Keywords
Pooled prevalence; Intestinal parasite; Pregnant women; Ethiopia
Background
Intestinal parasites (IP) are responsible for morbidity of about 450 million people worldwide; women of reproductive age and children in developing countries are disproportionately affected by IP. There is high burden of IP in list income countries because of poverty, lack of safe drinking water, poor hygiene, and malnutrition [1]. In developing countries, almost half the population does not have access to sanitary facilities and only most people of the world practice open defecation, which is the main risk factor for dissemination of IP in the community. In parts of Africa, only about 24% of the population residing in the rural parts of sub-Saharan Africa use improved sanitation system [2].
According to the World Health Organization (WHO) reports, more than one billion of the world’s populations are chronically infected with soil transmitted helminthes (STHs) [3]. About 40% of disease burden of all tropical infections is due to geo-helminths [4]. WHO established a prevalence of 20% as a minimum limit at which infections may be regarded as a generalized public health problem [5].
Pregnant women are at high risk of IP infection because of their close relationship with children. Also, most of these parasites are transmitted through contaminated soil and most of soil eating habit is commonly observed among pregnant women [6]. IP infections during pregnancy can affect the health of mother, fetus and child. Pregnant women often experience more severe infections than their non-pregnant counterparts because of immune-modulation and physiological changes that occur during pregnancy [7]. More than 44 million pregnancies are complicated by maternal Hookworm infection and 10 million pregnant women in Africa are infected with Schistosomiasis [8]. Several studies have been conducted to assess the prevalence and effects of intestinal helminthes among pregnant women and their children [8,9].
Untreated IP infection among pregnant women can influence the fetal immune system. In utero stimulation with helminth-derived antigens is believed to divert fetal immunity towards anergy, tolerance, or T helper 2 responses [10]. In utero exposure to helminth derived antigens has been considered as one of the risk factors in offspring for enhanced susceptibility to infections such as Tuberculosis [10]. Intestinal infection among pregnant women affects the health of mother and fetus; moreover, it shifts the immunity towards which is not protective for intracellular microbes. Therefore, we envisaged to determine the pooled prevalence of intestinal parasites among pregnant women in Ethiopia so that concerned bodies will take appropriate measure to reduce or prevent its transmission.
Methods
Search strategy
The search was conducted using six databases: PubMed, Google Scholar, HINARI and Cochrane Library by a special index search terms (medical subject headings (MeSH) "Intestinal diseases, parasitic" OR "intestinal" AND "diseases" AND "parasitic" OR "parasitic intestinal diseases" OR "intestinal" AND "parasite" OR "intestinal parasite" AND "epidemiology" OR "epidemiology" OR "prevalence" OR "prevalence" AND "gravidity" OR "gravidity" OR "pregnant" AND "ethiopia" OR "Ethiopia". The limit of language was English and search was restricted to humans only. We also screened reference lists of selected studies for any potentially relevant studies that had not been identified through the searches. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline was used to report the result of this systematic review and meta-analyses.
Inclusion criteria and exclusion criteria
Data extraction:
The data extraction was done by five researchers (SH, MM, MMA, BTT, TLA) using a standardized and pretested format. The data abstraction format included first author, study design, country, publication year, sample size, study population, the number who tested positive for intestinal parasite. Any disagreement that occurs during data extraction between researchers was handled through.
Quality assessment:
Nine point Joanna Briggs Institute (JBI) critical appraisal tool for systematic review was used to determine the quality of articles collected. The tool uses the following criteria [11]. Individual studies were assigned a score that was computed using different parameters in line with the review objectives. The responses were scored 1 for “Yes” and 0 for “Not reported”. Total scores ranged between 0 and 9. Studies with medium (fulfilling 50% of quality assessment parameter) and high qualities were included for analysis [11]. Accordingly, the overall score was 251.
Statistical analysis:
Data entry and analysis was done using STATA (version 14). The summary of the pooled prevalence of IP with 95% CI was obtained using the random effects model, due to the possibility of heterogeneity among the studies.
Results
Identified studies
Through electronic database search, we have found a total of 622 studies. Of which, 563 were excluded based on the titles and abstracts. Also 33 articles were excluded after full text review since they don’t reported prevalence of IP among pregnant women finally, 30 studies were found to be eligible and included in the meta–analysis (Figures 1 and 2). Included articles exhibited high heterogeneity according to Cochrane Q test (5820.71 test, p <0.0001) and I2 test (I2=99.5%), and Tau-squared (706.840) (P<0.0001) which is indicative to use random effects model. Eggers regression intercept test indicated evidence of publication bias (Figure 3).
Figure 1 Flow diagram of the studies included in the Metaanalysis.
