failure hope of a better quality of life (QOL) and the possibility of longer survival [94].
However, the success of kidney transplantation depends, in part, on the availability and stability of a nonprofessional primary caregiver, usually the spouse, to assist the patient in managing the needs of chronic kidney disease and subsequent transplantation [94]. According to these authors, the absence of this type of informal caregiving is considered by most kidney transplantation programs in the United States to be an absolute or a relative contraindication to transplant listing. Furthermore, evidence in other areas indicates that greater availability and/or higher quality support throughout the transplant process is associated with better psychological adjustment in patients, more optimal adherence behaviours, and longer survival [94].
Bolkhir et al. [95] stated that lack of caregiver support has been linked to patient depression in chronic disease, and patient depression has been linked to decreased survival after transplantation. Furthermore, the prevalence of moderate to severe depression in primary caregivers’ kidney transplant candidates is significant and screening for depression in caregivers could lead to clinical interventions that benefit caregivers and indirectly improve patient outcomes [95].
The study done by Rodrigue et al. [94] revealed that more than half of all kidney transplantation caregivers reported clinically significant caregiving strain. One possible explanation for the burden not being lower is that patients still have follow-up appointments, medical tests, rehospitalizations, medication regimens, and lifestyle modifications in the months after transplantation, all of which can affect perceived caregiving strain [94]. Some caregivers after kidney transplantation may continue to provide caregiving assistance because the patient has other comorbid health problems that were not ameliorated by transplantation and other similar problems are common for many kidney transplant recipients and these issues may increase caregiving strain as well [94].
Consequences
Graft failure not only results in (re)initiation of dialysis and the associated reduction in quality of life, it is also associated with increased health care costs [30]. Furthermore, expected survival is significantly lower when patients return to dialysis, and re-transplantation of the patient might be hampered by new HLA-antibodies [30]. Furthermore, a study by Frezza et al. [96] observed that in a long-term outcome after kidney transplantation, Glucocorticoids (GCs) resistant patients showed higher incidence of acute rejection episodes, lower acute rejection-free survival, poor response of acute rejection treatment, as well as higher incidence of renal allograft loss. However, no difference was found regarding patient mortality, wound, and vascular complications [63]. Moreover, greater frequency of more severe surgical complications and lower graft survival has been reported [63].
Non-adherence is a leading cause of preventable graft failure. It has been estimated that, on an annual basis, medication non-adherence contributes to US$100 billion in inpatients costs and US$2,000 per patient in excess physician visits [97]. Further, a report by the New England Healthcare Institute found that medication non-adherence contributed to avoidable health care spending of approximately US$290 billion annually [97]. In 2004, non-adherence to treatment in kidney transplantation alone cost the United States approximately $100 million annually. In addition to the economic burden, non-adherence to immunosuppressive medication in renal transplantation is a primary cause of kidney rejection with one meta-analysis finding that nonadherent transplant patients are seven times more likely to have graft failure than those who were adherent [49].
According to Naik et al. [53] first-year infections post transplantation range from a $17 691 marginal cost increase for UTI alone, $39 593 for pneumonia alone, and $53 965 for sepsis alone. First-year infections were also associated with significant downstream cost effects in years 2–3 after transplant, ranging from $8372 for UTI alone to $36 000–$38 000 for pneumonia with sepsis, or for combined UTI, pneumonia, and sepsis [53]. In addition to survival implications, infections also increase the intensity and cost of post-transplant care [53]. UTI, respiratory tract infections, and sepsis rank among the ten most common causes of rehospitalization in the first and second years after kidney transplantation [53].
A successful transplant offers freedom from the practical and psychological difficulties and restrictions of long-term dialysis; freedom from dependence upon the machine, fluid bag or partner; freedom from fluid and dietary restrictions; a return of sexual functioning and fertility with a possibility of parenthood; and a return to almost normal life [10].
