Keywords
Nutrition; Enteral nutrition; Parenteral nutrition; Oral feeding; Premature; Neonatal ICU
Introduction
The World Health Organization (WHO) [1] defines a premature infant as any newborn born before the 37th week of gestational age. It is estimated that 15 million premature children are born worldwide each year, representing 1 in 10 births. In Brazil, the prevalence of prematurity increased from 9.2% in 2010 [1] to 11.5% in 2011 [2]. The scientific and technological progress of neonatal care, associated with more humanized care, has allowed the survival of newborns (NBs) with ever lower gestational ages (GA) and birth weights [3]. However, prematurity is still a major public health problem and is the greatest risk factor for infant morbidity and mortality [4].
Being born prematurely predisposes the NB to nutritional risk, since at birth there is a sudden interruption of the nutritional supply to the fetus during the phase of greatest speed of growth and development [5]. Thus, the preterm newborn (PTNB) is harmed, partly or totally, depending on the gestational age, becoming more vulnerable to nutritional deficits, due to the high metabolic demand, inadequate nutritional reserves, immaturity of the physiological systems, and high presence of morbidity [6]. This condition requires complex adaptation to the extrauterine environment, which in many cases makes specialized care in the neonatal intensive care unit (NICU) necessary [7].
Neonatal nutrition plays a key role in the survival and growth and development processes of preterm infants [7]. According to the American Academy of Pediatrics (AAP), preterm nutrition aims to provide nutrients to achieve a postnatal growth rate, in anthropometric and body composition terms, similar to that of a normal fetus of the same gestational age [8]. However, this is still a significant challenge [6]. Review studies show that the nutritional and metabolic impact resulting from eating practices, whether appropriate or not, established in the initial period of life have repercussions in the short and long term [5-7]. Thus, nutritional care for preterm infants should have an emphasis on the assessment of nutritional practices and infant growth. The aim of this study was to compare feeding practices and anthropometric variables between preterm infants ≤ 33 and ≥ 34 weeks of gestational age admitted to a referral hospital for the care of high-risk newborns, as well as to correlate these practices with nutritional evolution.
Methods
This is a prospective longitudinal study carried out in the neonatal unit of a public hospital of reference in the care of high-risk newborns, in the city of Vitória de Santo Antão-PE. The study includes a convenience sample of 44 PTNBs of both sexes, hospitalized in the referred service, during the period from May 2016 to May 2017.
Newborns with GA less than 37 weeks, premature births according to the WHO classification [1] were included in the study. Exclusion criteria were the presence of congenital malformations, maternal or neonatal death shortly after delivery, laboratoryconfirmed congenital infections, genetic disorders, inborn errors of metabolism, neuropsychomotor diseases, and babies whose mothers were unable to breastfeed due to illness or use of medications and/or illicit toxic substances that contraindicate this practice.
Data collection was performed using a form prepared by properly trained researchers, which was completed based on the review of the patients' medical records. The information was collected at three moments: in the first 24 h postpartum, weekly during the period of stay in the neonatal unit, and at hospital discharge. The variables of interest were grouped as follows:
a) clinical birth variables: sex; birth weight (g); gestational age (full weeks); and nutritional status at birth, according to the classification of Fenton and Kim [9].
b) feeding practices: start and duration of enteral nutrition (days of life), start of full enteral nutrition (FEN) (150 ml/kg/day supply) [10] (days of life), start and duration of parenteral nutrition (days of life), start of oral feeding (days of life), type of milk in the first week of hospitalization, use of breast milk additive, start of breastfeeding on the free breast (days of life), and type of food at hospital discharge [11]. The premature infant was considered in enteral nutrition when they received food via a tube and the beginning of oral feeding when the PTNB started nutritional suction through stimulus with the breast, cup, or bottle feeding.
c) nutritional evolution: % of physiological weight loss, being adequate when less than 15%; [12] mean daily weight gain (g/ day), adequate when ≥ 25 g/d, [12] being calculated only for premature infants who spent more than a week in the neonatal unit, considering that in the first week of life physiological weight loss occurs; recovery of birth weight, being adequate when occurring between the 10th and 21st day of life [12] and length of hospital stay (days).
