Keywords
Hydroxychloroquine; COVID-19; Correlation; Mortality rate
Background
SARS-CoV-2, a newly emergent coronavirus first recognized in December 2019, is the causative agent of Coronavirus disease 2019 (COVID-19). While most people with COVID-19 develop only mild (40%) or moderate (40%) disease, approximately 15% develop severe disease that requires oxygen support, and 5% have critical disease with complications such as respiratory failure, acute respiratory distress syndrome (ARDS), sepsis and septic shock, thromboembolism, and/or multiorgan failure, including acute kidney injury and cardiac injury [1].
Currently there is a worldwide active search for vaccines and therapeutics to be used against the newly emergent disease, including repurposing of well known drugs. In vitro based evidence of suppression of activity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other coronavirus strains provoked increased interest in the use hydroxychloroquine and chloroquine for the treatment of COVID-19 [2-6].
In an attempt to contain and treat the spreading illness caused by the novel coronavirus responsible parties in almost every country worldwide issue recommendations and guidelines detailing instructions concerning hygiene, social distancing, diagnostics and drug therapies. A lot of these guidelines include hydroxychloroquine as a valuable treatment option for patients with COVID-19.
For example, a large number of countries in Europe have published documents with recommendations for the health professionals – Luxembourg, Germany, Romania, Russia, Belgium, Spain, France, Norway, Slovakia, Czech republic, Turkey, Ireland and the UK [7-19]. Although each and every one of these documents is based on similar sources and provides similar recommendations, there are some differences which are worth discussing. Hydroxychloroquine features as a first line treatment or at least as an option available to COVID-19 patients with different stages of the disease in most of the guidelines [7-12,14-17] while a recommendation against exists only in few [13,18,19]. Other countries do not have issued guidelines, following instead ministry of health statements [20,21] or treatment flowcharts [22].
The situation is similar in countries in Asia and Africa. The Lebanese Pulmonary Society, The Lebanese Society of Critical Care Medicine, The Lebanese Society of Anesthesiology issued a statement detailing a proposed protocol for diagnosis, triage and treatment of COVID-19 cases [23]. The Korean Society of Infectious Diseases, Korean Society for Antimicrobial Therapy, and The Korean Society for AIDS propose Interim Guidelines on Antiviral Therapy for COVID-19, published in June [24]. National guidelines have been provided in Morocco [25], Thailand [26], India [27], Pakistan [28], Saudi Arabia [29], China [30], Taiwan [31] and Egypt [32]. The common feature in all these directions is the use of hydroxychloroquine as one if not the first treatment option.
In North and Central America Canada and USA deny the use of hydroxychloroquine [33,34]. At the same time in Mexico guidelines provide dose regimes based on the experience from China [35]. Recommendations published by responsible parties in countries in South America generally support the application of hydroxychloroquine in the treatment of patients with COVID-19 [36-42]. Australia and New Zealand share the same guideline which features a strong recommendation against chloroquine and hydroxychloroquine for the treatment of COVID-19 [43].
Bearing in mind these differences in recommendations concerning the use of hydroxychloroquine against COVID-19, we aimed to determine if there is any connection between changes in the amounts hydroxychloroquine used and death rates from COVID-19 worldwide.
Methods
Data about amounts of hydroxychloroquine used. Changes in amounts hydroxychloroquine (tablets) sold were provided by IMS (Intercontinental Medical Statistics) Health for the period 01.2019 to 04.2020 including data for 56 countries worldwide (Table 1). Data is presented as average for each year with 2020 comprising data for only 4 month in accordance with data availability.
Table 1 Data about hydroxychloroquine use for 2019 and 2020, mortality from COVID-19 and recommendations for HQC use.
