Keywords
Health related quality of life; Outcome assessment (healthcare); Psychometric properties; Tuberculosis
Introduction
Tuberculosis (TB) is a major disease infected approximately 10.4 million new cases in the world with 1.4 million deaths reported in the year 2015 [1]. The customary clinical and biological indicators often fail to characterize self-observed functions, and physical and mental well-being of patients. Thus, an area, health related quality of life (HRQOL) has increased curiosity about dreadful diseases, like TB [2]. HRQOL mainly indicate the perception of patients about their physical and mental health [3].
HRQOL is defined as “the extent to which patient’s subjective perception of physical, mental and social wellbeing are affected on a day to day basis by a disease and its treatment [3]. It is known that patients with chronic diseases, in addition to pure physical health also place high value on their mental and social wellbeing [3]. As a result, evaluation of HRQOL has become an important health outcome and an area of concern for policy makers, health care professionals and researchers [3].
Generally, HRQOL is evaluated by self-administered questionnaires filled by patients. Therefore, these questionnaires are stated to as patient reported outcome measures (PROMs). HRQOL instruments can be generic or disease specific. Generic instruments don’t need any specific situation for interpretation of results. Thus, the comparisons with healthy individuals can be made easily without accessing other diseases. Also, diseases specific instruments are more sensitive and need a specific health situation [4].
Previous studies on health relate quality of life of TB patients before 2008 indicated the two major domains of quality of life [5]. However, most studies were focused on the use of only one reported HRQOL. A detailed study was performed on impact of quality of life in TB patients based on a specific subgroup [6]. Although, various standard instruments for HRQOL measurement are available [7] but the reliability, validity and awareness of these instruments in public is still limited. This review described the present scenario of awareness and development for HRQOL measurements in the area of TB research. We aimed to evaluate the most frequently used HRQOL instrument(s) in the patients of TB to demonstrate the properties and general recovery patterns based upon the Consensus-Based Standards for the assortment of health status measurement instruments (COSMIN) checklist [8].
Methodology
This literature review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta analyses (PRISMA) guidelines [9].
Literature search strategy
PUBMED, EMBASE and other data bases were searched for HRQOL articles of TB patients published from 1 January 2004 till December 2015. The keywords such as health status, health related quality of life (HRQOL), outcome measurements, quality of life, patient reported outcomes (PRO) in combination with terms used to find studies on measuring properties of HRQOL were searched. A reference list of the included articles was also updated for other publications. Further measurements were performed on the basis of expert’s suggestions.
Selection criteria
Inclusion/Exclusion criteria: The articles with the following details were included in the review.
• Which described the generic or disease specific HRQOL as primary or secondary outcome.
• One or more measurement properties of an instrument that measured physical, mental and social aspects of HRQOL were examined.
• Included measures could be completed by self, parent or clinician.
The articles not filling the above mentioned criteria were excluded from the review. The target population containing TB patients suffering from any type, cause and degree of the severity. All the text articles were written in English language.
Study selection
Two independent reviewers (S.K) and (A.I) thoroughly examined the full articles of all the studies and the title or abstract which meet the selection criteria were retrieved. All disagreements and discrepancies were resolved by a third reviewer (A.N.K).
Data extraction and quality assessment
The methodology quality was rated using COSMIN (Consensus based Standards for the selection of health Measurement Instruments) checklist [10]. The checklist consists of 9 boxes with a list of 5-18 items per box. This criteria list indicated whether the included study meets the standard for good methodology quality or not. The measuring property in the checklist includes reliability, validity, responsiveness and interpretability. Each item is scored on the rating scale (i.e., poor, fair, good and excellent) [11]. Table 1 gives definitions of these measuring properties.
Table 1 Definitions of these measuring properties.
| Reliability |
Defined as the extent to which the measurement is free from measuring error and it include internal consistency and reliability [12]. |
| Validity |
Defined as the degree to which a questionnaire measures the construct to claim and it include content and structural validity [12]. |
| Responsiveness |
Defined as the ability of an instrument to detect change over time and it include only one measurement property [12]. |
| Interpretability |
Defined as the extent to which one can assign qualitative meaning to an instrument quantitative scores or change in scores [12]. |
Results synthesis
Measuring properties of each instrument can be rated as positive, negative, and indeterminate, on the basis of level of evidence. Initially this criteria was used for systematic reviews on clinical trials but it can also be used in reviews on measurement properties [11] as shown in Table 2. The assessment of the result is based on the criteria set by Terwee et al. [13] as shown in Table 3. Approval of research ethics or institutional review board was not required because this was based upon the published data.