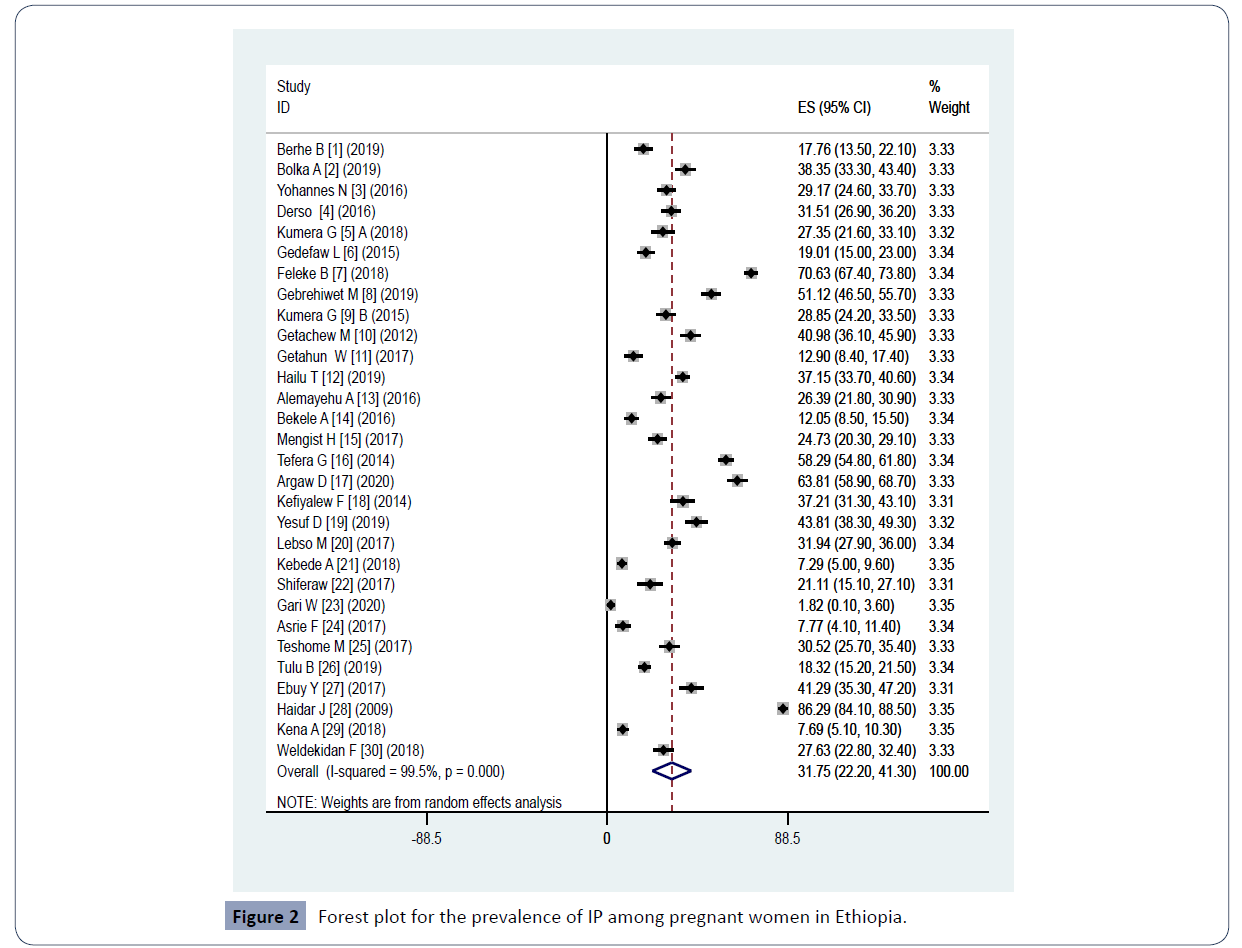
Figure 2 Forest plot for the prevalence of IP among pregnant women in Ethiopia.