Conclusion
Kidney transplantation is the only successful innovative way to provide a quality of life, for patients with end stage renal disease, which is free from dialysis. The long waiting lists, shortage of donor kidneys and the strict criteria to be considered for transplantation, make it imperative to prevent kidney allograft loss and aim at improving the graft survival. Identifying the contributing factors and consequences of kidney allograft loss is the gateway to successful, prolonged, quality life post kidney transplantation. The burden, which comes with end stage renal disease in terms of cost mortality and morbidity, can be conquered by promoting factors, which facilitate long-term graft survival.
Acknowledgements
The project received support from the University of KwaZulu Natal, school of nursing and public health. The review article is part of literature review of the PHD thesis entitled Development of an intervention model to improve on longterm graft survival post kidney transplantation in selected state hospitals in South Africa.
19570
References
- Mahendran AO, Barlow AD (2014) Kidney transplantation. Surgery (Oxford) 32: 364-370.
- Alachkar N (2012) Serum and urinary biomarkers in acute kidney transplant rejection. Nephrologie & therapeutique 8: 13-19.
- Naderi GH, Mehraban D, Kazemeyni, Darvishi SM, Latif MAH (2009) Living or deceased donor kidney transplantation: A comparison of results and survival rates among Iranian patients. In Transplantation proceedings 41: 2772-2774.
- Assounga A, Hariparshad S, Madala N (2012) Kidney diseases in an African setting. Reach Publishers.
- Ferreira SAL, Echer IC, Lucena (2014) Nursing diagnoses among kidney transplant recipients: Evidence from clinical practice. Int J Nursing Knowledge 25: 49-53.
- Ghaly M (2012) The ethics of organ transplantation: How comprehensive the ethical framework should be?
- Barsoum RS (2013) Burden of chronic kidney disease: North Africa. Kidney Int Suppl 3: 164-166.
- Marsicano DO, Fernandes DSE, Colugnati N, Grincenkov DS, Fernandes DSFFR, et al. (2013) Transcultural adaptation and initial validation of Brazilian-Portuguese version of the Basel assessment of adherence to immunosuppressive medications scale (BAASIS) in kidney transplants. BMC nephrology 14: 108.
- Gordon EJ, Wolf MS (2009) Health literacy skills of kidney transplant recipients. Progress in Transplantation 19: 25-34.
- El-Zoghby, Stegall ZM, Lager MD, Kremers DJ, Amer WK, et al. (2009) Identifying specific causes of kidney allograft loss. Am JTransplant 9: 527-535.
- Sellares J, De Freitas DG, Mengel M, Reeve J, Einecke G, et al. (2012) Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388-399.
- Qamri Z, Pelletier, Foste Rr, Kumar J, Momani S, et al. (2014) Early posttransplant changes in circulating endothelial microparticles in patients with kidney transplantation. Transplant immunology 31: 60-64.
- Solini, Aiello S, Cassis S, Scudeletti P, Azzollini P, et al. (2012) Prolonged cold ischemia accelerates cellular and humoral chronic rejection in a rat model of kidney allotransplantation. Transplant Int 25: 347-356.
- Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, et al. (2008) Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis 46: 840-846.
- Samaan, Requiao-Moura F, Pinheiro LR, Ozaki HS, Câmara KS, et al. (2011) Prevalence and progression of chronic kidney disease after renal transplantation. In Transplantation proceedings 43: 2587-2591.
- Khalkhali HR, Hajizadeh E, Kazemnejad A, Ghafari A (2010) Longterm progression pattern of chronic allograft dysfunction among kidney transplant recipients. Iran J Kidney Dis 4: 244.
- Gago M, Cornell LD, Kremers WK, Stegall MD, Cosio FG (2012) Kidney allograft inflammation and fibrosis, causes and consequences. Am J Transplant 12: 1199-1207.
- Shihab F, Christians U, Smith L, Wellen JR, Kaplan B (2014) Focus on mTOR inhibitors and tacrolimus in renal transplantation: Pharmacokinetics, exposure–response relationships, and clinical outcomes. Transpl Immunol 31: 22-32.
- Ingsathit A, Kamanamool N, Thakkinstian A, Sumethkul V (2013) Survival advantage of kidney transplantation over dialysis in patients with hepatitis C: A systematic review and metaanalysis. Transplantation 95: 943-948.