Table 2 Degree of assessment for quality of measurement property.
| Degree |
Assessment |
Measures |
| Strong |
+++ or --- |
Excellent and consistence finding in one or multiple studies of good methodology quality |
| Moderate |
++ or -- |
Good and consistence finding in one or multiple studies of fair methodology quality |
| Limited |
+ or - |
Single study of fair methodology quality |
| Conflicting |
± |
Contradictory findings |
| Unknown |
? |
Studies with poor methodology quality |
Source: Tulder et al. [13] where + (positive results, – (negative results).
Table 3 Criteria for quality measurement properties.
| Measure |
Assessment |
Criteria comment |
| Reliability |
| Internal consistency |
+ |
Unidimensional subscale and Cronbach’s alpha =0.07 |
| - |
Not unidimensional or Cronbach’s alpha<0.7 |
| ? |
Unknown dimension or Cronbach’s alpha not determined |
| Measuring error |
+ |
MIC>SDC or MIC |
| - |
MIC =SDC or MIC = LOA |
| ? |
Undefined MIC |
| Reliability |
+ |
ICC= 0.70 or Pearson’s r= 0.80 |
| - |
ICC<0.70 or Pearson’s r<0.80 |
| ? |
Undetermined ICC orPearson’s |
| Validity |
| Content validity |
+ |
For target population all questionnaires to be relevant and complete |
| - |
Target population consider questionnaires irrelevant and incomplete |
| ? |
Target population not involved |
| Construct validity |
+ |
Factors explain 50% variance |
| - |
Factors explain <50% variance |
| ? |
Unmentioned explained variance |
| Hypothesis testing |
+ |
Correlation with instrument = 0.50 or 75% in accordance to the hypothesis |
| - |
Correlation with instrument <0.50 or <75% in accordance to the hypothesis |
| ? |
Correlations with undetermined constructs |
| Responsiveness |
| Responsiveness |
+ |
Correlation with instrument = 0.50 or 75% in accordance to the hypothesis or AUC =0.70 |
| - |
Correlation with instrument <0.50 or <75% in accordance to the hypothesis or AUC <0.70 |
| ? |
Correlations with undetermined constructs |
Source: Terween et al. [13] where AUC=area under curve, ICC=intra class correlation coefficient, LOA=limits of agreements, SDC=smallest detectable change. +positive rating, - negative rating, ?indeterminate
Results
Literature selection
The database search showed 6020 unique titles of relevant articles (Figure 1). We selected 99 closely related articles which meet all selection criteria. After, second round of screening (Full reading) 68 of these articles did not match the inclusion criteria. Finally, 30 articles were selected for review that measured the HRQOL in patients with TB in general and six studies with the aim to validate a HRQOL instrument in patients with TB. The main reason of exclusion was not using a HRQOL instrument or that the population under study was not TB specific.
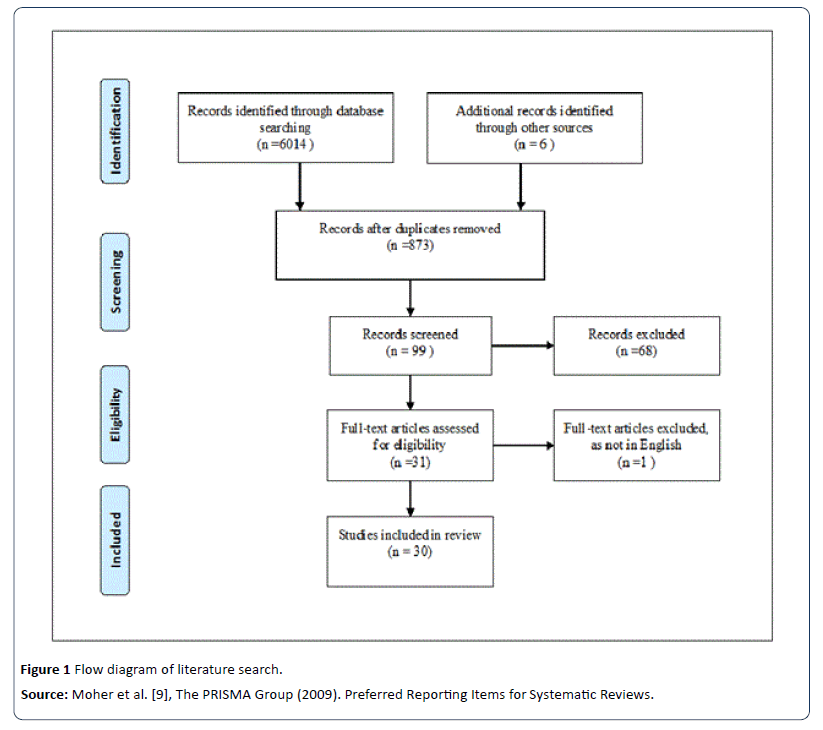
Figure 1: Flow diagram of literature search.
