Keywords
Human serum albumin (HSA); Neonatal Fc receptor (FcRn); Drug conjugate, Binding site, Drug delivery
Introduction
Most of the currently marketed drugs are small chemical molecules that are used to treat various kinds of human diseases. However, these small molecular drugs usually have disadvantages such as rapid degradation, short circulation duration, rapid renal clearance, non-specific distribution, and toxic accumulation in specific organ(s) [1,2]. Scientists have exploited different technologies and strategies to overcome these disadvantages, in order to enhance the efficacy of drugs and reduce their side effects. Particularly, some diseases such as cancers and immune disorders generally occur in specific tissues or organs. These characteristics require that the used drug can work as a “magic bullet”, as conceived by Paul Ehrlich over 100 years ago, that specifically recognizes and is selectively delivered to the targeted sites [1,2]. Therefore, certain strategies have been applied to link the non-specific small molecular drugs to unique drug delivery vehicles such as monoclonal antibodies, peptides and proteins [2]. These vehicles can make the “magic bullet” dream to come true via making these drugs targetable to specific sites. One of them is human serum albumin (HSA), which has been widely used for clinical treatments and drug delivery.
The human albumin gene is located on the long arm of chromosome 4. Human serum albumin (HSA) is produced in human hepatocytes and is the most dominant protein component in blood, making up about 50% of all serum proteins [3-5]. HSA functions in multiple critical biological roles. HSA has its antioxidant properties and regulates osmotic blood pressure. HSA can carry various endogenous or exogenous hydrophobic ligands [6]. For instance, HSA binds to fatty acids, amino acids, hormones, ions such as Ca2+, Na+ and K+, water and others [7,8]. Moreover, HSA can carry biomedical drugs by covalently linkages, to peptides/proteins by fusing them to its C- and N-termini [9], HSA has been widely applied to treat various health problems such as blood loss, burn, hemorrhage, hypovolemia, shock. Herein, we discuss HSA and its potential applications.
Albumin and its function structure
Human serum albumin (HSA) is a single chain consisting of 609 amino acids with a molecular weight of 66.5 kDa, including a signal peptide (1–18), a pro-peptide (19–24) and the active albumin (585 amino acids) [9]. The primary structure of HSA consists of one N-terminus, one C-terminus and three homologous domains named with domain I, II, and III. Each of these domains has two helical subdomains (IA and IB, IIA and IIB, IIIA and IIIB), respectively (Figure 1). Each of these subdomains contains 4 to 6 α-helices [8]. These six subdomains form a three dimensional (3D) structure (Figure 1). HSA is not glycosylated, unlike other blood proteins. Also, HSA is easily water-soluble due to its negatively charged surface, and is very stable in blood with a half-life of about 19 days due to its ability to diffuse out-and-in of blood vessels [3,10].
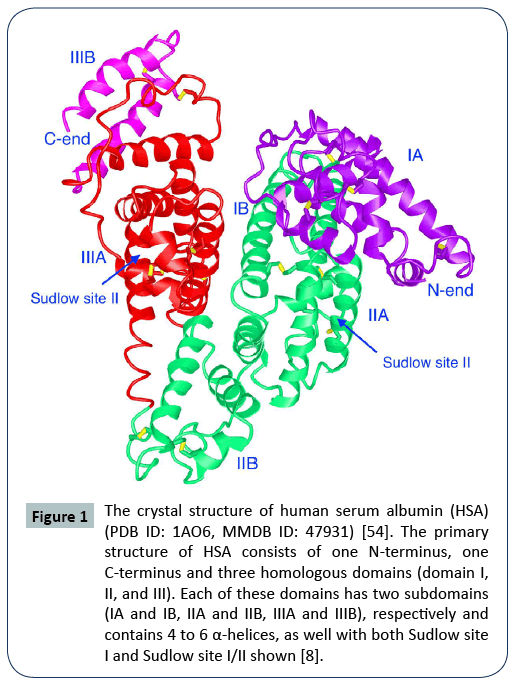
Figure 1: The crystal structure of human serum albumin (HSA) (PDB ID: 1AO6, MMDB ID: 47931) [54]. The primary structure of HSA consists of one N-terminus, one C-terminus and three homologous domains (domain I, II, and III). Each of these domains has two subdomains (IA and IB, IIA and IIB, IIIA and IIIB), respectively and contains 4 to 6 α-helices, as well with both Sudlow site I and Sudlow site I/II shown [8].
HSA displays its excellent capacity to carry various ligands mainly due to its hydrophobic packets, providing potential applications in drug delivery. According to Sudlow's nomenclature on albumin, there are two critical regions (Sudlow site I and Sudlow site II) that are two main drug-binding sites in the 3D structure of an albumin. Both Sudlow site I and Sudlow site II are located in the hydrophobic cavities in the subdomain IIA and the subdomain IIIA, respectively [8]. This unique structure of HSA provides the primary ligand-binding sites for various drugs. The bulky heterocyclic anions (the molecules with negative charges) and dicarboxylic acids prefer to bind to the Sudlow site I (mainly located in subdomains IIA) [8,9], with aromatic carboxylates preferring to bind to the Sudlow site II (mainly in subdomains IIIA). Also, there are seven fatty acid binding sites in HSA [7], providing the options for drugs to be linked to fatty acids (FA) and further extend their half life via forming HSA-FAdrug complex with the neonatal Fc receptor (FcRn) in blood. There are 35 cysteine residues in albumin, 34 of which form 17 internal disulfide bridges with one free amino acid cysteine. These intermolecular disulfide bridges play a key role in forming the 3D structure of albumin, and maintain its structural stability and biological activity. The free cysteine residue at the position 34 (Cys34) is located on the surface of albumin in its 3D structure and is far away from the drug-binding sites. This provides a free thiol group (-SH) that can be applied for covalent conjugation of drugs of interest [7]. The new drug conjugates will not interfere with the interactions of other ligands and HSA itself [5,7]. And also, there are several other residues such as lysine (Lys199) and histidine (His242) that areable to serve as the drug-linking sites [3]. Meanwhile, the two free terminal ends of albumin provide more options for coupling small molecules, peptides, or proteins of interest.
