Keywords
Mucoadhesive, Dorzolamide Hydrochloride, PolyAcrylic Acid, Xanthan Gum, Non-fickian.
Introduction
Eye is the most interesting organ due to its drug disposition characteristics. The poor accessibility of a number of ocular regions to systemic circulation makes local delivery via topical administration the preferred route for the treatment of ocular diseases. Typical conditions that require ocular administration include corneal disorders (i.e. glaucoma) and eye infections (i.e. conjunctivitis)[1] Ophthalmic drug delivery is one of the most interesting and challenging endeavours facing the pharmaceutical scientist. The biological barriers involved for ocular delivery are the permeability barriers posed by cornea and other regions, as well as the tear washout and blinking reflexes designed to remove foreign substances from the eye. A significant challenge to the formulator is to circumvent (bypass) the protective barriers of the eye without causing permanent tissue damage.[2] A significant challenge to the formulator is to circumvent (bypass) the protective barriers of the eye without causing permanent tissue damage.[2] Furthermore the ocular region is very sensitive and cannot withstand high local concentrations of drugs and vehicles without irritation. Because of these limitations, designing formulations and delivery systems for topically applied ophthalmic drugs is challenging. It requires thorough understanding of physiological basis of the protective mechanism designed by the eye which allows only 1- 10% of topically applied dose to be absorbed locally.[1]
Development of newer, more sensitive diagnostic techniques and novel therapeutic agents continue to provide ocular delivery systems with high therapeutic efficacy. Conventional ophthalmic formulations like solution, suspension, and ointment have many disadvantages which result into poor bioavailability of drug in the ocular cavity. The specific aim of designing a therapeutic system is to achieve an optimal concentration of a drug at the active site for the appropriate duration. Ocular disposition and elimination of a therapeutic agent is dependent upon its physicochemical properties as well as the relevant ocular anatomy and physiology. A successful design of a drug delivery system, therefore, requires an integrated knowledge of the drug molecule and the constraints offered by the ocular route of administration [2]
The various approaches that have been attempted to increase the bioavailability and the duration of the therapeutic action of ocular drugs can be divided into two categories. The first one is based on the use of sustained drug delivery systems, which provide the controlled and continuous delivery of ophthalmic drugs. The second involves maximizing corneal drug absorption and minimizing precorneal drug loss.[2] Ideal ophthalmic drug delivery system must be able to sustain the drug release and to remain in the vicinity of front of the eye for prolong period of time. Such systems should be more hydrophobic, minimize interference with blinking and exhibit pseudoplastic behaviour. A better approach of ocular product behaviour coupled with formulation optimization can lead the way to development of newer ocular drug delivery systems.[3]
Materials and Methods
Dorzolamide hydrochloride was obtained from Cipla Ltd.,Verna Goa. Xanthan Gum was obtained from Lucid Colloids Ltd., Mumbai. Carbopol 971 P from Lubrizol, Mumbai. HPMC K4M of pharma grade was procured by Colorcon Asia, Verna-Goa. Benzalkonium Chloride from Centaur Pharmaceuticals Karaswada Mapusa. Mannitol, Disodium edentate, Potassium dihydrogen ortho- phosphate, Sodium hydroxide all were laboratory reagent obtained from lobachemie. FTIR Spectrophotometer of Prestige- 21FTIR Shimadzu was used. UV Spectrophotometer of Perkin Elmer Lambda 25 was used.
Selection of Vehicle Solubility of Dorzolamide hydrochloride was tested in distilled water and a series of Phosphate Buffers of different pH. Phosphate buffer pH 6.8 was selected as vehicle as it formed sols having pH value close to pH 6 as using distilled water resulted acidic solutions having pH value less than 5.
Determination of In situ gelling concentration was done in Aqueous solutions of both the polymers decided to be used in the formulations, i.e. Xanthan Gum and Carbopol 971P were prepared in concentrations ranging from 0.1% to 1.0% (with and without 0.2% HPMC) and tested for gelling capacity. Gelling capacity was determined by placing a drop of the system in a vial containing 2 ml of artificial tear fluid freshly prepared and equilibrated to 370 C and visually assessing the gel formation and noting the time for gelation and the time taken for the gel form to dissolve.
In both the cases, the minimum concentrations at which the respective polymers showed gelling were noted. The results are shown in the Table No.1
| Polymer* |
Minimum Concentration Showing Gelling In STF (%W/V) |
| WITHOUT HPMC |
WITH HPMC |
| Xanthan Gum |
0.35 |
0.2 |
| Carbopol 971P |
0.5 |
0.3 |
Polymers were in combination with HPMC (0.4% w/v).