Summary of results
A total 30 articles were included to evaluate the 22 HRQOL measures in adult TB population (Table 4). All these articles were published in English. The data was based on comparative studies of TB population from China [14-34], two from Malaysia [18,23], five from India [14,15,29,35-38], four from Canada [5,24,25,30] one form each UK [17] Pakistan [35], Spain [36] Taiwan [28] South Africa [37] and Turkey [39]. Among the selected articles fourteen studies were cross sectional [5,14,26,27,30,33,34-40] and sixteen were prospective cohort based studies [15-26,29,31,32,41]. An article published by Chamla et al. [16] described the comparison of TB population group from general population. Another study used normative data from the Canadian population as the reference group and two studies included people with LTBI controls [20,30,39]. The sample size varied among all the studies and only one study reported statistical analysis of sample size [30]. In this review, all kinds of TB patients pulmonary TB, extra pulmonary TB, active TB disease, late TB infection, current TB and recurrent TB were included.
Table 4 Summary of included studies.
| Reference |
Country |
Scale used |
Study design |
Study Population |
Report |
Time points assessed |
Outcomes in HRQL domain |
| Abdulelah [24] |
Malaysia |
FACIT-TB |
Longitudinal cohort |
Pulmonary TB patients |
Clinic |
Unspecified time duringTB treatment |
Impaired HRQOL improves significantly |
| Aggarwal[15] |
India |
WHOQOL-BREF |
Cross-sectional |
Pulmonary TB patients |
Clinic |
Unspecified treatment of time |
Impaired HRQOL improves significantly with anti-tuberculosis treatment. Residual impairment is noticed in some patients at the end of treatment |
| Atif [19] |
Malaysia |
SF-36 v2 |
Longitudinalcohort |
Pulmonary TB patients |
Unspecified |
Baseline, end of intensive phase |
Impaired HRQOL improves significantly with anti-tuberculosis treatment. Scores in the physical and mental health components were still impaired after end of treatment |
| Balgude [16] |
India |
WHOQOL-BREF |
Longitudinal cohort |
Pulmonary TB patients |
Self |
Baseline and 6 months |
At baseline, HRQOL is significantly affected with physical and psychological domains most affected. All domains improve after 2 and 4 month treatment. |
| Baure[25] |
Canada |
SF36 v2 |
Longitudinal cohort |
TB patients |
Unspecified |
Unspecified time duringTB treatment |
Significant negative impact of TB on HRQOL |
| Baure [26] |
Canada |
SF-36, Standard Gamble |
Longitudinal cohort |
Active and latent TB patients |
Self |
Treatment initiation and 2 months |
HRQOL is significantly affected with physical domain and is improved after anti tuberculosis treatment |
| Babikako [27] |
Uganda |
MOS instrument, VAS |
Cross-sectional |
Active and latent TB patients |
Clinic |
Baselineand 2 months end of 8 months |
HRQOL of life improves with all the domains after treatment |
| Chamla [17] |
China |
SF-36 |
Longitudinal cohort |
Pulmonary and extra pulmonary |
Self |
Baseline,2 months end and 6 months |
HRQOL is impaired at baseline with physical scales most affected and improves due to treatment. |
| Chung [28] |
Taiwan |
WHOQOL-BREF |
Cross-sectional |
Pulmonary TB patients |
Self |
Baseline, end of treatment |
Impaired HRQOL improved with all the domains |
| Deribew[29] |
Ethiopia |
WHOQOL-HIV |
Longitudinal cohort |
TB patients |
Clinic |
Baseline and 6 months |
HRQOL is significantly affected with physical domain scale |
| Dhingra [42] |
India |
DR-12 |
Longitudinal cohort |
TB patients |
Clinic |
Baseline, end of intensive phase |
Improvement is significantly seen in all the domains of HRQOL after treatment |
| Dhuria [43] |
India |
WHOQOL-BREF |
Cross-sectional |
TB patients |
Clinic |
Baseline, 3months and end of treatment |
TB patients have an impaired HRQOL with significant improvement in all domains except social domain after treatment |
| Dino [31] |
Canada |
VAS and Standard Gamble |
Cross-sectional |