Albumin’s receptors
Albumin passes from blood into endothelial cells and then and returns from the extravascular area to the circulation in blood stream via the lymphatic system. Via binding to its receptors, Albumin can recycle in-and-out of circulation for about 28 times in the lymphatic system during its lifetime [4,6]. Therefore, HSA can serve as a promising drug delivery vehicle with its unique advances and its receptors play significant roles [7,8].
Nowadays, it has been demonstrated that there are several albumin-binding membrane proteins (or receptors) such as glycoprotein 18 (gp18), gp30, gp60 (albondin), cubilin, megalin, SPARC (Secreted Protein Acidic and Rich in Cysteine) and the neonatal Fc receptor (FcRn, Brambell receptor or Fc fragment of IgG receptor and transporter) (Figure 2) [7,8]. Particularly, FcRn has been demonstrated to be expressed in various cells and tissues, and to have a high binding affinity with the two most abundant serum proteins, HSA and IgG. FcRn can form complexes with these two proteins (Figures 3 and 4) and extend their half-lives, to protect them from lysosomal degradation and keep them recycling [11]. The receptor Gp60 being expressed in endothelium and epithelium can bind with albumin and help albumin transcytose through endothelial cells. Albumin can interact with receptors in renal proximal tubules and go through endocytosis in order to escape renal clearance and to circulate for longer time [7,8,12].
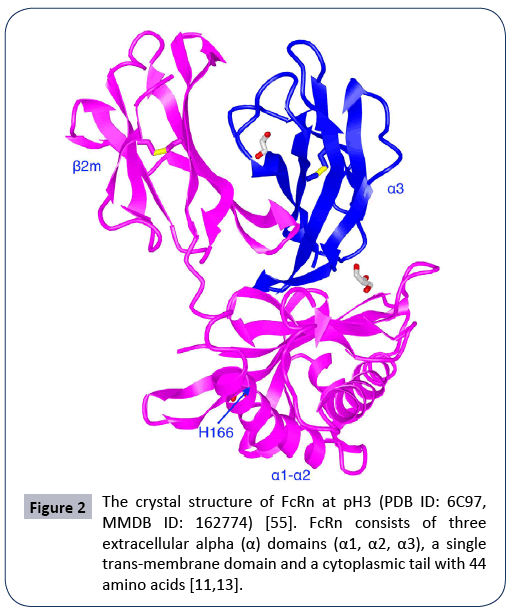
Figure 2: The crystal structure of FcRn at pH3 (PDB ID: 6C97, MMDB ID: 162774) [55]. FcRn consists of three extracellular alpha (α) domains (α1, α2, α3), a single trans-membrane domain and a cytoplasmic tail with 44 amino acids [11,13].
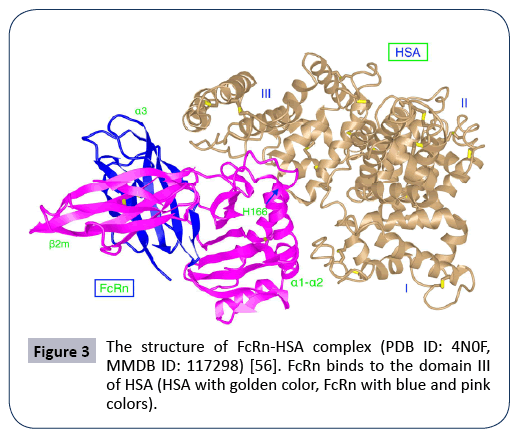
Figure 3: The structure of FcRn-HSA complex (PDB ID: 4N0F, MMDB ID: 117298) [56]. FcRn binds to the domain III of HSA (HSA with golden color, FcRn with blue and pink colors).
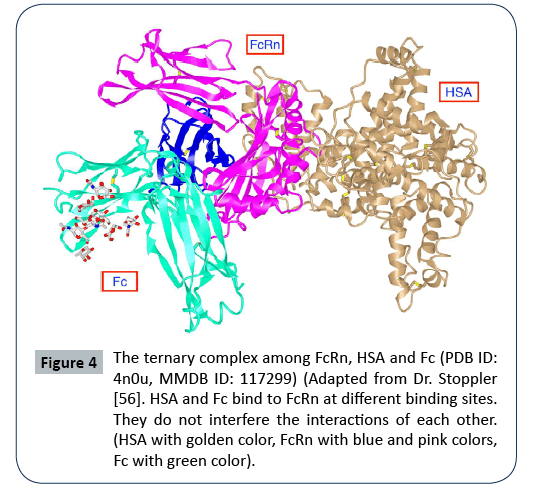
Figure 4: The ternary complex among FcRn, HSA and Fc (PDB ID: 4n0u, MMDB ID: 117299) (Adapted from Dr. Stoppler [56]. HSA and Fc bind to FcRn at different binding sites. They do not interfere the interactions of each other. (HSA with golden color, FcRn with blue and pink colors, Fc with green color).