- Jung N (2012) Incidence of post-transplant glomerulonephritis and its impact on graft outcome. kidney research and clinical practice. Kidney Res Clin Pract 31: 219-226.
- Marcén R, Fernández-Rodriguez A, Rodríguez-Mendiola N, Ponte B, Galeano C, et al. (2009) Evolution of rejection rates and kidney graft survival: a historical analysis. In Transplantation proceedings 41: 2357-2359.
- Nemati E, Taghipour M (2014) Factors associated with survival of kidney allografts. Iran J Kidney Dis 8: 166.
- Weng, Chandwani FL, Kurtyka S, Zacker KM, Chisholm-Burns C, et al. (2013) Prevalence and correlates of medication nonadherence among kidney transplant recipients more than 6 months post-transplant: A cross-sectional study. BMC nephrology 14: 261.
- Ponticelli C, Graziani G (2012) Proteinuria after kidney transplantation. Transpl Int 25: 909-917.
- Dharancy S, Giral M, Tetaz R, Fatras M, Dubel L, et al. (2012) Adherence with immunosuppressive treatment after transplantation: results from the French trial PREDICT. Clin Transplant 26: E293-E299.
- Obi Y, Ichimaru N, Kato T, Kaimori JY, Okumi M, et al. (2013) A single daily dose enhances the adherence to immunosuppressive treatment in kidney transplant recipients: a cross-sectional study. Clin Exp Nephrol 17: 310-315.
- Massey EK, Tielen M, Laging M, Beck DK, Khemai R, et al. (2013) The role of goal cognitions, illness perceptions and treatment beliefs in self-reported adherence after kidney transplantation: a cohort study. J Psychosom Res 75: 229-234.
- Pabst S, Bertram A, Zimmermann T, Schiffer M, de Zwaan M (2015) Physician reported adherence to immunosuppressants in renal transplant patients: prevalence, agreement, and correlates. J Psychosom Res 79: 364- 371.
- Morales JM, Marcén R, del Castillo D, Andres A, Gonzalez- Molina M, et al. (2012) Risk factors for graft loss and mortality after renal transplantation according to recipient age: A prospective multicentre study. Nephrology Dial Transplant 27: 39-46.
- Russell CL, Ashbaugh C, Peace L, Cetingok M, Hamburger KQ, et al. (2013) Time-in-a-bottle (TIAB): A longitudinal, correlational study of patterns, potential predictors, and outcomes of immunosuppressive medication adherence in adult kidney transplant recipients. Clin Transplant, 27: E580-E590.
- Muduma G, Shupo FC, Dam S, Hawken NA, Aballéa S, et al. (2016) Patient survey to identify reasons for non-adherence and elicitation of quality of life concepts associated with immunosuppressant therapy in kidney transplant recipients. Patient preference and adherence. 10: 27.
- Lin SY, Fetzer SJ, Lee PC, Chen CH (2011) Predicting adherence to health care recommendations using health promotion behaviours in kidney transplant recipients within 1–5 years posttransplant. J Clin Nurs 20: 3313-3321.
- Kumar BA, Mattoo SK (2015) Organ transplant & the psychiatrist: An overview. Indian J Med Res 141: 408.
- Guilfoyle SM, Goebel JW, Pai AL (2011) Efficacy and flexibility impact perceived adherence barriers in pediatric kidney posttransplantation. Families, Systems & Health 29: 44.
- Gordon EJ, Gallant M, Sehgal AR, Conti D, Siminoff LA (2009) Medication-taking among adult renal transplant recipients: barriers and strategies. Transplant Int 22: 534-545.
- Dew MA, DiMartini AF, Dabbs ADV, Myaskovsky L, Steel J, et al. (2007) Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplant 83: 858-873.
- Underwood PW, Sheetz KH, Cron DC, Terjimanian MN, Englesbe MJ, (2014) Cigarette smoking in living kidney donors: Donor and recipient outcomes. Clin Transplant 28: 419-422.