TB patients |
Clinic |
Baseline, end of 2 months |
After treatment ends significant improvement is seen |
| Dujaili [32] |
Iraq |
FACIT-TB |
Longitudinal cohort |
Pulmonary TB patients |
Self |
Baseline,2months and end of treatment |
Therapeutic intervention had a positive impact on HRQOL |
| Fu [33] |
China |
SAS |
Longitudinal cohort |
TB patients |
Self |
Unspecified time during TB treatment |
HRQOL is significantly associated with all the domains |
| Guo [5] |
Canada |
SF-36,HUI2/3,General Health VAS |
Cross-sectional |
TB patients |
Self |
Baseline, end of 2 months |
Impaired HRQOL improved with all the domains |
| Goday [34] |
Brazil |
asthma questionnaires 20 score |
Cross-sectional |
Pulmonary and MDRTB |
Self |
Unspecified time duringTB treatment |
Slight improvement in all domain of HRQOL after treatment |
| Hasain [35] |
Pakistan |
HAD, Illness Perception Questionnaires |
Cross-sectional |
TB patients |
Self |
Unspecified timeduring treatment |
Impaired HRQOL improved with all the domains |
| Kruijshaar [18] |
UK |
SF-36, EQ-5D |
Longitudinal cohort |
TB patients |
Clinic |
2 months of treatment |
Impaired HRQOL improves already after 2 month treatment, but is still below the UK norm score |
| Lopez-campas [36] |
Spain |
SRI |
Cross-sectional |
TB patients |
Clinic |
Unspecified time duringTB treatment |
After treatment ends significant improvement is seen in all the domains |
| Louw [37] |
South Africa |
SF-12 |
Cross-sectional |
TB patients |
Clinic |
Unspecified time duringTB treatment |
SIGNIFICANT improvement in mental and physical health after treatment ends |
| Magurie [23] |
Indonesia |
SGRQ |
Longitudinal cohort |
Pulmonary TB patients |
Self |
Baseline, 2months and 6 months |
Impaired HRQOL improves with treatment at 2 and 6 months |
| Mamani [20] |
Iran |
SF-36 |
Longitudinal |
Pulmonary and extra pulmonary |
Self |
Baseline, 2months and 6 months |
Impaired HRQOL improves due to treatment compared to controls |
| Marra [21] |
Canada |
SF-36,BDI |
Longitudinal cohort |
Active and latent TB patients |
Self |
Baseline,3 monthsand 6 months |
At baseline HRQOL is more affected in active than latent TB patients. Treatment improves HRQOL in active but not in latent TB. Patients with active TB have still impaired HRQOL after treatment completion compared to US norms |
| Masumoto [38] |
Philippines |
Short Form -8, Duke- UNC functional social support questionnaires, MRC |
Cross-sectional |
Pulmonary TB patients |
Clinic |
Unpecifiedtime duringTB treatment |
At baseline , HRQOL is significantly affected by physical and psychological score |
| Muniyandi [39] |
India |
SF-36 |
Cross-sectional |
Pulmonary and extra pulmonary |
Self |
Unspecified time duringTB treatment |
Improvement is significantly seen in all the domains of HRQOL after treatment |
| Pasipanodya[40] |
USA |
SGRQ |
Cross-sectional |
Pulmonary TB patients |
Clinic |
6 months and 8 months of TB treatment |
Scores of physical and mental health are still impaired after the end of treatment |
| Ralph [22] |
Indonesia |
SGRQ |
Longitudinal |
TB patients |
Clinic |
Baseline, 2 month end and 6 month |
Impaired HRQOL improved over treatment timebut morbidity does not end after 6 months |
| Rajeswari [42] |
India |
SF-36 |
Longitudinal cohort |
TB patients |
Self |
2 months and 6 months |
All domains are significantly impaired and improve after 2 months of treatment |
| Unalan [41] |
Turkey |
SF-36,BDI |
Cross-sectional |
TB patients |
Self |
Unspecified time duringTb treatment |
All domains of SF36 improve over treatment expect for social functioning |
The methodology quality of six studies [15,23,26,27,37,39] was validated by the use of COSMIN check list and results were demonstrated on the scale from poor to excellent (Tables 5 and 6). None of these studies included the measurement error while one study showed cross cultural validity [26]. Outcomes measures mostly focused on perceptions which are subjective and without a gold standard.