The neonatal Fc receptor (FcRn) (Figure 2) is encoded by FCGRT gene located on chromosome 19 (NC_000019.10). FcRn consists of three extracellular alpha (α) domains (α1, α2, α3), a single transmembrane domain, and a cytoplasmic tail (44 amino acids) (Figure 2) [11,13]. As said above, FcRn can function for the halflife extension of various ligands such as albumin, IgG and fatty acids, and protect them from enzymatic degradation [13,14]. FcRn is mainly located in acidic endosomes, where it binds its ligands and transports them via either a recycling pathway or a transcytosis pathway [8,12,13] . Albumin and IgG are the two most abundant proteins in blood (Figures 3 and 4). FcRn displays its high binding affinity to both in a pH-dependent manner, but the binding sites are different for the two proteins (Figure 4). The binding interactions of FcRn with HSA and FcRn with IgG do not interfere with one another. FcRn protects proteins like IgG and albumin from cellular catabolism via the pH-dependent recycling and transcytosis pathways [10]. In drug R&D, FcRn can help extend the half-life of FcRn-binding drugs and escape of renal clearance [8,13]. Via binding to FcRn, albumin becomes a more effective therapeutic deliverer and demonstrates slower renal clearance. Thus, albumin is frequently used for drug delivery.
The Interaction of HSA with FcRn and the Circulation of the Complex
Endosomes
Endosome acts as a key player in the process of albumin transportation and recycling. Endosomes are endocytic vacuoles and are classified into early endosomes, recycling endosomes and late endosomes. Various molecules internalize in endosomes during endocytosis Low pH or acidic environment in endosomes triggers the binding of FcRn with ligands such as albumin and IgG, resulting in recycling of the FcRn-ligand complex to cell surfaces with physiological environment (neutral pH), and favors exocytosis of ligands out of cells in a recycling manner. FcRn located on the surfaces of endosomes can mediate transcytosis of albumin in the FcRn-HSA complex across the cellular barriers and relocate HSA in the extracellular area [8]. The ligand albumin not bound to FcRn remain in late endosomes, and can subsequently pass to lysosomes where they are eventually degraded [7]. Thus, FcRn in endosomes is a critical regulatory factor of albumin homeostasis [4].
The producing and recycling of albumin
HSA is produced in hepatocytes and released into blood. HSA in blood (at neutral pH, pH7.4) is uptaken into endocytic vesicles by the plasma membrane of endothelial cells lining up inside of blood vessels (interior wall of vessels). This process is regarded as endocytosis. The endocytic vesicles further form early endosomes (at neutral pH), where HSA molecules may go through three pathways including recycling, transcytosis and degradation. The binding of HSA and FcRn is in a pH-dependent manner. One recycling pathway is that HSA recognizes and binds to FcRn at the acidic endosome (at acidic pH, pH5-6). FcRn protects albumin from degradation by binding albumin with high affinity at a low pH (pH 5-6) in acidic endosomes. Then FcRn-HSA complexes are sorted into recycling endosomes, HSA then dissociates from FcRn, releases and re-enters into blood at physiological pH (neutral pH). This process is the so-called exocytosis [8,10]. HSA recycles through this process again and again, resulting in the extension of half-life and escape from degradation. HSA can also bind the receptors Megalin/Cubilin to form the HSA-Megalin/ Cubilin complex that protect them from renal clearance [7,9]. Another transcytosis mechanism is that the FcRn-HSA complex, after FcRn sorted dissociates, releases HSA in an extravascular area (at physiological pH) [8,9]. At last, HSA not bound to FcRn are sorted in lysosomes and subsequently degraded (pH4-5). The circulation of albumin is disrupted when it is catabolized in organs such as the skin and muscles [4,6]. The degradation of HSA may provide nutrients for tumor growth [6,7]. Albumin can also transport across the vascular endothelium or endothelial cells via binding to the albumin receptor gp60 present on the cell surface, forming caveolae and vesicles. Or, albumin can directly pass through tumor vessels via leaky junctions or naturally go through the vascular endothelium, which serves as a drugdelivery barrier, to deliver drugs inside the tissues [10]. Unlike mature and well-organized normal blood vessels, tumor blood vessels are more immature, tortuous and leaky, resulting in the increased occurrence of vessel leakage. This enhances the ability of macromolecules such as albumin, to go through vessel wall, and accumulate in the tumor interstitium [6,10]. Albumin predominantly exists in the extravascular space and extracellular area (muscle, gut, skin), but less in intravascular area and intracellular area.
The key binding-site for FcRn is within the C-terminal DIII domain of albumin and the binding is pH-dependent. The three conserved histidine residues (H464, H510, and H535) located in this domain play a critical role in FcRn-albumin binding [8]. The histidine residue H166 is fully conserved and resides within α2-domain of the human FcRn. For FcRn-albumin binding, the contact sites are H166 in FcRn, and H464, H510 and H535 in albumin [6,15]. HSA undergoes the conformational change of albumin or the Neutral-to-Base transition of albumin (N-B transition of albumin) that occurred at physiological Ph [16-18], And the histidine residue H464 participates in the pH-dependent N-B transition of albumin between pH 5.0 and pH 7.0, suggesting a potential for histidine-mediated, pH-dependent interaction with FcRn [7,17]. The domain DI is also slightly involved in the interaction of FcRn and HSA, but DII does not. Particularly, the two histidine residues H510 and H535 located in the long loop between DIIIA and DIIIB affect the interaction via stabilizing the loop position at acidic pH [8,9]. Additionally, the involvement of the N-terminal DI, and two-surface exposed loops contribute to this pH-dependent binding to the receptor [9].