Premature infants were divided into two sample groups according to their physiological maturity, given their relationship with the establishment of nutritional behaviors: newborns of gestational age ≤ 33 weeks, including extreme, severe, and moderate premature infants; and premature infants of gestational age ≥ 34 weeks, including late preterm infants, according to the criteria of the Brazilian Society of Pediatrics (BSP) [13].
This study was approved by the Ethics Committee from the Federal University of Pernambuco (number 1692730). The parents of the infants signed a written informed consent form.
The data were organized in Microsoft Office Excel 2013 (Microsoft Corporation) and analyzed using GraphPad Prism 8 software (San Diego, USA). The distribution of variables was assessed using the Shapiro-Wilk test. Quantitative variables were expressed as mean and standard deviation, and compared by the Student's t test for independent samples, or as median and minimum and maximum values, compared by the Mann-Whitney test. Categorical variables were presented in absolute numbers and percentages and compared using the chi-square or Fisher's exact tests. Spearman's correlation test was used to study correlations between the variables of dietary practices and nutritional evolution. The level of significance adopted was 5% (p<0.05) for all tests.
Results
During the study period, 80 preterm newborns were potentially eligible, however, after exclusion of 36, the final sample contained 44 premature infants (Figure 1). Premature infants were divided into two groups: ≤ 33 (n=19) and ≥ 34 weeks of gestational age (n=25). All clinical birth variables were different between groups (Table 1).
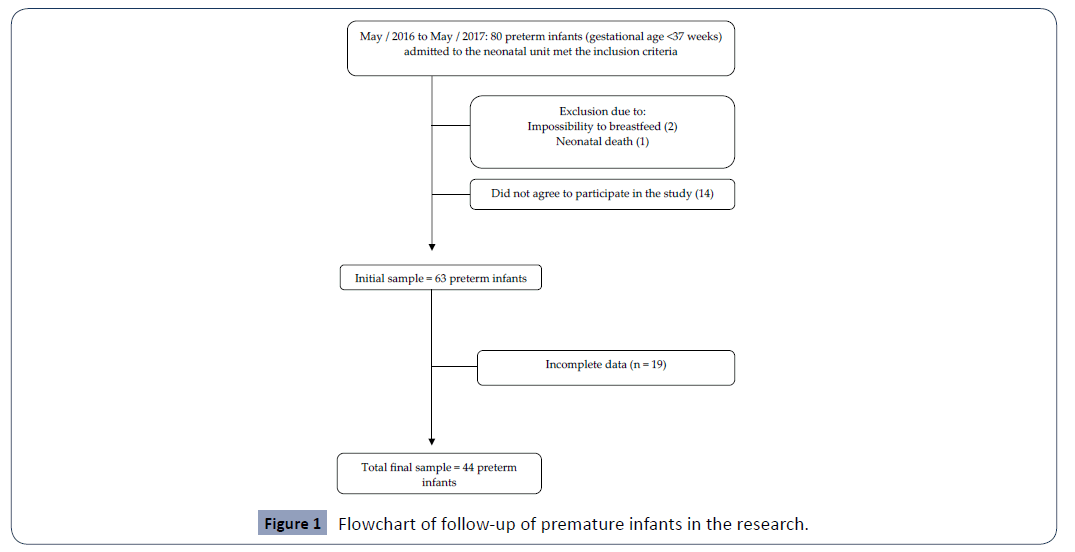
Figure 1: Flowchart of follow-up of premature infants in the research.