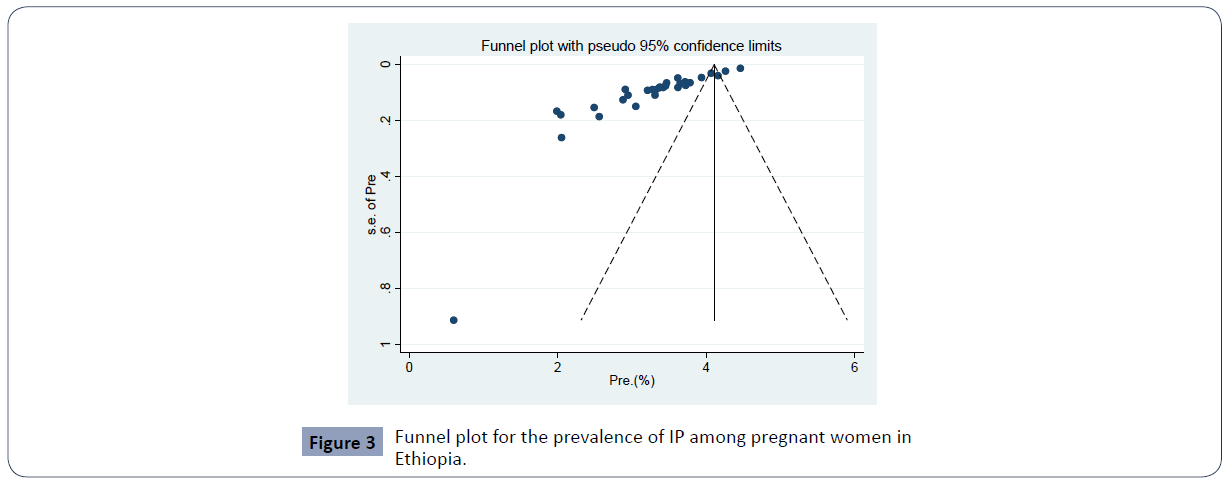
Figure 3 Funnel plot for the prevalence of IP among pregnant women in Ethiopia.
Study characteristics
Selected articles were published from 2009 to 2020. Also, all included publications were obtained from 5 regions and 1 Multicenter regional study, but no data was obtained from other regions (Afar, Benishangul-Gumuz and Somali). The total study populations involved in this systematic review and meta-analysis were 12,212. A total of thirty studies were considered eligible for quantitative syntheses (Table 1). A total of thirty studies were considered eligible for quantitative syntheses (Table 1). The prevalence of IP across study regions is as follows: Amhara region, 1285 (31.72%); Oromia region, 1062 (29.04%); Southern region, 868 (29.48%); Tigray, 427 (29.29%); population from Tigray; and Gambella, 95 (26.39%). The study population varied from 180 to 970,and was conducted between the years 2009-2019. The prevalence of IP in the community, and health institution was 27.37% and 53.43% respectively (Table 1).
| Author with ref. |
Pub. year |
Region |
Type of study |
study setting |
Sample size |
No,positive |
Pre.(%) |
Quality score |
| Berhe B [12] |
2019 |
Tigray |
Cross-sectional |
Health Facility |
304 |
54 |
17.8 |
8 |
| Bolka A [13] |
2019 |
Southern |
Cross-sectional |
Health Facility |
352 |
135 |
38.4 |
9 |
| Yohannes N [14] |
2016 |
Amhara |
Cross-sectional |
Health Facility |
384 |
112 |
29.2 |
7 |
| Derso[1] |
2016 |
Amhara |
Cross-sectional |
Health Facility |
384 |
121 |
31.5 |
9 |
| Kumera G [15] A |
2018 |
Amhara |
Cross-sectional |
Health Facility |
234 |
64 |
27.4 |
9 |
| Gedefaw L [16] |
2015 |
Southern |
Cross-sectional |
Health Facility |
363 |
69 |
19.0 |
8 |
| Feleke B [17] |
2018 |
Amhara |
Cross-sectional |
Community |
783 |
553 |
70.6 |
9 |
| Gebrehiwet M [18] |
2019 |
Tigray |
Cross-sectional |
Health Facility |
448 |
229 |
51.1 |
9 |
| Kumera G [19] B |
2015 |
Amhara |
Cross-sectional |
Health Facility |
364 |
105 |
28.8 |
9 |
| Getachew M [20] |
2012 |
Oromia |
Cross-sectional |
Community |
388 |
159 |
41.0 |
9 |
| Getahun W[21] |
2017 |
Southern |
Cross-sectional |
Health Facility |
217 |