HSA Serves as Drug Delivery Vehicles
HSA has been used as a drug delivery vehicle due to its characteristics like long half-life, recirculation, accumulation in tumor tissues, and its ease-of-diffusion across epithelia,. Particularly, the tumor blood vessels are immature, leaky and not well-organized compared to normal cells as tumor blood vessels are rapidly formed [7]. Albumin can pass across the leaky tumor blood vessels and this characteristic allows albumin to carry anticancer agents to pass into tumor sites [6,10]. Albumin in blood can be uptaken into endosomes where albumin binds to FcRn and is sorted for either recycling in blood or degradation in lysosomes [7]. Albumin can retain its binding efficacy of albumin- FcRn interactions after being covalently or non-covalently coupled with various drugs. However, albumin can be fused with a short peptide or a single-chain variable fragment (scFv) by genetic recombinant technology, which may influence pHdependent binding to FcRn, with more effects from fusion at its C-terminus [6,10].
Several strategies have applied for HSA to deliver drugs [19], including covalently linking drugs to C-terminus, N-termini of the albumin, or the site 34 (Cys34) on albumin with chemical techniques, fusing peptides or proteins to C-terminus and N-terminus with the recombinant technology, non-covalently binding drugs to the hydrophobic pockets of albumin, and ligand-drug complex via linking drugs to ligands of albumin, drugcarrying albumin nanoparticles [6,9,20]. Particularly, certain amino acid residues such cysteine and histidine are available for drugs to be conjugated to. Moreover, there are multiple lysine residues that are available for albumin conjugates.
Different strategies for the use of linkers/spacers in HSA conjugates
There are different strategies used for albumin drug conjugates. Especially, the linkers/spacers usually are different and critical for different drug conjugates. For instance, a disulfide bridge used to link albumin and drug of interest can easily result in the conjugate being degraded. An acid-sensitive hydrazine linker can maintain the albumin conjugate at a pH-dependent manner [6]. Additionally, a caspase-cleavable peptide spacer added in albumin-drug conjugate can take advantage of caspase-3 that is highly activated in apoptotic tumor cells and can degrade the conjugate. The peptide sequence Asp-Glu-Val-Asp (DEVD) is a well-known substrate of the enzyme caspase-3 and can be used as a spacer in a drug conjugate [21]. Cathepsins are lysosomal enzymes and are highly expressed in many malignant tumors. The sequence Ala-Leu-Ala-Leu-Ala is cathepsin-cleavable and can be used as a cathepsin-cleavable spacer [22]. The sequence Gly-Pro- Leu-Gly-Ile-Ala-Gly-Gln can be used as matrix metalloproteinase (MMP)-cleavable spacer that is recognized and cleaved by MMP2 and MMP9 [7,23].
Non-covalent HSA complex
There are two major drug-binding sites, or Sudlow site I and Sudlow site II, that are located in the subdomain IIA and the subdomain IIIA, respectively. As reported, certain molecules or drugs such as thyroxine, indoxyl sulphate, azidothymidine, indomethacine, warfarin, CMPF, azapropazone, oxyphenbutazone, phenylbutazone, DIS, TIB bind to the pocket at the subdomain IIA (Sudlow site I) (Figure 5), with thyroxine, diazepam, ibuprofen, CMPF, propofol, halothane, difunisal, indoxyl sulphate binding to the site of the subdomain IIIA (Sudlow site II) (Figure 6) [8]. Certain drugs can bind to other sites such as the subdomain IB and the subdomain IIIB. Such drugs as fusidic acid, hemin, lidocaine bind to the site of the subdomain IB, with thyroxine and propofol bind to the site of the subdomain IIIB. There are also multiple binding sites of metal ions (Zn2+, Co2+, Cu2+). And seven binding sites of fatty acids have been demonstrated as well. One strategy is to link drugs of interest to fatty acids (FAs), as the FA-albumin interactions to keep FAdrug complexes recycling [9]. The successful examples are the approval drugs Semaglutide (Rybelsus, Ozempic) and Liraglutide (Victoza) via linking glucagan-like peptide 1(GLP-1) to fatty acids (FAs) (Liraglutide: C16 fatty acid, Semaglutide: C18 fatty acid) [7]. These FA-GLP complexes extend GLP-1’s half-lives significantly via binding to albumin in blood. Albumin in turn binds to its receptor FcRn for further recycling and stability. Levemir [Insulin detemir] is another drug via linking insulin with a 14-carbon fatty acid. The FA-insulin complex binds endogenous albumin, keeps it stable in blood and retains a long-lasting effect to suppress glucose. Meanwhile, microRNA, Short interfering RNA (siRNA), oligoDNA can also be coupled to other albumin-binding vehicles. For instance, siRNA was linked to a diacyl lipid via a PEGylated linker. The peptide Trp-Gln-Arg-Pro-Ser-Ser-Trp (WQRPSSW) is an albumin-binding domain and is used to fuse with therapeutic peptides/proteins, such as human recombinant tumor necrosis factor-related apoptosis-inducing ligand (hTRAIL) and the insulinlike growth factor II [7,20].
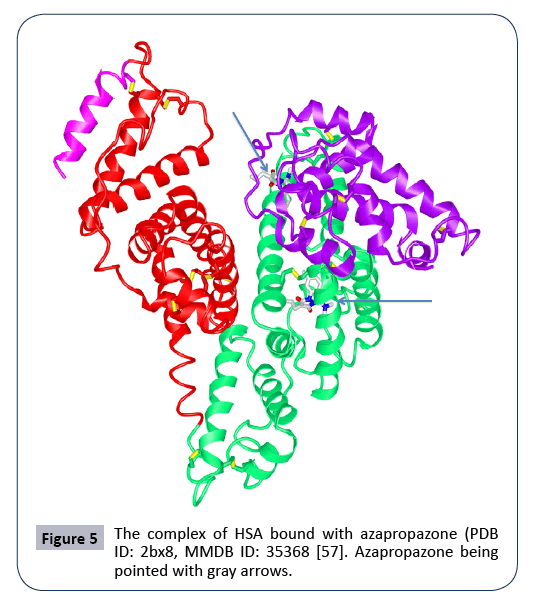
Figure 5: The complex of HSA bound with azapropazone (PDB ID: 2bx8, MMDB ID: 35368 [57]. Azapropazone being pointed with gray arrows.