Table 1 Characteristics of preterm infants according to gestational age. Vitória de Santo Antão, Pernambuco, Brazil, 2017 (n=44).
| Variables |
≤ 33 weeks (n=19) |
≥ 34 weeks (n=25) |
p-value |
| Sex (n, %) |
|
|
|
| Boys |
12 (63.2) |
11 (44.0) |
0.010 |
| Girls |
7 (36.8) |
14 (56.0) |
| Birth weight (g in mean ± SD) |
1669 ± 362.9 |
2078 ± 415.5 |
0.001 |
| Gestational age (weeks in median, min-max) |
31 (27-33) |
35 (34-36) |
<0.0001 |
| Nutritional status at birth (n,%) |
|
|
|
| Small for gestational age (SGA) |
1 (5.3%) |
7 (28.0%) |
<0.0001 |
| Appropriate for gestational age (AGA) |
17 (89.4%) |
18 (72.0%) |
| Large for gestational age (LGA) |
1 (5.3%) |
0 (0.0%) |
SD: standard deviation
Table 2 presents the eating practices during hospitalization. The groups were similar regarding parenteral nutrition practices and the beginning of full enteral nutrition. On the other hand, it was noted that premature infants with GA ≥ 34 weeks had an earlier onset (0; 0-1 versus 1; 0-2 days, p=0.002) and shorter time of use (7; 1-24 versus 25; 5-36 days of life, p<0.0001) of enteral nutrition, started oral feeding earlier (4.5; 1-24 versus 7.5; 2-32 days of life, p=0.019), and evolved earlier to the free maternal breast (7; 1-26 versus 18; 3-34 days of life, p=0.0006).
Table 2 Characteristics of feeding practices during the hospitalization of premature infants according to gestational age. Vitória de Santo Antão, Pernambuco, Brazil, 2017.
| Variables |
≤ 33 weeks (n=19) |
≥ 34 weeks (n=25) |
p-value |
| Parenteral nutrition |
| Start (days of life in median, min-max) |
2 (1-4)a |
2 (1-4)b |
>0.9999 |
| Duration (days in mean ± SD) |
5.8 ± 2.1c |
5.7 ± 0.6d |
0.922 |
| Enteral nutrition |
| Start (days of life in median, min-max) |
1 (0-2) |
0 (0-1)e |
0.002 |
| Start FEN (days of life in median, min-max) |
9.0 ± 4.0f |
7.5 ± 2.9g |
0.341 |
| Duration (days in mean ± SD) |
25 (5-36)h |
7.0 (1-24)i |
<0.0001 |
| Oral feeding |
|
|
|
| Start oral feeding(days of life in median, min-max) |
7.5 (2-32) j |
4.5 (1-24)k |
0.019 |
| Start FMB (days of life in median, min-max) |
18 (3-34)l |
7 (1-26)m |
0.0006 |
| Type of milk (oral / tube) in the 1st week of life (n,%) |
| Infant formula |
1 (5.3%) |
0 (0.0%) |
0.024 |
| Breast milk |
7 (36.8%) |
7 (30.4%) |
| Mixed |
11 (57.9%) |
16 (69.6) |
| Use of breast milk additive (n,%) |
11 (57.0%) |
4 (16.0%) |
<0.0001 |
| Type of food at hospital discharge (n,%) |
| Infant formula |
2 (12.5%) |
2 (9.5%) |
0.0017 |
| Exclusive breastfeeding |
9 (56.2%) |
16 (76.2%) |
| Mixed breastfeeding |
5 (31.3%) |
3 (14.3%) |
FEN: full enteral nutrition; FMB: Free maternal breast; SD: standard deviation.
a: n=7; b n=3; c: n=6; d: n=4; e: n=22; f: n=16; g: n=10; h: n=16; i: n=22; j: n=15; k: n=23; l: n=18; m: n=23.
In the first week of hospitalization, almost all preterm infants (97.6%) were receiving breast milk (exclusive or mixed).
Premature infants with gestational age ≥ 34 weeks presented less need for fortified human milk, when compared to PTNB with GA ≤ 33 weeks (16.0% versus 57.0%, p <0.0001). Premature infants with GA ≥ 34 weeks presented a higher prevalence of exclusive breastfeeding at the time of hospital discharge (76.2% versus 56.2%, p=0.0017), as shown in Table 2.