28 |
12.9 |
8 |
| Hailu T [22] |
2019 |
Amhara |
Cross-sectional |
Community |
743 |
276 |
37.1 |
8 |
| Alemayehu A [23] |
2016 |
Gambella |
Cross-sectional |
Health Facility |
360 |
95 |
26.4 |
8 |
| Bekele A [24] |
2016 |
Southern |
Cross-sectional |
Health Facility |
332 |
40 |
12.0 |
9 |
| Mengist H [25] |
2017 |
Oromia |
Cross-sectional |
Health Facility |
372 |
92 |
24.7 |
8 |
| Tefera G [26] |
2014 |
Oromia |
Cross-sectional |
Health Facility |
748 |
436 |
58.3 |
8 |
| Argaw D [27] |
2020 |
Southern |
Cross-sectional |
Health Facility |
373 |
238 |
63.8 |
9 |
| Kefiyalew F [28] |
2014 |
Oromia |
Cross-sectional |
Health Facility |
258 |
96 |
37.2 |
8 |
| Yesuf D [29] |
2019 |
Oromia |
Cross-sectional |
Health Facility |
315 |
138 |
43.8 |
8 |
| Lebso M [30] |
2017 |
Southern |
Cross-sectional |
Community |
504 |
161 |
31.9 |
9 |
| Kebede A [31] |
2018 |
Tigray |
Cross-sectional |
Health Facility |
480 |
35 |
7.3 |
8 |
| Shiferaw [32] |
2017 |
Amhara |
Cross-sectional |
Health Facility |
180 |
38 |
21.1 |
8 |
| Gari W [33] |
2020 |
Oromia |
Cross-sectional |
Health Facility |
220 |
4 |
1.8 |
8 |
| Asrie F [34] |
2017 |
Amhara |
Cross-sectional |
Health Facility |
206 |
16 |
7.8 |
8 |
| Teshome M [35] |
2017 |
Southern |
Case-control |
Health Facility |
344 |
105 |
30.5 |
9 |
| Tulu B [36] |
2019 |
Oromia |
Case-control |
Health Facility |
573 |
105 |
18.3 |
8 |
| Ebuy Y [37] |
2017 |
Tigray |
Case-control |
Health Facility |
264 |
109 |
41.3 |
8 |
| Haidar J [38] |
2009 |
Multicenter |
Cross-sectional |
Community |
970 |
837 |
86.3 |
8 |
| Kena A [39] |
2018 |
Oromia |
Cross-sectional |
Health Facility |
416 |
32 |
7.7 |
9 |
| Weldekidan F [40] |
2018 |
Southern |
Case-control |
Health Facility |
333 |
92 |
27.6 |
8 |
Table 1. Prevalence of intestinal parasitic infection among pregnant women in Ethiopia.
Meta-analysis of prevalence of IP