| Country |
AVG tablets HQC for 2019 |
AVG tablets HQC for 2020 |
% change in HQC use |
Mortality from COVID-19 % |
Recommendations for HQC use |
| Kuwait |
4 875 |
44 655 |
816,00% |
0,82 |
+* |
| Peru |
13 168 |
74 358 |
464,70% |
2,84 |
+* |
| Kazakhstan |
4 620 |
15 330 |
231,82% |
0,49 |
+* |
| Colombia |
75 376 |
232 611 |
208,60% |
3,29 |
+ [36] |
| Lebanon |
127 493 |
368 618 |
189,13% |
2,18 |
+ [23] |
| Belarus |
43 935 |
124 869 |
184,21% |
0,57 |
NG |
| Philippines |
161 710 |
401 630 |
148,36% |
4,24 |
NG |
| Uruguay |
90 255 |
190 013 |
110,53% |
2,72 |
+ [37] |
| Korea |
1 931 193 |
4 009 373 |
107,61% |
2,31 |
+ [24] |
| Puerto Rico |
191 275 |
381 550 |
99,48% |
6,45 |
NG |
| Lithuania |
89 948 |
171 165 |
90,29% |
4,21 |
+ [20] |
| Chile |
238 815 |
443 993 |
85,91% |
1,72 |
+ [38] |
| Luxembourg |
19 975 |
36 350 |
81,98% |
2,71 |
+ [7] |
| Portugal |
350 282 |
604 735 |
72,64% |
4,16 |
- [21] |
| Germany |
2 853 374 |
4 786 683 |
67,76% |
4,73 |
+ [8] |
| Argentina |
1 499 370 |
2 453 715 |
63,65% |
2,79 |
- [39] |
| Italy |
3 934 958 |
6 437 003 |
63,59% |
14,47 |
NG |
| Ecuador |
151 935 |
245 385 |
61,51% |
8,37 |
+ [40] |
| USA |
34 920 505 |
55 439 330 |
58,76% |
5,55 |
- [34] |
| Latvia |
33 510 |
52 530 |
56,76% |
2,46 |
NG |
| Mexico |
513 285 |
786 620 |
53,25% |
11,90 |
+ [35] |
| Romania |
611 610 |
926 550 |
51,49% |
6,49 |
+ [9] |
| Russia |
1 236 068 |
1 868 325 |
51,15% |
1,31 |
+ [10] |
| Brazil |
2 505 810 |
3 735 150 |
49,06% |
5,10 |
+ [41] |
| Sweden |
285 375 |
416 375 |
45,90% |
9,77 |
NG |
| Australia |
2 808 525 |
4 034 375 |
43,65% |
1,40 |
- [43] |
| Switzerland |
321 615 |
460 808 |
43,28% |
6,24 |
-* |
| Belgium |
575 362 |
805 092 |
39,93% |
16,13 |
+ [11] |
| Spain |
2 302 526 |
3 221 679 |
39,92% |
9,36 |
+ [12] |
| Slovenia |
24 315 |
32 310 |
32,88% |
7,32 |
-* |
| France |
2 822 521 |
3 733 846 |
32,29% |
18,86 |
- [13] |
| Morocco |
297 945 |
392 738 |
31,82% |
2,47 |
+ [25] |
| Thailand |
648 068 |
853 471 |
31,69% |
1,85 |
+ [26] |
| Norway |
275 400 |
361 025 |
31,09% |
2,81 |
+ [14] |
| Tunisia |
306 503 |
398 970 |
30,17% |
4,51 |
NG |
| Poland |
523 838 |
678 668 |
29,56% |
4,28 |
NG |
| India |
23 662 726 |
30 650 203 |
29,53% |
2,84 |
+ [27] |
| Slovakia |
250 470 |
318 900 |
27,32% |
1,82 |
+ [15] |
| Pakistan |
2 168 955 |
2 694 870 |
24,25% |
1,96 |
+ [28] |
| Finland |
794 275 |
980 625 |
23,46% |
4,59 |
NG |
| Ireland |
412 935 |
508 772 |
23,21% |
6,75 |
- [18] |
| Greece |
1 258 845 |
1 550 130 |
23,14% |
5,93 |
+ [22] |
| Czech republic |
502 125 |
611 355 |
21,75% |
3,33 |
+ [16] |
| Estonia |
74 625 |
85 020 |
13,93% |
3,50 |
NG |
| Canada |
5 480 850 |
5 974 100 |
9,00% |
8,20 |
- [33] |
| UK |
6 188 745 |
6 512 895 |
5,24% |
14,17 |
- [19] |
| New Zealand |
377 750 |
387 325 |
2,53% |
1,46 |
- [43] |
| Venezuela |
9 745 |
9 880 |
1,39% |
0,82 |
+ [42] |
| Netherlands |
1 681 120 |
1 523 558 |
-9,37% |
12,49 |
NG |
| Saudi Arabia |
383 450 |
344 120 |
-10,26% |
0,74 |
+ [29] |
| Turkey |
4 416 480 |
3 941 040 |
-10,77% |
2,74 |
+ [17] |
| China |
37 516 339 |
31 112 558 |
-17,07% |
5,58 |
+ [30] |
| Jordan |
70 690 |
56 155 |
-20,56% |
1,01 |
NG |
| Taiwan |
1 244 080 |
907 330 |
-27,07% |
1,58 |
+ [31] |
| Egypt |
2 638 685 |
1 561 210 |
-40,83% |
3,47 |
+ [32] |
| Algeria |
881 880 |
241 028 |
-72,67% |
7,00 |
NG |
AVG: average; HQC: hydroxychloroquine; + = positive recommendation in a guideline or other official document for the treatment of COVID-19; - = negative recommendation in a guideline or other official document for the treatment of COVID-19; * = recommendation not from a guideline or other official document for the treatment of COVID-19; NG = no guideline or other official document for the treatment of COVID-19 found.