Table 5 The measurement properties of specific HRQOL measures used in TB.
| HRQOL measures |
Internal consistency |
Reliability |
Measurement error |
Content validity |
Structural validity |
Hypothesis testing |
Responsiveness |
Cross cultural validity |
| Duke Health Profile (DUKE) [37] |
+ + + |
+ |
|
+ |
+ |
+ |
|
|
| Modified version of St. Georges Respiratory Questionnaire (SGRQ) [39] |
? |
+ |
|
|
+ + |
|
|
|
| Functional Assessment of Chronic Illness Therapy- Tuberculosis (FACIT-TB) [23] |
+ + + |
+ + + |
|
+ + |
+ + |
++ |
+ + + |
|
| MOS instrument [26] |
+ |
+ |
|
+ + |
- |
|
|
+ + + |
| SF 36 Health Survey (SF 36) [15] |
+ + + |
+ + + |
|
|
|
|
|
|
| World Health Organizations Quality of Life-BREF (WHOQOL BREF) [27] |
+ + |
+ + |
|
+ + |
+ |
+ + |
|
|
Table 6 The methodology quality of HRQOL measurement properties as described in the original development articles.
| HRQOL measure |
Ref |
Internal consistency |
Reliability |
Measurement error |
Content validity |
Structural validity |
Hypothesis testing |
Responsiveness |
Cross cultural validity |
| DUKE |
[37] |
Excellent |
Fair |
|
Fair |
Fair |
Fair |
|
|
| MOS instrument |
[26] |
Fair |
Fair |
|
Good |
Poor |
|
|
Excellent |
| FACIT-TB |
[23] |
Excellent |
Excellent |
|
Good |
Good |
Good |
Excellent |
|
| SF 36 Health Survey (SF 36) |
[15] |
Excellent |
Excellent |
|
|
|
|
|
|
| SGRQ |
[39] |
Poor |
Fair |
|
Good |
|
|
|
|
| WHOQOL BREF |
[28] |
Good |
Good |
|
Good |
Fair |
Good |
|
|
Internal consistency was excellent for Functional Assessment of Chronic Illness Therapy- Tuberculosis (FACIT-TB) in one of the study from Malaysia [23]. There results showed that the reliability of the subscales ranging from good to excellent as by the rule of thumb with a total score of 0.87 and for all the subscale ranging from 0.736-0.871 [23]. The validation study from Taiwan [27] used WHOHRQOL –BREF (World Health Organization Health Related Quality of Life- BREF) also showed an adequate reliability. However, only one HRQOL instrument showed cross culture adaptability [26].
Main findings
To the author's knowledge, this is the first systematic review of outcome measures based on TB population. The major highlights of this review are to examine the feasibility of measurement uses, and qualities of methodology. Among the fifteen HRQOL measures studied, eleven measures were generic and four diseases specific. All the measures were developed in English language and most frequently studied measure was SF-36.
Measurement properties of HRQOL instruments
Generic instrument measures were used in 10 studies with different language versions of SF-36 [5,16-20,24,25,38,40,41]. SF-36 is a generic health outcomes measure consisting of 36 items aggregated into eight sub-scales of PF (physical functioning), RP (role physical), BP (body pain), GH (general health), VT (vitality), SF (social functioning), RE (role emotion) and MH (mental health) [43]. A long medical outcome study (MOS) instrument was used in USA [39] which covered multiple dimensions including physical and emotional wellbeing [43].
SF-36 was developed from subsets from the MOS instrument [6]. In a previous study, the variance in SF-36 scores of patients with latent and active TB was described [5]. Results showed a worse mean PCS (Physical Component Summary) score of 44.8 and MCS (Mental Component Summary) score of 40.1 in the patients with active TB as compared to latent TB patients with mean PCS and MCS scores of 54.7 and 50.3 respectively [5]. Studies have reported that when patients were evaluated with SF-36, they showed significant improvements in HRQoL at the completion of intensive phase of treatment [17,20]. A validation study of SF36 was described by Chamla [16] which suggested that the validity and reliability scores were high at the end of the treatment with Cronchbachs score >0.7 [16].
Dhingra and Rajpal et al. [29] used the disease specific instruments DR-12. This instrument consists of a total 12 items, among them 7 cover TB symptoms and 5 are related to socio-psychological characteristics and exercise adaptation [29]. Response options were presented on 3 point scale and equal weightage were given to each item while calculating the 2 domain score and the total score [29]. Pasipanodya et al. [39] used St. George Questionnaires (SGRQ) in 100 patients with PTB. It is an extensively used specific instrument for measuring HRQOL in patients with COPD (chronic obstructive pulmonary disease) and other types of respiratory disorders [39]. Pasipanodya et al. had also reported a significant increase in SGRQ scores as compared to latent TB scores [39]. Magurie et al. [22] described changes in health status by using a modified version of SGRQ (Base line; 45.4) in 115 subjects diagnosed with smear TB positive from Indonesia. After 2 months of treatment, 94% improvement was recorded in at least 4 points [22].