In the assessment of nutritional evolution and length of stay in the neonatal unit (Table 3), no difference was found in the mean daily weight gain between the groups. However, the percentage of weight loss on the 7th day of life (9.6 ± 3.8% versus 6.8 ± 3.6%, p=0.021), recovery of birth weight (16.4 ± 3.7 versus 12.0 ± 4.6 days, p=0.025), and length of hospital stay (31; 10-55 versus 12.5; 3-55 days, p=0.0001) were longer in the group of premature infants with GA ≤ 33 weeks compared to those with GA ≥ 34 weeks.
Table 3 Characteristics of the nutritional evolution of preterm infants according to gestational age. Vitória de Santo Antão, Pernambuco, Brazil, 2017.
| Variables |
≤ 33 weeks (n=19) |
≥ 34 weeks (n=25) |
p-value |
| Weight loss on the 7th day of life (% in mean ± SD) |
9.6 ± 3.8a |
6.8 ± 3.6b |
0.021 |
| Mean daily weight gain (g in mean ± SD) |
6.1 ± 3.0c |
9.5 ± 8.7d |
0.145 |
| Recovery of birth weight (days of life in mean ± SD)c |
16.4 ± 3.7e |
12.0 ± 4.6f |
0.025 |
| Length of stay (days in median, min-max) |
31 (10-55) |
12.5 (3-55) |
0.0001 |
SD: standard deviation
a: n= 18; b: n=22; c: n=18; d: n=11; e: n=13; f: n=14
Table 4 shows the study of correlations between variables related to dietary practices and nutritional evolution. There was a positive correlation between the beginning of enteral nutrition and the length of hospital stay (r=0.410, p=0.007).
Table 4 Correlation between variables related to dietary practices and nutritional evolution. Vitória de Santo Antão, Pernambuco, Brazil, 2017.
| Related variables |
r |
p-value |
| Start of PN (days of life) and daily weight gain (g) |
-0.193 |
0.669 |
| Start of EN (days of life) and recovery of birth weight (days of life) |
0.052 |
0.821 |
| Start of EN (days of life) and length of stay (days) |
0.410 |
0.007 |
| Start of FMB (days of life) and daily weight gain (g) |
0.207 |
0.288 |
| Start of oral feeding (days of life) and recovery of birth weight (days of life) |
0.058 |
0.800 |
| Daily weight gain (g) and length of stay (days) |
0.230 |
0.238 |
| Physiological weight loss (%) and length of stay (days) |
0.086 |
0.626 |
PN: parenteral nutrition; EN: enteral nutrition; FMB: free maternal breast.
Discussion
In the present study, we compared feeding practices and anthropometric variables among preterm newborns admitted to a neonatal unit in the interior of Pernambuco, according to the degree of prematurity, and correlated these practices with nutritional evolution.
The nutrition of preterm newborns has been widely discussed in the literature and it is noted that the nutritional practices in the NICU vary between hospitals [5-7,14,15]. In our study, PN and EN started in the first days of life, similar to what was observed in premature infants admitted to an NICU in northeastern Brazil [16]. There is sufficient evidence that parenteral nutrition in the first days of life prevents the initial loss of protein and hypoglycemic peaks, and maintains the growth of organs and tissues [6]. Enteral nutrition meanwhile promotes the functional and structural integrity of the gastrointestinal tract, favoring maturation of motor activity and resulting in rapid weight gain and obtention of full enteral nutrition earlier [14]. Late onset of FEN is seen as a risk factor for increased morbidity and mortality in the perinatal period and beyond [7].
The time required to achieve FEN was adequate (up to 14 days of life) [10]. Menezes et al. [16] followed the clinical evolution of 137 preterm infants in a public maternity hospital in the northeastern region of Brazil and also observed that babies reached FEN by the second week of life (10 ± 5 days of life). However, the authors did not specify the volume of diet needed to achieve FEN.