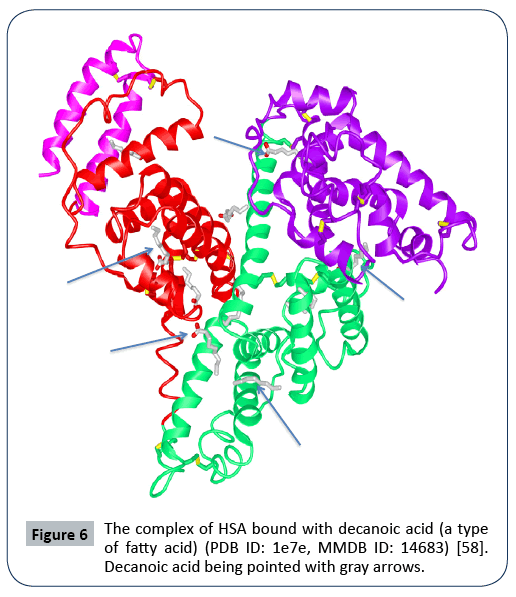
Figure 6: The complex of HSA bound with decanoic acid (a type of fatty acid) (PDB ID: 1e7e, MMDB ID: 14683) [58]. Decanoic acid being pointed with gray arrows.
Chemically covalent HSA conjugates
By serving as a drug delivery vehicle, HSA provides its multiple specific sites (cysteine, lysine and histidine) on the surface of HSA and the terminal sites of HAS [19]. For instance, the only one free amino acid cysteine located on the surface of albumin and away from the other drug-binding sites provides a free thiol group (-SH) for covalent conjugation of drugs of interest. Crucially, these conjugates will not interfere with albumin’s binding affinity and biological activity. A variety of compounds such as methotrexate (MTX), doxorubicin (DOX), Toluene diisocyanate (TDI), piperacillin, docetaxel (DTX), paclitaxel (PTX), hexamethylene diisocyanate(HDI) were coupled to albumin at different strategies [22]. These albumin conjugates of MTX-HSA, DOX-HSA, TDI-HSA, piperacillin-HSA, DTX-HSA, PTX-HSA, HDI-HSA showed more potent efficacy and longer circulation durations [9,21,24-27].
Aldoxorubicin is a DOX-HSA complex via selectively coupling doxorubicin (DOX, Adriamycin) to the residue cysteine at the site 34 (Cys34) of albumin with an acid-sensitive linker (N-ε- maleimidocaproic acid hydrazide, or EMCH). This complex effectively delivered DOX into the tumors and result in potent tumor suppression. In another example, Dr. Chung coupled DOX to HSA via a self-immolative linker and a caspase-3-sensitive peptide Asp-Glu-Val-Asp (DEVD) spacer to obtain the HSA-DEVDS- DOX conjugate. This conjugate displayed much less in vitro toxic activity, but fully recovered DOX’s toxic activity after free DOX was released from the conjugate by caspase-3 [21]. Methotrexate (MTX) is a well-known anticancer agent. Dr. Urger covalently linked MTX to the residue lysine of HSA at a ratio of drug:HSA to 1:1. This MTX-HSA conjugate, under a single injection, (12.5 mg/ kg) displayed broad antitumor activity against tumors of breast, lung, bladder and prostate cancers, osteosarcoma and sarcoma. In another example, the chemotherapeutic agent docetaxel (DTX), which is poorly soluble in water, was conjugated to HSA and was subsequently made more water-soluble [28]. Oleanolic acid (OA, oleanic acid) is a natural hydroxyl pentacyclic triterpenoic acid (HPTA) and shows its anti-oxidant, anti-bacterial, anti-tumor and anti-inflammatory activities [29]. Oleanolic acid (OA) was coupled to HSA with an aminie-terminated linker and via esterification of carboxylic acid [30].
Besides conjugated with small molecular compounds, HSA was also used as a delivery vehicle for macromolecules such as peptides or proteins. For example, the tumor necrosis factorrelated apoptosis-inducing ligand (TRAIL) was coupled to HSA via a bi-functional PEG derivative, and the TRAIL-HSA conjugate was used to treat rheumatoid arthritis [31]. Gonadotropin releasing hormone (GnRH) is a peptide hormone to regulate the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), but is rather unstable. The HSA-Cys-Gly-GnRH conjugate was made with GnRH linkage to HSA and shows a much longer half-life compared to GnRH itself [32]. Exendin-4 is a peptide consisting of 39 amino acids and is structurally similar to GLP-1. Insulin is a peptide consisting of two chains connected each other with two disulfide bridges (Chain A with 21 amino acids, chain B with 30 amino acids). These peptides were conjugated to the Cys34 site of HSA in the formation of a disulfide bridge, respectively. The formation of these peptide conjugates (HSA-PEG-Ex4, Insulin- HSA) extended their half-life greatly in circulation and thus enhanced their biological functions [33,34].