Chung et al. [27] assessed the quality of life by using 4 domain model of WHOQOL-BREF in TB affected population of Taiwan. Internal consistency reliability coefficients scores were 0.92 and 0.93 for the subjects and control referents, correspondingly [27]. Babikako et al. [26] carried out a validation study for the feasibility to use MOS instrument. All subscales have adequate internal consistency with Cronbach’s alpha >0.7. Construct validity varied with different stages of treatment in functional status and wellbeing of TB patients [26].
Abdulelah et al. [24] used a disease specific instrument (FACIT-TB, Functional Assessment of Chronic Illness Therapytuberculosis) for the measurement of the quality of life in TB patients. FACIT-TB (Functional Assessment of Chronic Illness Therapy- tuberculosis) consist of 27 items and their subsets described the disease symptoms associated to the infection site, adverse effects, and additional QOL dimensions (fatigue, social stigma, economic burden) of the ilHOlness. Factor analysis confirmed that the FACIT-TB construct comprised of five domains which are comparatively brief, easy to manage, easy to score, and suitable for the use in clinical trials [23].
Discussion
The strategy of evaluating the HRQOL in TB patients is an important outcome to measure the efficacy of new treatments or interventions. Typically, HRQOL measures have been developed and used to describe the mean scores for specific groups. During the last decade a rapid increase in the number of measures to assess the HRQOL in TB patients was recorded. We identified 22 different HRQOL measures with internal consistency, reliability, measurement error, content validity and responsiveness depending on the information obtained from the literature. Some of the HRQOL measures showed some aspects of psychometric strength especially construct validity. However, these have different characteristics and most of them did not complete the required properties proposed by Terwee et al. [10]. Notably, the SF-36, a commonly used HRQOL measure, was not fully validated in the original study [16]. Furthermore FACIT-TB is a shorter protocol comparatively which made its use popular worldwide [23]. We used COSMIN check list to evaluate the methodology quality of the original HRQOL measures in development studies. This included evaluation of different properties such as reliability, internal consistency, content validity, structural validity, responsiveness and measurement error. Using the criteria of COSMIN checklist most of the studies were rated as fair to poor because of insufficient information or these did not match the required standards. Thus, our results suggested that high quality studies are required for the proper evaluation of the measurement quality. The use of COSMIN checklist criteria in systematic reviews of outcome measures has increased. Though, a major limitation of COSMIN is that it cannot be used for the evaluation of old measures. The inconsistency in the measurement properties is due to disagreement in the definitions and their different criteria. Moreover, the questionnaires still need to meet the criteria of validity and reliability and should be described comprehensively. The selected studies assessed by COSMIN, these showed a poor methodology quality and missing items. The measuring properties will be rated fair even if these are not well defined. Most of the HRQOL instruments developed recently and additional study is required for their validation and reliability.
The COSMIN checklist and the quality criteria for two reviewers can be different. In such cases of disagreements a third reviewer can be consulted. Our research is limited to English language only so there is possibility of missing the measure developed in other languages. As far author’s knowledge the data was included from the TB population of non-English speaking countries and we did not find any non- English HRQL measure. Previous reports of HRQOL measures in the TB patients have included a limited number of measure and only a single concept of multidimensional HRQOL [6,44].
Till date, the evaluation of the methodology quality and instrument properties of HRQOL measures has not been reviewed systematically in literature. The major strength of this systematic review is to consider the related concepts like disease burden, productivity, fatigue and social impact. We preformed the literature search in an organized manner to identify all HRQOL measurements used in TB population. To the best of our knowledge this is the first review of HRQOL measures in TB which evaluated the properties and methodological qualities using a robust and standardized approach. This also described the detailed comparison between the HRQOL measures and properties of quality measurement. This review will guide the use of HRQOL in various clinical and research studies. It will also help the clinicians, researcher and general public to assess the scientific literature on HRQOL measures easily. Several new HRQOL measures are emerging and our study showed that most of the HRQOL are supported by evidence of at least one type of reliability or validity and further validation studies might support their use. The choice of HRQOL measure in future will depend on the context for which hit will be used (e.g. social or disease burden). Until then, the FACIT-TB [23] has the strongest published evidence of reliability and validity and is well established in literature.