- Sung RS, Althoen M, Howell TA, Ojo AO, Merion RM (2001) Excess risk of renal allograft loss associated with cigarette smoking. Transplant 71: 1752-1757.
- Akchurin OM, Melamed ML, Hashim BL, Kaskel FJ, Del Rio M (2014) Medication adherence in the transition of adolescent kidney transplant recipients to the adult care. Pediatr Transplant 18: 538-548.
- Kostakis ID, Moris DN, Barlas A, Bokos I, Darema M, et al. (2013) Impact of donor and recipient age difference on long-term allograft survival after living donor renal transplantation: analysis of 478 cases. Clin Transplant 27: 838-843.
- Patzer RE, Amaral S, Klein M, Kutner N, Perryman JP, et al. (2012) Racial disparities in pediatric access to kidney transplantation: does socioeconomic status play a role? Am J Transplant 12: 369-378.
- Hatamizadeh P, Molnar MZ, Streja E, Lertdumrongluk P, Krishnan M, et al. (2013) Recipient-related predictors of kidney transplantation outcomes in the elderly. Clin Transplant 27: 436-443.
- Sankaranarayanan J, Collier D, Furasek A, Reardon T, Smith LM, et al. (2012) Rurality and other factors associated with adherence to immunosuppressant medications in community dwelling solid-organ transplant recipients. Res Social Adm Pharm 8: 228-239.
- Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, et al. (2011) Association between age and graft failure rates in young kidney transplant recipients. Transplant 92: 1237-1243.
- Meier-Kriesche HU, Arndorfer JA, Kaplan B (2002) The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplant 73: 70-74.
- Ortega F, Otero A, Crespo JF, Delgado JF, Borro JM, et al. (2013) Satisfaction and adherence with immunosuppressant treatment in renal transplant patients living with a working graft. J Nephrol 26: 297-305.
- Cukor D, Rosenthal DS, Jindal RM, Brown CD, Kimmel PL (2009) Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int 75: 1223-1229.
- Massey EK, Tielen M, Laging M, Beck DK, Khemai R, et al. (2013) The role of goal cognitions, illness perceptions and treatment beliefs in self-reported adherence after kidney transplantation: A cohort study. J Psychosom Res 75: 229-234.
- Lee SY, Chu SH, Oh EG, Huh KH (2015) Low Adherence to Immunosuppressants Is Associated With Symptom Experience Among Kidney Transplant Recipients. In Transplantation proceedings 47: 2707-2711.
- Kwan JM, Hajjiri Z, Metwally A, Finn PW, Perkins DL (2016) Effect of the obesity epidemic on kidney transplantation: Obesity is independent of diabetes as a risk factor for adverse renal transplant outcomes. PloS one 11: e0165712.
- Naik AS, Sakhuja A, Cibrik DM, Ojo AO, Samaniego-Picota MD, et al. (2016) The impact of obesity on allograft failure after kidney transplantation: A competing risks analysis. Transplant 100: 1963-1969.
- Schweer T, Gwinner W, Scheffner I, Schwarz A, Haller H, et al. (2014) High impact of rejection therapy on the incidence of post-transplant diabetes mellitus after kidney transplantation. Clin Transplant 28: 512-519.
- Nath J, Mastoridis S, van Dellen D, Guy AJ, McGrogan DG, et al. (2015) Complex kidneys for complex patients: the risk associated with transplantation of kidneys with multiple arteries into obese patients. In Transplantation proceedings, Elsevier 47:373-378.
- Counts CS (2008) Core Curriculum for Nephrology Nursing. (5th edn). American Nephrology Nurse’s Association.
- Tancredi DJ, Butani L (2014) Pretransplant serum albumin is an independent predictor of graft failure in pediatric renal transplant recipients. J pediatr 164: 602-606.
- Pourmand G, Dehghani S, Rahmati MR, Mehrsai A, Gooran S, et al. (2015) Does hypertension remain after kidney transplantation?. Acta Medica Iranica 53: 297-300.