Table 1: Determination of polymer concentration.
| INGREDIENTS |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
| DORZOLAMIDE HYDROCHLORIDE |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
| CARBOPOL 971P |
0.3 |
0.4 |
0.5 |
--- |
--- |
--- |
0.2 |
| XANTHAN GUM |
--- |
--- |
--- |
0.2 |
0.25 |
0.3 |
0.1 |
| HPMC K 4M |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
| DISODIUM EDETATE |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
| BENZALKONIUM CHLORIDE |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
| MANNITOL |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
| AQUEOUS BUFFER BASE |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
Table 2: Composition of Formulations (all the quantities are in %w/v).
Methodology adopted in which Polymer solution was prepared by soaking HPMC K4M and the polymer in 50 ml of phosphate buffer pH 6.8 in a beaker. Dorzolamide hydrochloride was dissolved in another portion of phosphate buffer. Benzalkonium chloride (BKC), edetate disodium (EDTA) and mannitol were then added to the above drug solution. The drug solution was added to the polymer solution with stirring taking precaution not to incorporate air bubbles. The final solution was then transferred to a 100 ml volumetric flask and the volume was made up with Phosphate buffer.
Estimation of dorazamide hydrochloride spectrophotometric method based on the measurement of absorbance at 253nm in solvent phosphate buffer pH 7.4 was used to estimate Dorzolamide Hydrochloride.
A) Preparation of Simulated Tear Fluid- Phosphate buffer pH 7.4
The buffer was prepared by mixing 50 ml of 0.2M Monobasic Potassium di hydrogen Ortho Phosphate with 39.1 ml of 0.2M Sodium Hydroxide and diluting it with distilled water to produce 200 ml.
B) Standard calibration curve of Dorzolamide Hydrochloride
Accurately weighed 10mg of Dorzolamide hydrochloride was dissolved in 100 ml phosphate buffer pH 7.4 to get a stock solution of 100 μg/ml. From this stock solution aliquots of 3, 6, 9, 12, 15, 18, 21 and 24ml were withdrawn and further diluted to 100ml with buffer to obtain a concentrations range of 3 to 24 μg/ml.
The absorbance of the resulting solutions was measured at 253nm on a UV spectrophotometer using phosphate buffer pH 7.4 as blank. The standard curve was obtained by plotting a graph of absorbance v/s concentration (μg/ml). From the standard curve, the amount of Dorzolamide hydrochloride in the formulations was estimated.
Aqueous solutions of Carbopol 971P and Xanthan gum coupled with added viscolizers are attractive in-situ gel forming systems, promising controlled ocular drug delivery of Dorzolamide Hydrochloride. All the systems exhibited zero order kinetics suggesting drug release in a controlled manner and release profile was diffusion associated with erosion of the polymer. Devised formulations were found to be effective in vitro and ex vivo in management of related ocular pathological conditions.
Clarity is one of the important characteristic features of ophthalmic preparations.
pH is one of the most important parameter involved in the ophthalmic preparations.
The sols were further converted to gels using phosphate buffer pH 7.4 and the physical appearance of the gel was taken note off. pH is one of the most important parameter involved in the ophthalmic formulation.
Measurement of pH was carried out for sols and the gels (obtained by neutralizing sols to gels with phosphate buffer pH 7.4) at room temperature with the help of a pH meter.The measurement of the time taken to form the gel and the extent of gellation was determined.The test was carried out in test tubes wherein the formulations were mixed with Simulated Tear Fluid (maintained at 37°C) in the proportion 25:7. The proportion is so chosen because the application volume of eye drops is usually 25 μl and the volume of tear fluid present is 7 μl. Also the time for which the formed gel retains its integrity was noted. Isotonicity is an important characteristic of ophthalmics. Formulation was mixed with few drops of blood and observed under microscope at 45X magnification and compared with standard marketed ophthalmic formulation containing dorzolamide.
FT-IR spectra of the individual drug and the drug polymer mixtures were taken using FTIR-IR (Prestige-21FTIR Shimadzu Sr. no. A21004401461) and the spectra were run from 400 to 2000 cm-1 to rule out any possible incompatibilities in solid state. Calorimetric characterization of Dorzolamide hydrochloride alone and in combination with Carbopol, xanthan and HPMC K4M were carried out using a DSC 823e instrument (Mettler Toledo). Nitrogen was used as the purging gas at a rate of 10 ml/min. All experiments were performed using non-hermetic aluminium pans, into which samples were accurately weighed, and then simply covered with a lid. Culture media chosen must necessarily support growth of different microorganisms viz bacteria, moulds and yeasts. Therefore Soyabean Casein Digest Media (SCDM) as fungal media and Fluid Thioglycollate Media (FTG) as bacterial media, were used in a sterile condition after autoclaving at 1210C for 20 minutes at 15 psi. Ex vivo was done using goat skin using the permeability studies.