Conclusion
Conclusively, there is no ideal HRQOL measure for the use in TB as the validation studies. The outcome measurements in TB were hardly ever carried out and there are no specific HRQOL measures for the use in TB population. In the light of development in the field of patient reported outcomes, it is necessary to develop a combination of measures which are important to the individual, family and society. The whole purpose of this study is to improve clinical care and to evaluate services potentially for academic and research purposes [8].
Acknowledgement
We would like to thank Institute of Postgraduate Studies (IPS) of Universiti Sains Malaysia for providing fellowship support Ref.no.P-FD0009/15(R).
Conflict of Interest
None
18409
References
- Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL (2005) Methods for the economic evaluation of health care programmes. Oxford University Press, Oxford, UK.
- Ahmad N, Javaid A,Sulaiman AZ, Basit A, Afridi AK, et al. (2016) Effects of multidrug resistant tuberculosis treatment on patients’ health related quality of life: Results from a follow up study. PLOS ONE 11: e0159560.
- Patrick DL, Deyo RA (1989) Generic and disease-specific measures in assessing health status and quality of life. Med Care 27:217-232.
- Guo N, Marra CA, Marra F, Moadebi S, Elwood RK, et al. (2008) Health state utilities in latent and active tuberculosis. Value Health 11:1154-1161.
- Bauer M, Leavens A, Schwartzman K (2013) A systematic review and meta-analysis of the impact of tuberculosis on health-related quality of life. Qual Life Res 22: 2213-2235.
- EuroQol (1990) A new facility for the measurement of health related quality of life. The EuroQol Group health Policy 16:199-208.
- Mokkink L, Terwee C, Patrick D, Alonso J, Stratford PW, et al. (2010) The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 19:539-549.
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J ClinEpidemiol 62: 1006-1012.
- Terwee CB, Mokkink LB, Knol DL, Ostelo WJG, Bouter LM, et al. (2011) Rating the methodological quality in systematic reviews of studies on measurement properties: A scoring system for the COSMIN checklist. Qual Life Res 21: 651-657.
- Schellingerhout JM, Verhagen AP, Heymans MW, Koes BW, De Vet HC, et al. (2012) Measurement properties of disease-specific questionnaires in patients with neck pain: A systematic review. Qual Life Res 21: 659-670.
- Tulder V, Furlan A, Bombardier C, Bouter L (2003) Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine 28: 1290-1299.
- Terwee CB, Bot S, De Boer MR, Van der Windt DA, Knol DL, et al. (2007) Quality criteria were proposed for measurement properties of health status questionnaires. J ClinEpidemiol 60: 34-42.
- Aggarwal AN, Gupta D, Janmeja AK, Jindal SK (2013) Assessment of health related quality of life in patients with pulmonary tuberculosis under programme conditions. Int J Tuberc Lung Dis 17:947-953.
- Balgude A, Sontakke S (2012) Study of impact of antitubercular therapy on quality of life. Indian J Med Sci 66:71-77.
- Chamla D (2004) The assessment of patients’ health-related quality of life during tuberculosis treatment in Wuhan, China. Int J Tuberc Lung Dis8:1100-1106.
- Kruijshaar ME, Lipman M, Essink-Bot ML, Lozewicz S, Creer D, et al. (2010) Health status of UK patients with active tuberculosis. Int J Tuberc Lung Dis 14:296-302.
- Atif M, Sulaiman SA, Shafie AA, Asif M, Sarfraz MK, et al. (2014) Impact of tuberculosis treatment on health-related quality of life of pulmonary tuberculosis patients: A follow-up study. Health Qual Life Outcomes 12:19.
- Mamani M, Majzoobi MM, Ghahfarokhi SM, Esna-Ashari F, Keramat F (2014) Assessment of health-related quality of life among patients with tuberculosis in Hamadan, Western Iran. Oman Med J 29:102-105.
- Marra CA, Marra F, Colley L, Moadebi S, Elwood RK, et al. (2008) Health related quality of life trajectories among adults with tuberculosis: differences between latent and active infection. Chest 133:396-403.
- Ralph AP, Kenangalem E, Waramori G, Pontororing GJ, Sandjaja, et al. (2013) High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena. PLoS One 8:e80302.
- Maguire GP, Anstey NM, Ardian M, Waramori G, Tjitra E, et al. (2009) Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. Int J Tuberc Lung Dis 13:1500-1506.
- Abdulelah J, Sulaiman AZ, Hassali MA, Blebil AQ, Awaisu A, et al. (2015) Development and psychometric properties of a tuberculosis specific multidimensional health-related quality-of-life measure for patients with pulmonary tuberculosis. Vl in HelthReg 6: 53-59.