Genetic HSA fusion macromolecule
The binding affinity of FcRn with albumin or IgG is pH-dependent. And a peptide fused to C- or N- terminal ends of HSA may have impact on FcRn binding in a pH-dependent manner [35-37]. As reported, HSA has been fused with various peptides or protein fragments of interest [9] such as Glucagon-like peptide 1 (GLP-1) [38,39], Interleukin-2 [40], IFN-α [41-43], Somatostatin [36]. Glucagon-like peptide 1 (GLP-1) is peptide hormone consisting of 30 or 31 amino acids. GLP-1 can enhance the secretion of insulin from β cells while reducing the release of glucose. Its derivatives such as exenatide and lixisenatide, that were successfully used for the clinical treatments of diabetes. Albumin was also used to deliver GLP-1. In the HSA-GLP conjugate, two tandem repeats of GLP-1 molecules with each containing 30 amino acids and the residue alanine at position 2 being replaced with glycine in order to resist the enzymatic digestion of dipeptidyl peptidase-4 (DPP-4) were fused to the N-terminus of albumin and named as Albiglutide (brand name Tanzeum). Albiglutide extends the halflife of GLP-1 from several minutes to 5 days. Thus, Albiglutide was approved to treat diabetes with weekly administration [38,39,44-46].
Alpha-melanocyte-stimulating hormone (α-MSH) is another peptide hormone with a half-life of only several minutes. α-MSH displays potent anti-inflammatory and protective effects in brain damage. Dr. Wang and his coworkers fused α-MSH with HSA and the human immunodeficiency virus Tat protein (TAT). The new conjugate TAT-HSA-α-MSH can effectively pass through the blood brain barrier (BBB) and regulate brain inflammation [47]. Somatostatin (SST) is a growth hormone inhibitory factor and regulates the secretion of various hormones. Naturally, there are two subtypes SST-14 and SST-28. Many of its analogs have been developed with some being approved for clinical uses, including Octreotide, Lanreotide, Vaprotide, Seglitide, Pasireotide and Somatoprim [48]. Natural SST has very short half-life (only a few minutes). These analogs are more stable with only the limited extension of their half-life. SST fused with HSA, on the other hand, could greatly extend its half-life. Dr. Ding et al. fused SST to C- and N- terminus of HSA with two or three tandem repeats of SST-14. Three fusion proteins (SS14)2-HSA, (SS14)3-HSA, and HSA- (SS14)3, were acquired. They displayed similar biological activity to SST-14, with (SS14)2-HSA being more productive and effective than other two [36].
Thrombopoietin (TPO) is a regulator of megakaryopoiesis and thrombopoiesis. Two tandem repeats of TPO analogs or TPO mimetic peptides (TMPs) were fused to the C-terminus or N-terminus of HSA, respectively, and formed the fusion proteins HSA-TMP-TMP and TMP-TMP-HAS [49]. Interleukin-2 (IL-2) is a kind of cytokine and participates in immune regulation. IL-2 was genetically fused with HAS [40,50]. Dr. Melder et al. fused IL-2 to HSA to form the IL2-HSA conjugate that demonstrated its immunemodulatory effects and enhanced the infiltration of CD4+/CD8+ T cells [40]. IFN-α is a type of interferon and was widely used to treat human cancers and viral diseases. Scientists constructed the IFN-α-HSA conjugates [37,41-43,51]. Dr. Zhao fused IFN-α2b to C- or N- terminus of HSA and acquired the fusion proteins IFN- α2b-HSA and HAS-IFN-α2b, respectively [37,51]. Some more HSA fusion proteins were also constructed with therapeutic proteins such as Thymosin-α1, Granulocyte colony stimulating factor (G-CSF) [35,52]. These conjugates displayed their advantages over their respective native proteins.
Conclusion and Prospective
Human serum albumin (HSA) has demonstrated its versatile and functional properties with significant advantages like abundance in blood, high concentration in tumor area, superior binding efficacy with various ligands, and extension of circulatory half life [8,9,12]. These predict that HSA could broadly serve as an excellent drug delivery vehicle via non-covalently binding, covalently binding and genetic fusion strategies. Indeed, some HSA-based or HSA-binding drugs such as Albiglutide, Semaglutide, Abraxane and Levemir have been successfully developed and clinically applied [7]. Many more promising drug candidates are currently under investigation around the world. We are also constructing different HSA-based drugs in the fields of cancers and immune diseases. These drug candidates displayed their potent efficacy and are receptor-selective. Nowadays, albumin is gradually becoming the hot topic and is expected to be the next-generation drug R&D platform. However, There are still certain challenges or limits for albumin to act as a drug delivery vehicle. For example, albumin can be uploaded with different cargoes. Their interactions may impact the binding of albumin with endogenous ligands and their homeostasis. Thus, it is critical to optimize the albumin-based drug design. And also, albumin may not be suitable for all cancers and for all drugs to be coupled. Particularly, the drugs need to be of quick clearance [7,53-58]. These unknown issues need to be further explored.
Disclosure of conflict of interest
None
Acknowledgement
We would greatly acknowledge the supports from Shenzhen Science and Technology Program. (Grant No: KQTD20170810154011370), Xiangtan Institute of Industrial Technology Collaborative Innovation, and Xiangtan Science and Technology Bureau.
26640
References
- Sun LC, Coy DH (2011) Somatostatin receptor-targeted anti-cancer therapy. Curr Drug Deliv 8: 2-10.
- Sun LC, Mackey LV, Luo J, Fuselier JA, Coy DH (2008) Targeted chemotherapy using a cytotoxic somatostatin conjugate to inhibit tumor growth and metastasis in nude mice. Clin Med Oncol 2: 491-499.
- Bern M, Sand KM, Nilsen J, Sandlie I, Andersen JT (2015) The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J Control Release 211: 144-162.
- Roopenian DC, Low BE, Christianson GJ, Proetzel G, Sproule TJ, et al. (2015) Albumin-deficient mouse models for studying metabolism of human albumin and pharmacokinetics of albumin-based drugs. MAbs 7: 344-351.