Results and Discussions
All the seven formulations were prepared and packed in 10 ml amber colored vials and sealed. Turbidity was noticed in the formulations, immediately after sterilization, which disappeared after cooling. The turbidity can be assigned to the precipitation of HPMC at high temperatures due to thermo-reversible gelation, which then disappeared after cooling. The Standard curve of Dorzolamide hydrochloride was recorded. Calibration table for the spectrophotometric determination of Dorzolamide hydrochloride is as given below which gives the absorbance values of Dorzolamide hydrochloride standard solutions containing 2-10μg/ml of drug in phosphate buffer pH 7.4. Figure 1 shows the standard calibration curve with slope 0.0569 and regression coefficient 0.9998. The calculations of Drug Content Uniformity, In vitro drug release studies and stability studies are based on this calibration curve.
| Concentration in µg/ml |
Absorbance at 253nm |
| 3 |
0.1694 |
| 6 |
0.3306 |
| 9 |
0.5134 |
| 12 |
0.6756 |
| 15 |
0.8574 |
| 18 |
1.0249 |
| 21 |
1.1798 |
| 24 |
1.3682 |
Table 3: Concentration vs Absorbance
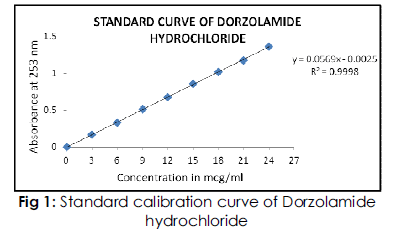
Fig 1: Standard calibration curve of Dorzolamide hydrochloride.
Graph of concentration vs absorbance in which a linear increase in absorbance is achieved with increase in concentration. Both quantities are directly proportional.
Evaluation for the gelling system was done w.r.t clarity and appearance. The physical appearance of all seven formulations showed that, they all were free flowing liquids and slightly milky in appearance. The milky appearance can be attributed to the polymers present in the formulations. The pH of all sols were very close to the physiological pH while the gels were completely neutral. Viscosity studies were done using Brookfield Viscometer. Rhelogical studies The Formulation was shear thinning and an increase in shear stress was observed with increase in angular velocity (pseudo plastic rheology).The drug content uniformity was calculated for all seven formulations, the results of which are tabulated as below. For the various formulations the drug content uniformity varies between 97.62% to 99.33%.
Solid state compatibility studies were carried out on Drug-Polymer mixtures. The study indicates that the peaks in the physical mixture correlate with the drug spectrum ranging from 4000-400cm- 1. Also there were neither alterations in the major/ characteristic peaks nor the appearance of any extra peals indicating that the drug is compatible with all the added excipients.
DSC thermogram of Dorzolamide HCl and physical mixtures of Drug with the polymers. Dorzolamide HCl showed a long and sharp characteristic endothermic peak at 278.720C due to its phase transition system. The physical mixture of drug with polymers shows characteristic peak at 273.57, 261.45 and 271.44. The slight change in characteristic peak may be due to fusion of excipient present in the Physical mixture. From this result, it is clear that there is no interaction between Drug and the excipients.
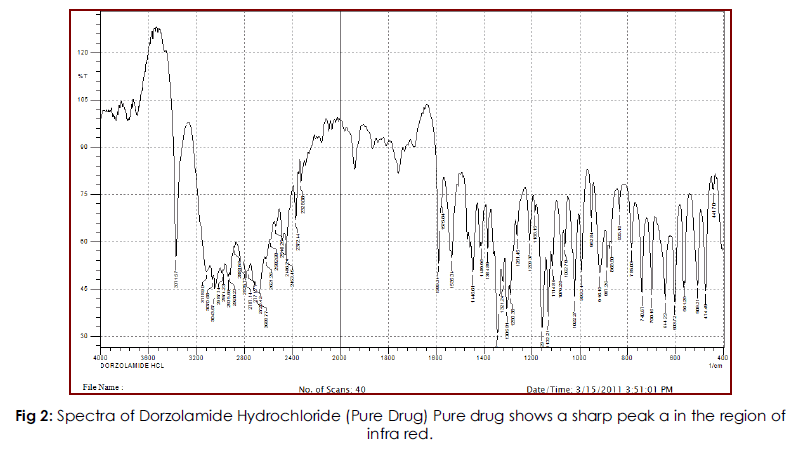
Fig 2: Spectra of Dorzolamide Hydrochloride (Pure Drug) Pure drug shows a sharp peak a in the region of infra red.