- Bauer M, Ahmed S, Benedetti A, Greenaway C, Lalli M, et al. (2015) Health-related quality of life and tuberculosis: A longitudinal cohort study. Health Qual Life Outcomes27:65.
- Bauer M, Greenaway C, Lalli M, Leavens A, Wynee A, et al. (2011) Health related quality of life in active and latent tuberculosis patients, and a matched comparison group. Annual International Meeting of the American Thoracic Society, Denver.
- Babikako HM, Neuhauser D, Katamba, A, Mupere E (2010) Feasibility, reliability and validity of health-related quality of life questionnaire among adult pulmonary tuberculosis patients in urban Uganda: cross-sectional study. Health Qual Life Outcomes 8:93.
- Chung WS, Lan YL, Yang MC (2012) Psychometric testing of the short version of the world health organization quality of life (WHOQOL-BREF) questionnaire among pulmonary tuberculosis patients in Taiwan. BMC Public Health 12:630.
- Deribew A, Deribe K, Reda AA, Tesfaye M, Hailmichael Y, et al. (2013) Change in quality of life: a follow up study among patients with HIV infection with and without TB in Ethiopia. BMC Public Health 13:408.
- Dhingra VK, Rajpal S (2005) Health related quality of life (HRQL) scoring (DR-12 score) in tuberculosis–additional evaluative tool under DOTS. J Commun Dis 37:261-268.
- Dion MJ, Tousignant P, Bourbeau J, Menzies D, Schwartzman K (2004) Feasibility and reliability of health-related quality of life measurements among tuberculosis patients. Qual Life Res 13:653-665.
- Dujaili JA, Sulaiman SA, Hassali MA, Awaisu A, Blebil AQ, et al. (2015) Health-related quality of life as a predictor of tuberculosis treatment outcomes in Iraq. Int J Infect Dis 31:4-8.
- Fu G, Sheng C, Li Y (2016) Analysis on related factors influencing life satisfaction of patients with tuberculosis. Shanghai Nurs J 1: 1-4.
- Godoy MD, Mello FC, Lopes AJ, Costa W, Guimarães FS, et al. (2012) The functional assessment of patients with pulmonary multidrug-resistant tuberculosis. Respir Care 57:1949-1954.
- Husain MO, Dearman SP, Chaudhry IB, Rizvi N, Waheed W (2008) The relationship between anxiety, depression and illness perception in tuberculosis patients in Pakistan. ClinPractEpidemiolMent Health 4:4.
- López-Campos JL, Failde I, Masa JF, Benítez-Moya JM, Barrot E, et al. (2008) Factors related to quality of life in patients receiving home mechanical ventilation. Respir Med 102:605-612.
- Louw J, Peltzer K, Naidoo P, Matseke G, Mchunu G, et al. (2012) Quality of life among tuberculosis (TB), TB retreatment and/or TB-HIV co-infected primary public health care patients in three districts in South Africa. Health Qual Life Outcomes 10:77.
- Masumoto S, Yamamoto T,Ohkado A, Yoshimatsu S, Querri AG, et al. (2014) Factors associated with health-related quality of life among pulmonary tuberculosis patients in Manila, the Philippines. Qual Life Res 23:1523-1533.
- Muniyandi R, Rajeswari R, Balasubramanian C, Nirupa PG, Gopi K, et al. (2007) The union evaluation of post-treatment health-related quality of life (HRQoL) among tuberculosis patients M. Int J Tuberc Lung Dis 11:887-892.
- Pasipanodya JG, Miller TL, Vecino M, Munguia G, Bae S, et al. (2007) Using the St. George respiratory questionnaire to ascertain health quality in persons with treated pulmonary tuberculosis. Chest 132:1591-1598.
- Unalan D, Soyuer F, Ceyhan O, Basturk M, Ozturk A (2008) Is the quality of life different in patients with active and inactive tuberculosis? Indian J Tuberc 55:127-137.
- Rajeswari R, Muniyandi M, Balasubramanian R, Narayanan PR (2005) Perception of tuberculosis patients about their physical, mental and social well-being. SocSci Med 60: 1845-1853.
- Dhuria M, Sharma N, Singh NP, Jiloha CR, Saha R, et al. (2009) A study of the impact of tuberculosis on the quality of life and the effect after treatment with DOTS. Asia Pac J Public Health 21:312-320.
- Hays RD, Morales LS (2001)The RAND-36 measure of health-related quality of life. Ann Med 33:350-357.
- Chang B, Wu AW, Hansel NN, Diette GB (2004) Quality of life in tuberculosis: a review of the English language literature. Qual Life Res 13:1633-1642.