- Leblanc Y, Berger M, Seifert A, Bihoreau N, Chevreux G (2019) Human serum albumin presents isoform variants with altered neonatal Fc receptor interactions. Protein Sci 28: 1982-1992.
- Merlot AM, Kalinowski DS, Richardson DR (2014) Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol 5: 299.
- Hoogenboezem EN, Duvall CL (2018) Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev 130: 73-89.
- Sand KM, Bern M, Nilsen J, Noordzij HT, Sandlie I, et al. (2014) Unraveling the Interaction between FcRn and Albumin: Opportunities for Design of Albumin-Based Therapeutics. Front Immunol 5: 682.
- Larsen MT, Kuhlmann M, Hvam ML, Howard KA (2016) Albumin-based drug delivery: harnessing nature to cure disease. Mol Cell Ther 4: 3.
- Foss S, Grevys A, Sand KMK, Bern M, Blundell P, et al (2016) Enhanced FcRn-dependent transepithelial delivery of IgG by Fc-engineering and polymerization. J Control Release 223: 42-52.
- Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, et al. (2010) Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol 30: 777-789.
- Toh WH, Louber J, Mahmoud IS, Chia J, Bass GT, et al. (2019) FcRn mediates fast recycling of endocytosed albumin and IgG from early macropinosomes in primary macrophages. J Cell Sci 133.
- Pyzik M, Sand KMK, Hubbard JJ, Andersen JT, Sandlie I, et al. (2019) The Neonatal Fc Receptor (FcRn): A Misnomer? Front Immunol 10: 1540.
- Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, et al. (2011) Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc Natl Acad Sci USA 108: 9927-9932.
- Kuo TT, Aveson VG (2011) Neonatal Fc receptor and IgG-based therapeutics. MAbs 3: 422-430.
- Dockal M, Carter DC, Ruker F (2000) Conformational transitions of the three recombinant domains of human serum albumin depending on pH. J Biol Chem 275: 3042-3050.
- Wanwimolruk S, Birkett DJ (1982) The effects of N-B transition of human serum albumin on the specific drug-binding sites. Biochim Biophys Acta 709: 247-255.
- Kaneko K, Fukuda H, Chuang VT, Yamasaki K, Kawahara K, et al. (2008) Subdomain IIIA of dog albumin contains a binding site similar to site II of human albumin. Drug Metab Dispos 36: 81-86.
- Low BE, Wiles MV (2016) A Humanized Mouse Model to Study Human Albumin and Albumin Conjugates Pharmacokinetics. Methods Mol Biol 1438: 115-122.
- An FF, Zhang XH (2017) Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 7: 3667-3689.
- Chung SW, Choi JU, Lee BS, Byun J, Jeon OC, et al. (2016) Albumin-binding caspase-cleavable prodrug that is selectively activated in radiation exposed local tumor. Biomaterials 94: 1-8.
- Schmid B, Chung DE, Warnecke A, Fichtner I, Kratz F (2007) Albumin-binding prodrugs of camptothecin and doxorubicin with an Ala-Leu-Ala-Leu-linker that are cleaved by cathepsin B: synthesis and antitumor efficacy. Bioconjug Chem 18: 702-716.
- Mansour AM, Drevs J, Esser N, Hamada FM, Badary OA, et al. (2003) A new approach for the treatment of malignant melanoma: enhanced antitumor efficacy of an albumin-binding doxorubicin prodrug that is cleaved by matrix metalloproteinase 2. Cancer Res 63: 4062-4066.
- Park HS, Lee SK, Lee YM, Kim SS, Nahm DH (2002) Longitudinal study of specific antibodies to toluene diisocyanate (TDI)-human serum albumin (HSA) conjugate in patients with TDI-induced asthma. Korean J Intern Med 17: 249-251.
- Kim JE, Kim SH, Kim JH, Bahn JW, Jin HJ, et al. (2011) A case of piperacillin-induced occupational anaphylaxis: detection of serum IgE to piperacillin-HSA conjugate. J Korean Med Sci 26: 682-685.
- Dosio F, Arpicco S, Brusa P, Stella B, Cattel L (2001) Poly(ethylene glycol)-human serum albumin-paclitaxel conjugates: preparation, characterization and pharmacokinetics. J Control Release 76: 107-117.
- Taheri A , Dinarvand R, Atyabi F, Nouri F, Ahadi F, et al. (2011) Targeted delivery of methotrexate to tumor cells using biotin functionalized methotrexate-human serum albumin conjugated nanoparticles. J Biomed Nanotechnol 7: 743-753.
- Nateghian N, Goodarzi N, Amini M, Atyabi F, Khorramizadeh MR, et al. (2016) Biotin/Folate-decorated Human Serum Albumin Nanoparticles of Docetaxel: Comparison of Chemically Conjugated Nanostructures and Physically Loaded Nanoparticles for Targeting of Breast Cancer. Chem Biol Drug Des 87: 69-82.
- Nataraju A, Saini D, Ramachandran S, Benshoff N, Liu W, et al. (2009) Oleanolic Acid, a plant triterpenoid, significantly improves survival and function of islet allograft. Transplantation 88: 987-94.
- Yang Y, He HJ, Chang H, Yu Y, Yang MB, et al. (2018) Multivalent oleanolic acid human serum albumin conjugate as nonglycosylated neomucin for influenza virus capture and entry inhibition. Eur J Med Chem 143: 1723-1731.
- Byeon HJ, Min SY, Kim I, Lee ES, Oh KT, et al. (2014) Human serum albumin-TRAIL conjugate for the treatment of rheumatoid arthritis. Bioconjug Chem 25: 2212-2221.