The estimated pooled prevalence of IP among pregnant women in the current review and meta-analysis was 31.75% (95% CI: 22.20-41.30) (Figure 2). Pooled prevalence of IP among regions were as follows: Amhara, 31.72% (95% CI: 16.35-47.10); Oromia, 29.04% (95% CI; 14.09-43.99); South Ethiopia, 29.48% (95% CI: 18.27-40.70); Tigray, 29.29% (7.96-50.61); Gambella, 26.39% (21.84-30.94. The pooled prevalence within a year 2009-2016 was 34.80% (95% CI: 16.72-52.88) and within 2017-2020 29.97% (95% CI: 20.31-39.62) (Table 2). A pooled prevalence of 27.37% (20.48-34.26) from health facility and 53.43% (30.19-76.68) from community were found (Figure 4). Moreover, meta-regression model was conducted among the above study parameters to identify the possible source of heterogeneity though none of them was statistically significant.
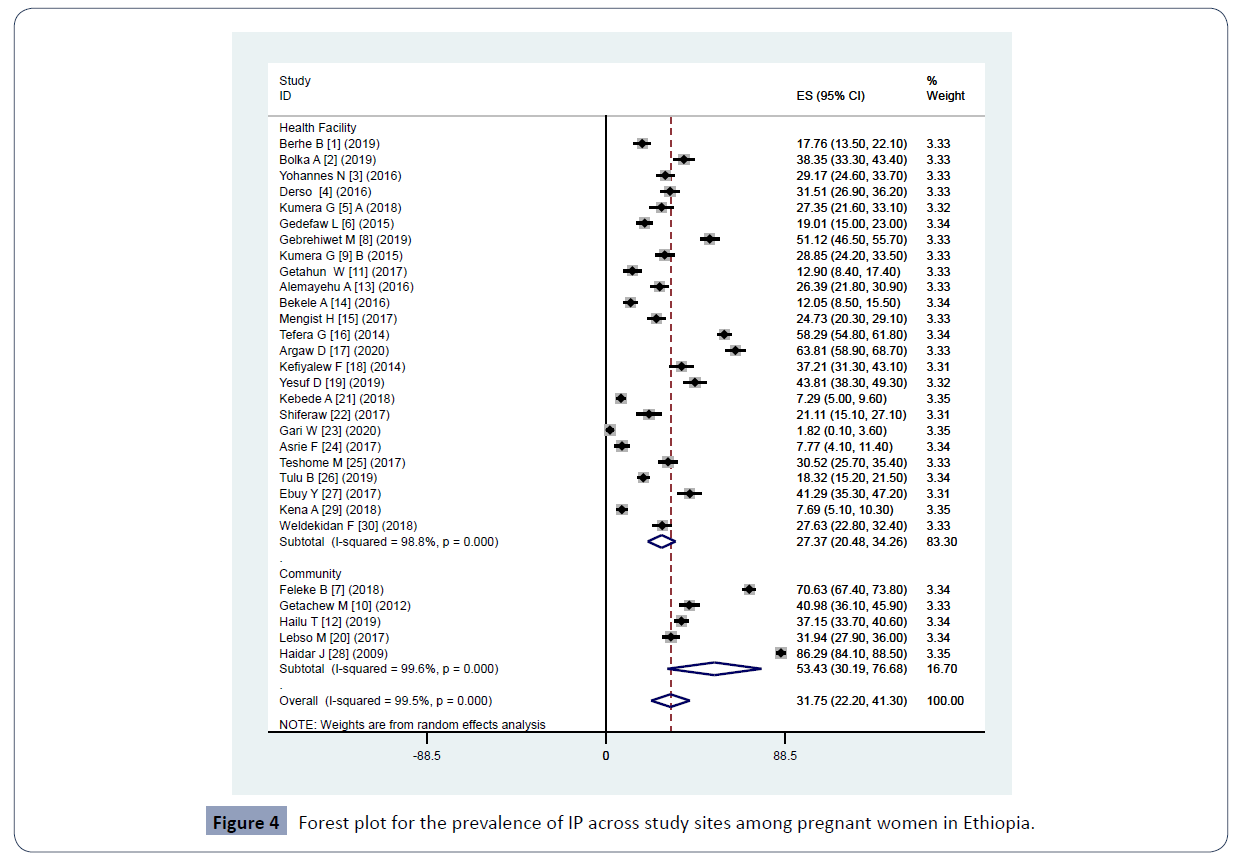
Figure 4 Forest plot for the prevalence of IP across study sites among pregnant women in Ethiopia.
| Categories |
Subgroup |
Studies included |
No tested |
No positive |
Prevalence %
(95% CI) |
I2% |
P-V |
| Region |
Tigray |
4 |
1496 |
427 |
29.29(7.96-50.61) |
99.1 |
<0.0001 |
| Southern |
8 |
2818 |
8 868 |
29.48(18.27-40.70) |
98.1 |
<0.0001 |
| Amhara |
8 |
3278 |
1285 |
31.72 (16.35-47.10) |
99.0 |
<0.0001 |
| Oromia |
8 |
3290 |
1062 |
29.04 (14.09-43.99) |
99.4 |
<0.0001 |
| Gambella |
1 |
360 |
95 |
26.39(21.84-30.94) |
--- |
--- |
| Year of study |
2009-2016 |
11 |
4293 |
1974 |
34.80(16.72-52.88) |
99.6 |
<0.0001 |
| 2017-2020 |
19 |
7661 |
2504 |
29.97(20.31-39.62) |
99.3 |
<0.0001 |
| Study setting |
Community |
25 |
3388 |
1986 |
27.37(20.48-34.26) |
98.8 |
<0.0001 |
| Health Facility |
5 |
8824 |
2588 |
53.43(30.19-76.68) |
99.6 |
<0.0001 |
Table 2. Subgroup meta-analysis of IP prevalence in Ethiopia from 2009-2020.
Subgroup analysis of IP among pregnant women
The pooled prevalence of Hook worm was 11.46% (95%CI; 8.62- 14.30) and A. lumbricoides was 10.44% (95%CI; 8.27,12.60) (Table 3).
| Parasites |
Studies included |
Prevalence % (95% CI) |
I2% |
P-V |
| H. worm |
21 |
11.46 (8.62-14.30) |
98.3 |
<0.0001 |
| A. lumbricoides |
21 |
10.44 (8.27,12.60) |
98.7 |
<0.0001 |
| E. histolytica |
23 |
1.84 (1.35,2.34) |
95.9 |
<0.0001 |
| G. lamblia |
23 |
1.18(0.82,1.54) |
92.3 |
<0.0001 |
| T.t richiura |
23 |
1.27(0.84,1.71) |
94.7 |
<0.0001 |
| S. mansoni |
22 |
1.19(0.73,1.66) |
94.8 |
<0.0001 |
Table 3. The pooled Prevalence of different intestinal parasites among pregnant women in Ethiopia from 2009 to 2020.