Mortality rates of COVID-19
Case fatality rate from COVID-19 is calculated as a ratio of total deaths to total number of cases for each country as provided by the website of Worldometer (https://www.worldometers.info/ coronavirus/) on the 10 July 2020 (date of site access) for the 56 countries with the largest number of registered cases (above 10 000).
Statistical methods
The following statistical methods were used to describe and summarize data.
1. Pearson’s product moment correlation coefficient (r). Interpretation of the value of this coefficient was done in terms of percent of explained variation. The hypothesis about difference from zero was checked at significance level of 0.05. Scatterplot presentation was used to show the form of relation.
2. The hypothesis that two groups that the mortality rate in two independent groups of countries: those with > 50% increase in use of hydroxychloroquine and these with ≤ 50% increase in use of hydroxychloroquine do not differ was tested by Student’s t-test. Significance level was fixed at 0.05.
Cluster analysis is used to classify objects into homogenous groups in an formal way. K-means algorithm was used.
Differences between clusters determinate by cluster-analysis were checked for significance by one-way ANOVA model.
Eta-coefficient (η) was used to discover the non-linearity relationship between use of hydroxychloroquine and mortality. The formal measure in percent was (η2-r2)*100%.
IBM® SPSS® Statistics, ver. 26, package was used for statistical data analysis.
Results
We have presented data for changes in hydroxychloroquine use for 56 countries worldwide for the period January 2019 to April 2020 available from the IMS Health database. It should be noted that changes have been both in the direction of increase and decrease of use of hydroxychloroquine. Largest positive increase in the amount of hydroxychloroquine tablets sold has been reported in Kuwait and Peru – 816% and 464% respectively, while smallest positive change has been registered in Venezuela – 1.39%. Countries where use of hydroxychloroquine has decreased include the Netherlands, Egypt and Algeria among others (Table 1).
Mortality rate from COVID-19 was extrapolated as a case fatality rate from data about total deaths to total number of registered cases for each country as provided by the website of Worldometer. Mortality rate ranges from 0.49% in Kazakhstan to 18.86% in France (Table 1). The high mortality rate in some countries or the low one in others respectively may be due to a number of factors. We attempted to determine if the use of hydroxychloroquine is one of them.
When we researched recommendations for hydroxychloroquine use we established that in 32 of these 56 countries it is considered a treatment option. In 29 of these 32 there are published guidelines or other official documents backing the use of hydroxychloroquine. In 11 countries issued guidelines deny the use of hydroxychloroquine for treatment of COVID-19 patients (Table 1). 13 countries have no official recommendations for treatment of the novel coronavirus disease issued.
In an attempt to determine the existence of a linear relation between changes in hydroxychloroquine use and mortality rate from COVID-19 we performed a correlation analysis. The scatter plot (Figure 1) suggests a weak linear correlation between the two variables. However, the Pearson’s product moment correlation coefficient is -0.215. Together with the p value of 0.112, it points to lack of statistical significance. This means that the correlation, if it exists is most likely nonlinear. Additionally, the Pearson’s product moment correlation coefficient is <0 which is an indicator of a negative direction of the correlation.