Oral feeding in the neonatal period requires the coordination of suction, swallowing, and breathing movements, which takes place around the 34th week of GA. Before this, it is necessary to use enteral nutrition probes for feeding [17,18]. In our research, the beginning of oral feeding and the free maternal breast were earlier among premature GI ≥ 34 weeks, who also passed less time using enteral nutrition. Scochi et al [19] identified a negative correlation between GI at birth and the duration of the food transition to oral route, showing that the more immature PTNBs make the transition in a longer time. It is worth noting that this factor alone should not be decisive for the beginning of oral feeding, as the clinical stability and neurological maturity of the PTNB should also be evaluated [18].
The food of choice for the PTNB is their own mother’s milk [8]. In the first week of life, almost all preterm infants were already receiving breast milk (exclusive or mixed). The advantages of offering breast milk to premature infants are well established in the literature, such as the provision of intestinal maturation factors and antioxidants, immunological protection, pain relief, interaction between mother and child, reduced risk of rehospitalization after discharge, and prevention of necrotizing enterocolitis and delayed sepsis [5-7,20,21].
The continuous supply of nutrition through fortified breast milk is important during neonatal hospitalization since human milk as the only source of nutrition does not fully meet the nutritional needs of very premature babies with birth weight <1,500 g [5]. Although it is possible to supply the energy and protein needs with larger amounts of milk without additives, this does not necessarily meet the needs for calcium, phosphorus, or other micronutrients [6]. Due to premature GA ≤ 33 weeks being more vulnerable to nutritional deficits, greater use of breast milk additive was observed in this group. Martins and Krebs [22], when studying the effects of the use of additives in the mother's own human milk in PTNB infants ≤ 34 weeks hospitalized in a neonatal unit, confirmed that the premature infants in the intervention group presented better growth, with a significant increase in length and head circumference. Maintaining exclusive breastfeeding until hospital discharge is a major challenge faced by mothers of preterm infants, since the deprivation of spontaneous contact between mother and child arouses a feeling of anxiety, fear, and uncertainty regarding the newborn's survival. In addition to witnessing their children's daily struggle for life, they need to be persistent to overcome this difficult experience, seeking to remain calm, within the possibilities, so as not to interfere with milk production [23]. Maciel, Almeida and Braga [24] emphasize the importance of humanized and educational assistance with mothers, stating that when education is properly offered, it can reconstruct meanings and dispel some myths, thus being able to promote breastfeeding.
The frequency of exclusive breastfeeding at hospital discharge can vary between 10% and 56.2% [16,25]. In our study, the prevalence of exclusive breastfeeding at hospital discharge was higher in premature infants with GA> 34 weeks (76.2%). However, a study carried out with PTNB of GI <32 weeks found that the mother's own breast milk supply on the 7th postnatal day is associated with EBF at 36 weeks corrected GA (OR=1.18 per 10 mL/kg of breast milk; 95% CI=1.06-1.32), showing that it is possible to achieve good exclusive breastfeeding rates in these premature babies [26].
Expectations regarding the postnatal growth of the PTNB are that there will be an initial weight loss, followed by recovery of birth weight. The intensity and duration of these phases is inversely proportional to the GA and the birth weight and directly proportional to the severity of the newborn's condition, also being influenced by the nutritional practice [7,27]. During the first weeks of life, preterm infants have high energy expenditure [16], using their limited body reserves to maintain energy production and, consequently, their vital functions, at the expense of growth [6].
Our data showed that physiological weight loss and birth weight recovery occurred as expected in both groups, being longer among premature infants with GA ≤ 33 weeks, who also had lower birth weight. A study carried out with 61 preterm infants suitable and small for gestational age (birth weight below 1,500 grams or gestational age <32 weeks) admitted to a NICU in Rio de Janeiro, also showed a similar evolution of postnatal growth among preterm infants suitable for gestational age, who also had a gestational age <32 weeks (29 ± 1.3 weeks) [28].