Discussion
Parasitic infection among pregnant women can be asymptomatic or can cause anemia, deficiency of nutrients, and can affect the immune response of child [12-41]. IP infections can severely affect affect both the pregnant women and fetus. As a result, WHO recommended treatment of pregnant women for helminthic infections [41]. There are several studies addressing IP among children and pregnant women in Ethiopia; however, there is no summarized report among pregnant women so as to inform policymakers and health care providers. In this review and meta-analysis we aimed to estimate pooled prevalence of IP among pregnant women in Ethiopia.
The overall pooled prevalence of IP among pregnant women in Ethiopia is 31.75% which is higher than the lower limit prevalence (20%) set by WHO to consider IP as a public health concern [5]. The finding of this review and meta-analysis is lower than proportion of intestinal parasite among pregnant women reported from worldwide study [42]. In contrast to this review and meta-analysis, higher prevalence of intestinal parasites (73.9%) was reported from Venezuelan pregnant women [43]. It is also relatively low compared to prevalence of intestinal parasite infection (37.3%) reported from the Northwestern parts of Ethiopia [22]. Our finding is in line with finding reported Ghana (30.5%) [44].
The most prevalent parasite identified in this review and meta-analysis was Hookworm (11.46%) followed by A. lumbricoides (10.44%). The prevalence of the rest parasite such as E. histolytica, G. lamblia, T. trichiura, S. mansoni was less than 2%. Unlike the current review and meta-analysis, a higher prevalence of A. lumbricoides (57%) and low prevalence of Hookworm (8.1%) was reported from Venezuelan pregnant women [43]. High prevalence of Hookworm and A. lumbricoides could be due to the suitability of the environment along with the high fertility of A. lumbricoides and the stability of eggs in the soil.
In contrast to this review, a high prevalence of Hookworm (49.8%) was reported from pregnant women living in Gojam, Ethiopia. Moreover, the prevalence of E. histolytica/dispar (40.8%) and G. lamblia (19.1%) reported from pregnant women residing in West Gojjam is higher than our finding [22]. Study from other places also reported a high prevalence of Hookworm (19%), A. lumbricoides (17%) and T. trichiura (11%), and E. histolytica/dispar (9%) and G.lamblia (8%) [45]. These variations in prevalence of IP and proportion of parasite among pregnant women due to socio-economic differences, environmental sanitation, habits of shoe wearing, and Laboratory methods.
In this review, the pooled prevalence of IP varies slightly across different regions of Ethiopia with the highest prevalence from Amhara (31.72%) followed by Southern Ethiopia (29.48%), Tigray (29.29), Oromia (29.04%) and Gambella (26.39%). We observed relative reduction of pooled prevalence of IPs from 34.8% in 2009-2016 to 29.97% in 2017-2020 indicating some work has be done to reduce burden of IP among pregnant women in various parts of Ethiopia.
A higher prevalence of IP (53.43%) was observed among studies conducted in the community as compared to those which was conducted in health facilities (27.37%). This indicates high number of intestinal parasites are being circulated in the community and missed by health care workers. This emphasizes the importance of mass screening of pregnant women in the community to come up with true burden of IP among pregnant women plan for suitable prevention and control.
Moreover, considering that as there is no ‘gold standard’ test for detection of intestinal helminths, a variety of parasitological methods have been utilized in different parts of Ethiopia such as formalin–ether concentration technique, Kato-Katz methods which may be responsible for variation of prevalence observed in this analysis.
Conclusion
This review and meta-analysis found a high prevalence of IP among pregnant women which is greater than the minimum limit set by WHO to be considered as public health concern. The pooled prevalence we found in this review varied across different regions and study sites with highest from Amhara and community respectively. There was slight reduction of IP parasites among pregnant women from 2009-2016 to 2017-2020.
Conflicts of Interest
The authors declare there is no competing interest.
Funding Statement
We did not receive any funding for this work.
38696
References
- Derso A, Nibret E, Munshea A (2016) Prevalence of intestinal parasitic infections and associated risk factors among pregnant women attending antenatal care center at Felege Hiwot Referral Hospital, northwest Ethiopia. BMC Inf Dis 16:530.
- Yimam YT, Gelaye KA, Chercos DH (2014) Latrine utilization and associated factors among people living in rural areas of Denbia district Northwest Ethiopia. Pan Afr Med J18:1-10.
- WHO (2012) Eliminating soil-transmitted helminthiases as a public health problem. Geneva: WHO Library Cataloguing-in-Publication Data.
- Wekesa AW, Mulambalah CS, Muleke CI, Odhiambo R (2014) Intestinal helminth infections in pregnant women attending antenatal clinic at Kitale District Hospital, Kenya. J Parasitol Res 2014:823923.
- World Health Organization (2011) Pan American Health Organization, SABIN Vaccine Institute, UIBD, Global Network for Neglected Tropical Diseases. A call to action: addressing soil-transmitted helminths in Latin America and the Caribbean. Geneva: World Health Organization.