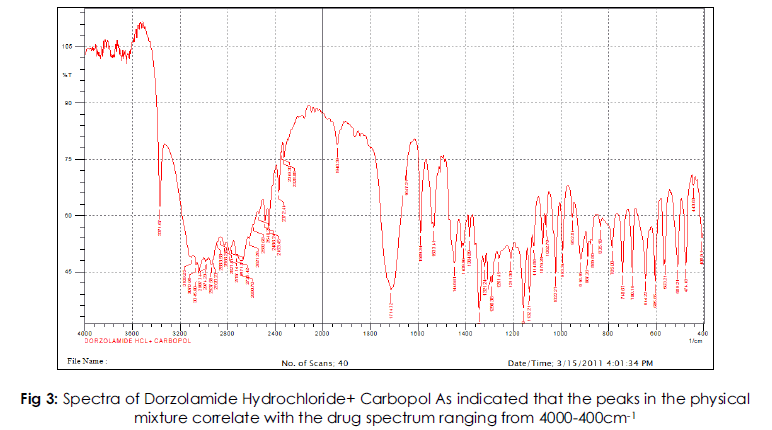
Fig 3: Spectra of Dorzolamide Hydrochloride+ Carbopol As indicated that the peaks in the physical mixture correlate with the drug spectrum ranging from 4000-400cm-1.
All the seven formulations showed an initial burst release. The cumulative percent drug release from formulations F1, F2 and F3 containing Carbopol was found to be 82.68%, 88.64% and 80.66% respectively. And that from formulations F4, F5 and F6 containing xanthan gum was found to be 90.84%, 88.84% and 83.08% respectively. It can be seen from above results that the drug release from the batches containing lower polymer content was higher than the batches containing higher polymer content (F3, F6). This happened probably because higher concentrations of polymers show higher extent of gelling and hence the drug cannot penetrate the gel matrix and come out in the surrounding dissolution medium.Out of the two polymers used, Xanthan gum has shown the highest and the fastest drug release. The formulation F4 containing 0.2% w/v of Xanthan Gum has shown the drug release of 90.84% and hence this batch is taken as the best batch. The lowest release (78.98%) is shown by formulation F7 which is prepared by using both the polymers in combinations by reducing their concentrations to half. The formulation F2, containing Carbopol 971 P NF has also shown a good release of 88.64%. As expected from Carbopol, it combined with HPMC to give a reasonably sustained drug release. The release data was plotted for Zero Order and First Order kinetics and these plots were subjected to linear regression analysis. The regression coefficients of both the plots were calculated for comparison purposes. Zero Order plots for all the formulations were found to be linear. The regression coefficients predict that the release from the formulations best fit the Zero Order plots. Hence it can be concluded that the all the drug release from all the formulations follow the Zero order kinetics. The drug release data was also plotted for Peppa’s model. These plots were also linear and the slope values (n) were more than 0.5 indicating that the drug release is by Non-Fickian diffusion.
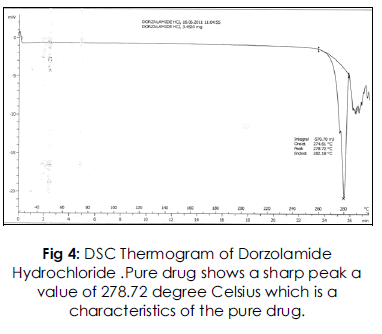
Fig 4: DSC Thermogram of Dorzolamide Hydrochloride .Pure drug shows a sharp peak a value of 278.72 degree Celsius which is a characteristics of the pure drug.
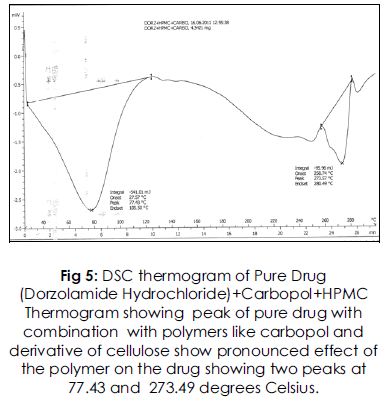
Fig 5: DSC thermogram of Pure Drug (Dorzolamide Hydrochloride)+Carbopol+HPMC Thermogram showing peak of pure drug with combination with polymers like carbopol and derivative of cellulose show pronounced effect of the polymer on the drug showing two peaks at 77.43 and 273.49 degrees Celsius.