- Kubo MN, Kayima JK, Were AJ, McLigeyo SO, Ogola EN (2015) Factors associated with uncontrolled hypertension among renal transplant recipients attending nephrology clinics in Nairobi, Kenya. J Transplant.
- Karatzas T, Bokos J, Katsargyris A, Diles K, Sotirchos G, et al. (2011) Advanced donor age alone is not a risk factor for graft survival in kidney transplantation. In Transplantation proceedings, Elsevier 43: 1537-1543.
- Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, et al. (2010) Perioperative mortality and long-term survival following live kidney donation. JAMA 303: 959-966.
- Vavallo A, Simone S, Lucarelli G, Rutigliano M, Galleggiante V, et al. (2014) Pre-existing type 2 diabetes mellitus is an independent risk factor for mortality and progression in patients with renal cell carcinoma. J Med 93: e183.
- Barba J, Zudaire JJ, Robles JE, Rosell D, Berian JM, et al. (2013) Complications of kidney transplantation with grafts from expanded criteria donors. World J Urol 31: 893-899.
- Reese PP, Caplan AL, Kesselheim AS, Bloom RD (2006. Creating a medical, ethical, and legal framework for complex living kidney donors. Clin J Am Soc Nephrol 1: 1148-1153.
- Mazaris EM, Warrens AN, Smith G, Tekkis P, Papalois VE (2011) Live kidney donation: attitudes towards donor approach, motives and factors promoting donation. Nephrol Dialysis Transplant 642.
- Shin M, Moon HH, Kim JM, Park JB, Kwon CHD, et al. (2013) Importance of donor–recipient age gradient to the prediction of graft outcome after living donor liver transplantation. In Transplantation proceedings, Elsevier 45: 3005-3012.
- Codas R, Danjou F, Dagot C, Martin X, Morelon E, et al. (2014). Influence of allograft weight to recipient bodyweight ratio on outcome of cadaveric renal transplantation. Nephrol 19: 420-425.
- Laging M, Kal-van Gestel JA, van de Wetering J, IJzermans JN, Weimar W, et al. (2012) The relative importance of donor age in deceased and living donor kidney transplantation. Transplant Int 25: 1150-1157.
- Glorie K, Haase-Kromwijk B, Klundert J, Wagelmans A, Weimar W (2014) Allocation and matching in kidney exchange programs. Transplant Int 27: 333-343.
- Crespo M, Heidt S, Redondo D, Pascual J (2015) Monitoring B cell subsets and alloreactivity in kidney transplantation.Transplant Rev 29: 45-52.
- Emmanouilidis N, Boeckler J, Ringe BP, Kaltenborn A, Lehner F, et al. (2017) Risk balancing of cold ischemic time against night shift surgery possibly reduces rates of reoperation and perioperative graft loss. J Transplant.
- Kienzl-Wagner K, Schneiderbauer S, Bösmüller C, Schneeberger S, Pratschke J, et al. (2013) Nighttime procedures are not associated with adverse outcomes in kidney transplantation. Transplant Int 26: 879-885.
- Bozkurt B, Kumru AO, Dumlu EG, Tokaç M, Koçak H, et al. (2013) Patient and graft survival after pre-emptive versus non-preemptive kidney transplantation: A single-center experience from turkey. In Transplantation proceedings, Elsevier 45: 932-934.
- Liu J, Liu D, Li J, Zhu L, Zhang C, et al. (2017) Efficacy and safety of everolimus for maintenance immunosuppression of kidney transplantation: A meta-analysis of randomized controlled trials. PloS one 12: e0170246.
- Mitterhofer AP, Tinti F, Pietropaolo V, Umbro I, Anzivino E, et al. (2014) Role of BK virus infection in end-stage renal disease patients waiting for kidney transplantation–viral replication dynamics from pre-to post-transplant. Clin Transplant 28:299-306.
- Smedbråten YV, Sagedal S, Leivestad T, Mjøen G, Osnes K, et al. (2014) The impact of early cytomegalovirus infection after kidney transplantation on long-term graft and patient survival. Clin Transplant 28: 120-126.