- Baidoo SE, Tay SC, Abruquah HH (2010) Intestinal helminth infection and anaemia during pregnancy: A community-based study in Ghana. African J Microbiol Res 4:1713-1718.
- Roopal N, Supriya P, Avani K, Gita N, Preeti M (2020) Correlation of sociodemographic factors and intestinal parasites in pregnant women. Int J Res Med Sci 8:244-251.
- Fuseini G, Edoh D, Kalifa BG, Hamid AM, Knightn D, et al. (2010) Parasitic infections and anemia during pregnancy in Kassena-Nankana district of northern Ghana. J Pub Health Epidemiol2:48-52.
- Ndibazza J, Muhangi L, Akishule D, Kiggundu M, Ameke C, et al. (2010) Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled trial. Clin Infect Dis 50:531-540.
- Gebreegziabiher D, Desta K, Desalegn G, Howe R, Abebe M (2014) The Effect of Maternal Helminth Infection on Maternal and Neonatal Immune Function and Immunity to Tuberculosis. PLoS One 9: e93429.
- Yeshitila YG, Kassa GM, Gebeyehu S, Memiah P, Desta M (2021) JBIcritical appraisal checklist for studies reporting prevalence data 2016.
- Berhe B, Mardu F, Legese H, Gebrewahd A, Gebremariam G, Tesfay K, et al. (2019) Prevalence of anemia and associated factors among pregnant women in Adigrat General Hospital, Tigrai, northern Ethiopia, 2018. BMC Research Notes 12:1-6.
- Bolka A, Gebremedhin S (2019) Prevalence of intestinal parasitic infection and its association with anemia among pregnant women in Wondo Genet district, Southern Ethiopia: a cross-sectional study. BMC Infectious Diseases19:1-8.
- Yohannes N, Leykun N, Abebe W, Worku L, Asrie F (2016) Burden of intestinal parasites and associated risk factors among pregnant women attending antenatal care service at Gondar University Hospital, Northwest, Ethiopia. Ethiopian Journal of Laboratory Medicine.
- Kumera G, Haile K, Abebe N, Marie T, Eshete T (2018) Anemia and its association with coffee consumption and Hookworm infection among pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia. PloS one 13:e0206880.
- Gedefaw L, Ayele A, Asres Y, Mossie A (2015) Anaemia and associated factors among pregnant women attending antenatal care clinic in Walayita Sodo town, Southern Ethiopia. Ethiop J Health Sci 25:155-164.
- Feleke BE, Jember TH (2018) Prevalence of helminthic infections and determinant factors among pregnant women in Mecha district, Northwest Ethiopia: a cross sectional study. BMC infectious diseases 18:1-6.
- Gebrehiwet MG, Medhaniye AA, Alema HB (2019) Prevalence and associated factors of soil transmitted helminthes among pregnant women attending antenatal care in Maytsebri primary hospital, North Ethiopia. BMC Research Notes 12:1-6.
- Kumera G, Awoke T, Melese T, Eshetie S, Mekuria G, et al. (2015) Prevalence of zinc deficiency and its association with dietary, serum albumin and intestinal parasitic infection among pregnant women attending antenatal care at the University of Gondar Hospital, Gondar, Northwest Ethiopia. BMC Nutrition 1:1-1.
- Getachew M, Yewhalaw D, Tafess K, Getachew Y, Zeynudin A (2012) Anaemia and associated risk factors among pregnant women in Gilgel Gibe dam area, Southwest Ethiopia. Parasites & vectors 5:1-8.
- Getahun W, Belachew T, Wolide AD(2017) Burden and associated factors of anemia among pregnant women attending antenatal care in southern Ethiopia: cross sectional study. BMC Research Notes 10:1-7.
- Hailu T, Kassa S, Abera B, Mulu W, Genanew A (2019) Determinant factors of anaemia among pregnant women attending antenatal care clinic in Northwest Ethiopia. Tropical diseases, travel medicine and vaccines 5:1-7.
- Alemayehu A, Gedefaw L, Yemane T, Asres Y (2016) Prevalence, severity, and determinant factors of Anemia among pregnant women in south Sudanese refugees, Pugnido, Western Ethiopia. Anemia.
- Bekele A, Tilahun M, Mekuria A (2016) Prevalence of anemia and Its associated factors among pregnant women attending antenatal care in health institutions of Arba Minch town, GamoGofa Zone, Ethiopia: A Cross-sectional study. Anemia.