The length of hospital stay can be considered an indicator of severity for PTNB. Lima et al [29] demonstrated that the increase of one day in the length of hospital stay for very low birth weight preterm infants increased the chance of growth restriction of the head circumference and weight at discharge (z score ≤ −2 for corrected age), by 3 % and 2%, respectively. In our study, premature infants with GA ≤ 33 weeks also stayed longer in the neonatal unit. However, the period was lower than that found by Abranches et al [28] when studying premature infants with GA <32 weeks suitable for gestational age (39.7 ± 13.8 days). A possible explanation for this finding is that the physiological skills considered essential for hospital discharge are reached by most preterm infants at around 36 to 37 weeks of corrected age. Thus, the lower the GA, the greater the time to achieve these skills, which implies a prolonged stay in the neonatal unit [12].
The correlation observed between the start of enteral nutrition and the length of hospital stay demonstrated that the later the premature infant starts enteral nutrition, the longer the hospital stay. This correlation was also observed in other studies carried out in Brazil [25] (r=0.41; p=<0.026) and in Spain [30] (r=0.409; p=<0.001). It should be noted that a correlation does not necessarily imply a causal relationship. However, a review study showed that early enteral nutrition strategies, in addition to being well tolerated by low weight preterm infants, result in a rapid recovery of lost weight and reduced hospitalization time [7].
Conclusion
Premature infants with lower GI spent more time on enteral nutrition; started the transition to oral feeding and free maternal breast later; and showed a greater need for fortified breast milk, lower frequency of EBF at hospital discharge, and longer hospital stay. The initial weight loss, recovery of birth weight, and days necessary to reach NEP occurred within the expected ranges in both groups, although this was more accentuated among premature GI ≤ 33 weeks. In the current study, the early onset of enteral nutrition was correlated with a shorter hospital stay.
The definition of sample groups according to the degree of prematurity allows a closer look by the professional to detect the group at greatest risk and adopt nutritional strategies that promote growth in the appropriate standards. It is noted that in the routine of the NICU in question, practical measures are already taken, whose benefits to the PTNB are scientifically proven, such as the establishment of nutrition in the first 24 hours of life, and use of human milk and breast milk additives.
The present study brings valuable information contributing to the improvement in nutritional care for PTNBs. However, it also has limitations. The lack of quantitative variables in the diet and clinical profile of the premature infants made it impossible to assess the influence on nutritional evolution. Finally, the continuity of studies of this nature are recommended with expansion of the studied sample and which allow follow-up after hospital discharge, in order to understand the influence of neonatal nutrition on the long-term growth process.
Funding: This research received no external funding.
Acknowledgments: We thank all premature infants, surviving or not, and their mothers, for participating in this study and especially for the examples of perseverance, determination, and overcoming odds. We also thank the members of the “Premature” project, for their dedication and collaboration in the research.
Conflicts of interest: The authors declare no conflict of interest.
32676
References
- Leal MC, Esteves-Pereira AP, Nakamura-Pereira M, Torres JA, Theme-Filha M, et al. (2016) Prevalence and risk factors related to preterm birth in Brazil. Reprod Health 13: 127.
- de Miranda AM, Cunha DIB, Gomes SMF (2010) A influência da tecnologia na sobrevivência do recém-nascido prematuro extremo de muito baixo peso: revisão integrativa. Rev Min Enferm 14: 435-442.
- Liu L, Oza S, Hogan D, Chu Y, Perin J, et al. (2016) Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388: 3027-3035.
- Su BH (2014) Optimizing nutrition in preterm infants. Pediatr Neonatol 55: 5-13.
- Hay WW (2018) Nutritional support strategies for the preterm infant in the neonatal intensive care unit. Pediatr Gastroenterol Hepatol Nutr 21: 234-247.
- Ximenes Neto FRG, Damasceno JR, Machado MMT, Silva ASR, SIilva RCC da, et al. (2014) Nutrition in premature and low birth weight infants: an integrative review. Rev Soc Bras Nurse Ped 14: 40-46.
- Kleinman RE, Greer FR (2013) Pediatric Nutrition, 7th Edition (Sponsored Member Benefit). Am Acad Pediatrics.