- Toyoda M, Shin BH, Ge S, Mirocha J, Thomas D, et al. (2017) Impact of desensitization on antiviral immunity in hla-sensitized kidney transplant recipients. J Immunol Res.
- Schlott F, Steubl D, Hoffmann D, Matevossian E, Lutz J, et al. (2017) Primary cytomegalovirus infection in seronegative kidney transplant patients is associated with protracted cold ischemic time of seropositive donor organs. PloS one 12: e0171035.
- Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, et al. (2008) Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis 46: 840-846.
- Le Page AK, Jager MM, Kotton CN, Simoons-Smit A, Rawlinson WD (2013) International survey of cytomegalovirus management in solid organ transplantation after the publication of consensus guidelines. Transplant 95: 1455-1460.
- Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ (2002) Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med 347: 103-109.
- Cia CT, Li MJ, Li CW, Lee NY, Chang SS, et al. (2016) Communityonset bacteremia in kidney transplant recipients: The recipients fare well in terms of mortality and kidney injury. J Microbiol, Immunolo, Infect 49: 685-691.
- Wong G, Chakera A, Chapman JR, Chadban SC, Pilmore H, et al. (2017) Cytomegalovirus and cancer after kidney transplantation: Role of the human leukocyte antigen system? Transplant Infect Disease 19: 1.
- Ariza-Heredia EJ, Beam EN, Lesnick TG, Cosio FG, Kremers WK, et al. (2014) Impact of urinary tract infection on allograft function after kidney transplantation. Clinical Transplant 28: 683-690.
- El-Agroudy AE, Alarrayed SM, Al-Ghareeb SM, Farid E, Alhelow H, et al. (2017) Efficacy and safety of early tacrolimus conversion to sirolimus after kidney transplantation: Long-term results of a prospective randomized study. Ind J Nephrol 27: 28.
- Savikko J, Teppo AM, Taskinen E, von Willebrand E (2014) Different effects of tacrolimus and cyclosporine on PDGF induction and chronic allograft injury: Evidence for improved kidney graft outcome. Transplant Immunol 31: 145-151.
- Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, et al. (2012) Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 367: 329-339.
- Shah T, Arjang K, Edmund H, Rick H, Brian Y, et al. (2006) "Risk factors for development of new-onset diabetes mellitus after kidney transplantation. Transplant 82: 1673-1676
- Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR (2007) Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: A cohort study of 15 183 recipients. American J Transplant 7: 2140-2151.
- Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM (2001) Post-transplant diabetes mellitus: increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int 59:732-737.
- Saunders MR, Cagney KA, Ross LF, Alexander GC (2010) Neighborhood poverty, racial composition and renal transplant waitlist. Am J Transplant 10: 1912-1917.
- Waterman AD, Rodrigue JR, Purnell TS, Ladin K, Boulware LE (2010) Addressing racial and ethnic disparities in live donor kidney transplantation: priorities for research and intervention. Semin Nephrol 30: 90-98.
- Schold JD, Heaphy ELG, Buccini LD, Poggio ED, Srinivas TR, et al. (2013) Prominent impact of community risk factors on kidney transplant candidate processes and outcomes. Am J Transplant 13: 2374-2383.
- Rodrigue JR, Dimitri N, Reed A, Antonellis T, Pavlakis M, et al. (2010) Spouse caregivers of kidney transplant patients: quality of life and psychosocial outcomes. Progress in Transplant 20: 335-342.
- Bolkhir A, Loiselle MM, Evon DM, Hayashi PH (2007) Depression in primary caregivers of patients listed for liver or kidney transplantation. Progress in Transplant 17: 193-198.
- Frezza G, Colli LM, De Antonio SR, De Castro M (2014) Glucocorticoid resistance in dialysis patients reduces long- term graft survival after kidney transplantation. Transplant Immunol 30: 145-148.
- Chisholm-Burns MA, Spivey CA, Tolley EA, Kaplan EK (2016) Medication therapy management and adherence among US renal transplant recipients. Patient preference and adherence 10: 703.