- Mengist HM, Zewdie O, Belew A (2017) Intestinal helminthic infection and anemia among pregnant women attending ante-natal care (ANC) in East Wollega, Oromia, Ethiopia. BMC Research Notes 10:1-9.
- Tefera G (2014) Determinants of anemia in pregnant women with emphasis on intestinal helminthic infection at Sher-Ethiopia Hospital, Ziway, Southern Ethiopia. Immunol Infect Dis 2:33-39.
- Argaw D, Kabthymer RH, Birhane M (2020) Magnitude of Anemia and Its Associated Factors Among Pregnant Women Attending Antenatal Care in Southern Ethiopia: A Cross-Sectional Study. J Blood Med 11:335.
- Kefiyalew F, Zemene E, Asres Y, Gedefaw L (2014) Anemia among pregnant women in Southeast Ethiopia: prevalence, severity and associated risk factors. BMC Research Notes 7:1-8.
- Yesuf DA, Abdissa LT, Gerbi EA, Tola EK (2019) Prevalence of intestinal parasitic infection and associated factors among pregnant women attending antenatal care at public health facilities in LaloKile district, Oromia, Western Ethiopia. BMC Research Notes 12:1-6.
- Lebso M, Anato A, Loha E (2017) Prevalence of anemia and associated factors among pregnant women in Southern Ethiopia: A community based cross-sectional study. PloS one 12:e0188783.
- Kebede A, Gerensea H, Amare F, Tesfay Y, Teklay G (2017) The magnitude of anemia and associated factors among pregnant women attending public institutions of Shire Town, Shire, Tigray, Northern Ethiopia, 2018. BMC Research Notes 11:1-6.
- Shiferaw MB, ZegeyeAM, Mengistu AD (2017) Helminth infections and practice of prevention and control measures among pregnant women attending antenatal care at Anbesame health center, Northwest Ethiopia. BMC Research Notes 10:1-5.
- Gari W, Tsegaye A, Ketema T (2020) Magnitude of Anemia and Its Associated Factors among Pregnant Women Attending Antenatal Care at Najo General Hospital, Northwest Ethiopia. Anemia 2020: 8851997
- Asrie F (2017) Prevalence of anemia and its associated factors among pregnant women receiving antenatal care at Aymiba Health Center, northwest Ethiopia. J Blood Med 8:35-40.
- Teshome MS, Meskel DH, Wondafrash B (2020) Determinants of Anemia Among Pregnant Women Attending Antenatal Care Clinic at Public Health Facilities in KachaBirra District, Southern Ethiopia. J Multidiscip Healthc 13:1007–1015.
- Tulu BD, Atomssa EM, Mengist HM (2019) Determinants of anemia among pregnant women attending antenatal care in Horo Guduru Wollega Zone, West Ethiopia: Unmatched case-control study. PloS one 14:e0224514.
- Ebuy Y, Alemayehu M, Mitiku M, Goba GK (2007) Determinants of severe anemia among laboring mothers in Mekelle city public hospitals, Tigray region, Ethiopia. PloS one 12:e0186724.
- Haidar JA, Pobocik RS (2009) Iron deficiency anemia is not a rare problem among women of reproductive ages in Ethiopia: a community based cross sectional study. BMC Blood Disord 9:1-8.
- Kenea A, Negash E, Bacha L, Wakgari N (2018) Magnitude of anemia and associated factors among pregnant women attending antenatal care in public hospitals of ilu Abba Bora zone, south west Ethiopia: a cross-sectional study. Anemia 2018:9201383.
- Weldekidan F, Kote M, Girma M, Boti N, Gultie T (2018) Determinants of anemia among pregnant women attending antenatal clinic in public health facilities at Durame Town: unmatched case control study. Anemia 2018: 8938307.
- Blackwell AD (2016) Helminth infection during pregnancy: insights from evolutionary ecology. International journal of women's health 8:651-661.
- Taghipour A, Ghodsian S, Jabbari M, Olfatifar M, Abdoli A, et al. (2020) Global prevalence of intestinal parasitic infections and associated risk factors in pregnant women: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 115:457-470.
- Rodríguez-Morales AJ, Barbella RA, Case C, Arria M, Ravelo M, et al. (2006) Intestinal parasitic infections among pregnant women in Venezuela. Infect Dis Obstet Gynecol 2006:23125.
- Ahenkorah B, Nsiah K, Baffoe P, Ofosu W, Gyasi C, et al. (2020) Parasitic infections among pregnant women at first antenatal care visit in northern Ghana: A study of prevalence and associated factors. Plos one 15:e0236514.
- Abdoli A, Pirestani M (2014) Are pregnant women with chronic helminth infections more susceptible to congenital infections? Front Immunol 5:53.