- Finnerty M, et al. (1994) Immunization of bull calves with a GnRH analogue-human serum albumin conjugate: effect of conjugate dose, type of adjuvant and booster interval on immune, endocrine, testicular and growth responses. J Reprod Fertil 101: 333-343.
- Kim I, Kim TH, Ma K, Lee ES, Kim D, et al. (2010) Synthesis and evaluation of human serum albumin-modified exendin-4 conjugate via heterobifunctional polyethylene glycol linkage with protracted hypoglycemic efficacy. Bioconjug Chem 21: 1513-1519.
- Thibaudeau K, Léger R, Huang X, Robitaille M, Quraishi O, et al. (2005) Synthesis and evaluation of insulin-human serum albumin conjugates. Bioconjug Chem 16: 1000-1008.
- Zhao S, Zhang Y, Tian H, Chen X, Cai D, et al. (2013) Extending the serum half-life of G-CSF via fusion with the domain III of human serum albumin. Biomed Res Int 2013: 107238.
- Ding Y, Fan J, Li W, Yang R, Peng Y, et al. (2013) The effect of albumin fusion patterns on the production and bioactivity of the somatostatin-14 fusion protein in Pichia pastoris. Appl Biochem Biotechnol 170: 1637-1648.
- Zhao HL, Yao XQ, Xue C, Wang Y, Xiong XH, et al. (2008) Increasing the homogeneity, stability and activity of human serum albumin and interferon-alpha2b fusion protein by linker engineering. Protein Expr Purif 61: 73-77.
- Bush MA, Matthews JE, De Boever EH, Dobbins RL, Hodge RJ, et al. (2009) Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes Obes Metab 11: 498-505.
- Poole RM, Nowlan ML (2014) Albiglutide: first global approval. Drugs 74: 929-938.
- Melder RJ, Osborn BL, Riccobene T, Kanakaraj P, Wei P, et al. (2005) Pharmacokinetics and in vitro and in vivo anti-tumor response of an interleukin-2-human serum albumin fusion protein in mice. Cancer Immunol Immunother 54: 535-547.
- Subramanian GM, Fiscella M, Lamousé-Smith A, Zeuzem S, McHutchison JG (2007) Albinterferon alpha-2b: a genetic fusion protein for the treatment of chronic hepatitis C. Nat Biotechnol 25: 1411-1419.
- Hu J, Wang G, Liu X, Gao W (2015) Enhancing Pharmacokinetics, Tumor Accumulation, and Antitumor Efficacy by Elastin-Like Polypeptide Fusion of Interferon Alpha. Adv Mater 27: 7320-7324.
- Osborn BL, Olsen HS, Nardelli B, Murray JH, Zhou JX, et al. (2002) Pharmacokinetic and pharmacodynamic studies of a human serum albumin-interferon-alpha fusion protein in cynomolgus monkeys. J Pharmacol Exp Ther 303: 540-548.
- Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, et al. (2003) Development and characterization of a glucagon-like peptide 1-albumin conjugate: the ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes 52: 751-759.
- Trujillo JM, Nuffer W (2014) Albiglutide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Ann Pharmacother 48: 1494-1501.
- Young MA, Wald JA, Matthews JE, Scott R, Hodge RJ, et al. (2014) Clinical pharmacology of albiglutide, a GLP-1 receptor agonist. Postgrad Med 126: 84-97.
- Wang M, Zhi D, Wang H, Ru Y, Ren H, et al. (2016) TAT-HSA-alpha-MSH fusion protein with extended half-life inhibits tumor necrosis factor-alpha in brain inflammation of mice. Appl Microbiol Biotechnol 100: 5353-5361.
- Sun L, Coy DH (2016) Somatostatin and its Analogs. Curr Drug Targets 17: 529-537.
- Ru Y, Zhi D, Guo D, Wang Y, Li Y, et al. (2016) Expression and bioactivity of recombinant human serum albumin and dTMP fusion proteins in CHO cells. Appl Microbiol Biotechnol 100: 7565-7575.
- Lei J, Guan B, Li B, Duan Z, Chen Y, et al. (2012) Expression, purification and characterization of recombinant human interleukin-2-serum albumin (rhIL-2-HSA) fusion protein in Pichia pastoris. Protein Expr Purif 84: 154-160.
- Zhao HL, Xue C, Wang Y, Li XY, Xiong XH, et al. (2007) Circumventing the heterogeneity and instability of human serum albumin-interferon-alpha2b fusion protein by altering its orientation. J Biotechnol 131: 245-252.
- Chen JH, et al. (2010) Bioactivity and pharmacokinetics of two human serum albumin-thymosin alpha1-fusion proteins, rHSA-Talpha1 and rHSA-L-Talpha1, expressed in recombinant Pichia pastoris. Cancer Immunol Immunother 59: 1335-1345.
- Fasano M, Curry S, Terreno E, Galliano M, Fanali G, et al. (2005) The extraordinary ligand binding properties of human serum albumin. IUBMB Life 57: 787-796.
- Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K (1999) Crystal structure of human serum albumin at 2.5 A resolution. Protein Eng 12: 439-446.
- Stoppler D, Macpherson A, Smith-Penzel S, Basse N, Lecomte F, et al. (2018) Insight into small molecule binding to the neonatal Fc receptor by X-ray crystallography and 100 kHz magic-angle-spinning NMR. PLoS Biol 16: e2006192.
- Oganesyan V, Damschroder MM, Cook KE, Li Q, Gao C, Wu H, et al. (2014) Structural insights into neonatal Fc receptor-based recycling mechanisms. J Biol Chem 289: 7812-7824.
- Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, et al. (2005) Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol 353: 38-52.
- Bhattacharya AA, Grüne T, Curry S (2000) Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol 303: 721-732.