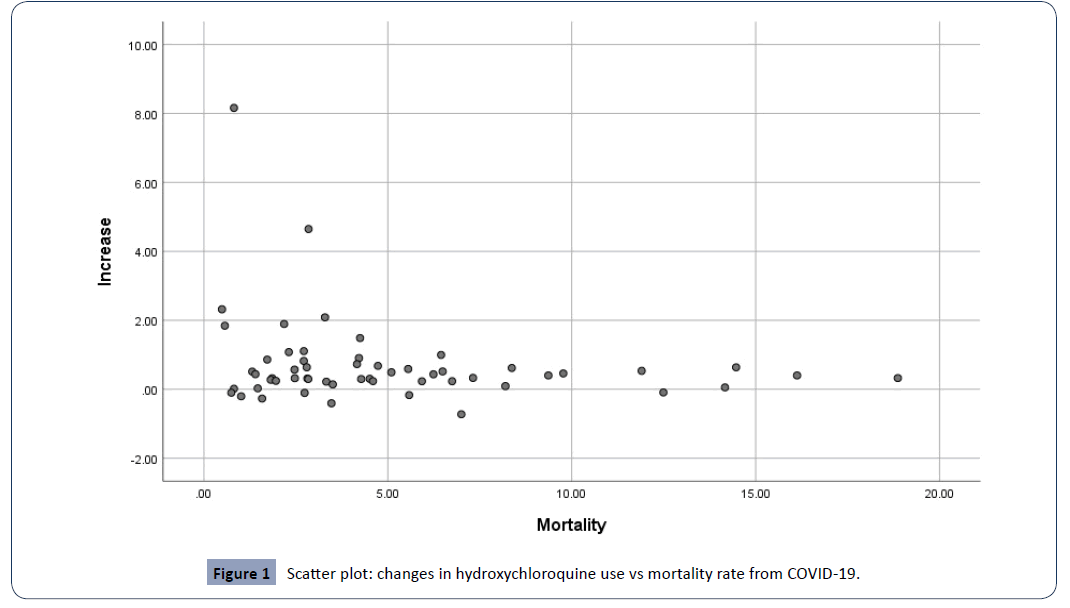
Figure 1: Scatter plot: changes in hydroxychloroquine use vs mortality rate from COVID-19.
We attempted to compare the mortality rate in two independent groups of countries: those with >50% increase in use of hydroxychloroquine and these with ≤ 50 % increase in use of hydroxychloroquine. Mean mortality rate for the first group including 32 countries with >50 % increase in use of hydroxychloroquine was 5.47 % (SD 4.63 %) and for the second group with ≤ 50 % increase in use of hydroxychloroquine comprising 23 countries: 4.21 % (SD 3.48 %). We used Student’s T-test in order to determine the statistical significance of the difference between the two groups (Table 2). The difference between the two groups is 1.26 % and the p=0.255 means it is statistically non-significant.
Table 2 Independent Samples Test.
| |
t-test for Equality of Means |
| t |
df |
Sig. (2-tailed) |
Mean Difference |
Std. Error Difference |
95% Confidence Interval of the Difference |
| Lower |
Upper |
| Mortality |
1.151 |
52.852 |
0.255 |
1.26 |
1.09 |
- 0.93579 |
3.45328 |
t – t-value; df – degrees of freedom
We performed cluster analysis in order to get an intuition about the structure of the data. Using K-means clustering, we formed three clusters. The first one contains 5 cases, the second one – 8 cases and the third one – 43 cases (Table 3).
Table 3 Cluster membership.