- Fenton TR, Kim JH (2013) A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13.
- Margotto PR (2013) Assistance to the Newborn at Risk (3rd Edn). Brasília ESCS.
- Básica CA (2009) Child health: child nutrition: breastfeeding and complementary feeding. Ministry of Health Brasília.
- Silveira RC (2012) Outpatient follow-up of preterm: nutritional monitoring. Porto Alegre Soc Bras Pediatrician.
- Pediatria SB (2009) Nutritional assessment of children and adolescents - Manual of Guidance. Brazilian Society of Pediatrics.
- Castro MJ, Totta G, García F, Marcano J, Ferrero JL (2013) Nutritional management of premature babies. Venezuelan Archives of Childcare and Pediatrics 76: 111-118.
- Ho MY, Yen YH (2016) Trend of nutritional support in preterm infants. Pediatr Neonatol 57: 365-370.
- Menezes MA, Garcia DC, de Melo EV, Cipolotti R (2014) Premature newborns assisted by the Kangaroo Method: evaluation of a cohort from birth to six months. Rev Paul Pediatr 32: 171-177.
- Mizuno K, Ueda A (2003) The maturation and coordination of sucking, swallowing, and respiration in preterm infants. J Pediatr 142: 36-40.
- Yamamoto RCC, Prade LS, Bolzan GP, Weinmann ARM, Keske-Soares M (2017) Readiness for oral feeding and oral motor function in preterm infants. Rev CEFAC 19: 503-509.
- Scochi CGS, Gauy JS, Fujinaga CI, Fonseca LMM, Zamberlan NE (2010) Oral food transition in preterm infants at a Baby Friendly Hospital. Acta Paul Nursing 23: 540-545.
- Eidelman AI, Schanler RJ (2012) Breastfeeding and the use of human milk. Pediatrics 129: e827-e841.
- Schanler RJ (2015) In time: Human milk is the feeding strategy to prevent necrotizing enterocolitis. Rev Paul Pediatr 33: 131-133.
- Martins EC, Krebs VLJ (2009) Efeitos do uso de aditivo no leite humano cru da própria mãe em recém-nascidos pré-termo de muito baixo peso. J Pediatr 85: 157-162.
- Paiva CVA, Saburido KAL, de Vasconcelos MN, da Silva MAM (2013) Breastfeeding a hospitalized newborn: Difficulties of mothers with children in neonatal intensive and intermediate care units. Reme Rev Min Enferm 17: 924-931.
- Maciel IVL, de Almeida CS, Braga PP (2014) Breastfeeding in the context of prematurity: The Maternal speech 8: 1178-1184.
- Lopes CC, Machado RC, de Lima GCF, Reis D, Saunders C, et al. (2018) Enteral nutrition practices in preterm infants in the neonatal unit of a public maternity ward. World of Health 42: 696-709.
- Wilson E, Christensson K, Brandt L, Altman M, Bonamy AK (2015) Early provision of mother’s own milk and other predictors of successful breast milk feeding after very preterm birth: a regional observational study. J Hum Lact 31: 393-400.
- Cardoso-Demartini AA, Bagatin AC, Silva RPGVC, Boguszewski MCS (2011) Crescimento de crianças nascidas prematuras. Bras Endocrinol Metab 55: 534-540.
- de Abranches AD, Soares FVM, Villela LD, Méio MDBB, Zin OA, et al. (2018) Energy expenditure, growth, and nutritional therapy in appropriate and small for gestational age preterm infants. J Pediatr (Rio J) 94: 652-657.
- Azara P, Lima T, Carvalho M De, Carolina A (2014) Variables associated with extra uterine growth restriction in very birth weight infants. J Pediatr (Rio J) 90: 22-27.
- Izquierdo ELO, Lobato ES, Pérez IC, Sánchez MSH, Vilaplana LC (2012) Delayed acquisition of suck-swallow-respiration in the preterm period; effects of early stimulation. Nutr Hosp 27: 112-1126.