Further, to ascertain whether the drug release is also prompted by erosion mechanism, the data was subjected to Erosion Plot. The regression coefficients were calculated which indicate that the data is best fitted to Erosion Plot for all formulations.
Hence it can be concluded that all the batches showed fairly high drug release because of presence of both diffusion and erosion mechanisms.
5761
References
- Hill J M, O’ Callaghan R J, Hobden J A, Kaufman H E. Corneal Collagen Shields for ocular Delivery. In: Mitra A K, eds. Ophthalmic drug delivery systems, New York: Marcel Dekker Inc., 1993;261-273
- Mundada AS, Avari JG, Mehta SP, Pandit SS, Patil AT. Recent advances in ophthalmic drug delivery system. Pharm Rev 2008; 6(1).
- Wagh VD, Inamdar B, Samanta MK. Polymers used in ocular dosage form & drug delivery system. Asian J Pharm 2008; 2(1):12-7.
- Gilbert S. Banker and Christopher T. Rhodes; Modern Phamaceutics Chapter 13, 3rd Edition; Volume 72; pages 489-546.
- Ophthalmic Preparations; Remington’s: The Science and Practice of Pharmacy; 19th Edition, Volume 2, Chapter 59; page 1563
- N. K. Jain, Ocular Drug Delivery, Controlled and Novel Drug Delivery, 1st edition, CBS publishers, pages 82-99.
- https://www.ivy- rose.co.uk/HumanBody/Eye/Anatomy_Eye.php.( Access on date 08/08/2009; 4.30 pm.)
- Hosoyaa K, Vincent HL, Kim KJ .Roles of the conjunctiva in ocular drug delivery: a review of conjunctival transport mechanisms and their regulation. Eur J Pharm Biopharm 2005; 60:227– 40.
- Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev 2006; 58:1131–35.
- New Ophthalmic Drug Delivery System. Drug Development and Industrial Pharmacy, 1995; 21 (1): 19-59,
- Jtirvinena K, Tomi J, Urttia SA. Ocular absorption following topical delivery.Adv Drug Deliv Rev 1995; 16:3-19.
- Nanjawade BK, Manvi FV, Manjappa AS. In situ- forming hydrogels for sustained ophthalmic drug delivery. J Control Release 2007; 122:119–34.
- Sarim Imam, AbhishekBansal, Bushetti S S, Arpita Singh, Himansu Chopra. Novel ocular dosage form in the treatment of glaucoma. The Pharma Research 2009; 01: 72-81
- Ophthalmic, Nasal, Otic and Oral preparations applied topically pharmaceutical dosage forms and drug delivery systems 6th edition Edited by Howard Ansel, Chapter 11, pg.396-411.
- Industrial Pharmacy by Lachmann, Verghese Publishing house, 3rd edition, 1987, pg.760-788
- Ludwig A, Van Ootenhgm M; Influence of Viscolyzers on the Residence of Ophthalmic Solutions Evaluated by Slit Lamp Fluorophotometry; S.T.P. Pharmaceutical Sciences 1992; 2; pages 81-87
- Davies N.M, Farr S.J, Hadgraft J, Kellaway I.W; Evaluation of Mucoadhesive Polymers in Ocular Drug Delivery. I. Viscous Solutions; Pharmaceutical Research 1991; 8; pages 1039- 1043.
- Sasaki H, Yamamura K, Mukai T, Nishida K, Nakamura M; Enhancement of Ocular Drug Penetration; Critical Reviews In Therapeutic drug Carrier Systems 1999; 16; pages 85-146.
- Rathore K. S., Nema R. K.; An Insight Into Ophthalmic Drug Delivery System; International Journal Of Pharmaceutical Sciences And Drug Research 2009; 1(1); pages 1-5.
- https://bocapremierhomes.com/boca-raton/a- review-on-topical-ophthalmic-drug-delivery- systems
- Ding S, Recent Developments in Ophthalmic Drug Delivery; PSTT 1998; 1; pages 328-335
- Gurtler F, Kaltsatos V, Biosrame B, Gurny R; Long Acting Soluble Bioadhesive Ophthalmic Drug Insert (BODI) Containing Gentamicin For Veterinary Use: Optimization And Clinical Investigation; Journal Of Controlled Release 1995; 33; pages 231-236.