| Case Number |
Country |
Cluster |
| 1 |
Kuwait |
1 |
| 2 |
Peru |
1 |
| 3 |
Kazakhstan |
1 |
| 5 |
Lebanon |
1 |
| 6 |
Belarus |
1 |
| 47 |
Italy |
2 |
| 49 |
Mexico |
2 |
| 50 |
Sweden |
2 |
| 51 |
Belgium |
2 |
| 52 |
Spain |
2 |
| 53 |
France |
2 |
| 55 |
UK |
2 |
| 56 |
Netherlands |
2 |
| 4 |
Colombia |
3 |
| 7 |
Philippines |
3 |
| 8 |
Uruguay |
3 |
| 9 |
Korea |
3 |
| 10 |
Puerto Rico |
3 |
| 11 |
Lithuania |
3 |
| 12 |
Chile |
3 |
| 13 |
Luxembourg |
3 |
| 14 |
Portugal |
3 |
| 15 |
Germany |
3 |
| 16 |
Argentina |
3 |
| 17 |
USA |
3 |
| 18 |
Latvia |
3 |
| 19 |
Romania |
3 |
| 20 |
Russia |
3 |
| 21 |
Brazil |
3 |
| 22 |
Australia |
3 |
| 23 |
Switzerland |
3 |
| 24 |
Slovenia |
3 |
| 25 |
Morocco |
3 |
| 26 |
Thailand |
3 |
| 27 |
Norway |
3 |
| 28 |
Tunisia |
3 |
| 29 |
Poland |
3 |
| 30 |
India |
3 |
| 31 |
Slovakia |
3 |
| 32 |
Pakistan |
3 |
| 33 |
Finland |
3 |
| 34 |
Ireland |
3 |
| 35 |
Greece |
3 |
| 36 |
Czech republic |
3 |
| 37 |
Estonia |
3 |
| 38 |
New Zealand |
3 |
| 39 |
Venezuela |
3 |
| 40 |
Saudi Arabia |
3 |
| 41 |
Turkey |
3 |
| 42 |
China |
3 |
| 43 |
Jordan |
3 |
| 44 |
Taiwan |
3 |
| 45 |
Egypt |
3 |
| 46 |
Algeria |
3 |
| 48 |
Ecuador |
3 |
| 54 |
Canada |
3 |
The resulting statistics for the three clusters concerning the changes in hydroxychloroquine use and the mortality rate from COVID-19 are presented in Table 4.
Table 4 Cluster statistics.
| Cluster |
N |
Mean |
Standard Deviation |
Maximum |
Median |
Minimum |
| Increase |
1 |
5 |
3.77 |
2.711 |
8.16 |
2.32 |
1.84 |
| 2 |
8 |
0.34 |
0.244 |
0.64 |
0.40 |
- 0.09 |
| 3 |
43 |
0.40 |
0.502 |
2.09 |
0.32 |
- 0.73 |
| Mortality |
1 |
5 |
1.38 |
1.067 |
2.84 |
0.82 |
0.49 |
| 2 |
8 |
13.39 |
3.197 |
18.86 |
13.33 |
9.36 |
| 3 |
43 |
3.79 |
2.090 |
8.37 |
3.33 |
0.74 |
The analysis of the differences between the two parameters in the three clusters shows that there is a statistically significant differences both when it comes to mortality rates and to changes in the use of hydroxychloroquine. However, the correlation seems to be non-linear and difficult to characterize with the limited data we have available now. The eta coefficient and the eta squared are 0.750 and 0.563 for the increase in the use of hydroxychloroquine and 0.853 and 0.727 for the mortality rate respectively. The high values of the eta coefficient suggest the existence of a non-linear correlation.
Results from the dispersion analysis have been summarized in Table 5.
Table 5 ANOVA output.
| |
Sum of Squares |
df |
Mean Square |
F |
Sig. |
| Increase |
Between Groups |
51.974 |
2 |
25.987 |
34.086 |
0.000 |
| Within Groups |
40.407 |
53 |
0.762 |
|
|
| Total |
92.381 |
55 |
|
|
|
| Mortality |
Between Groups |
692.368 |
2 |
346.184 |
70.673 |
0.000 |
| Within Groups |
259.616 |
53 |
4.898 |
|
|
| Total |
951.984 |
55 |
|
|
|
Discussion
There are currently more than 20 million cases of people infected with the novel coronavirus SARS-CoV-2 worldwide with more than 700 000 deaths so far. Despite widespread measures of heightened hygiene, social distancing and travel restrictions, the pandemic does not show any tendency towards weakening with second waves of increased number of cases in many countries.
Although it is proven that certain measures can slow down the spread of the virus and help protect vulnerable subgroups of the population, so far there has not been much of advancement in the field of plausible treatments. Mostly, scientists and doctors have been focused on the repurposing of old drugs with the hope of using them against COVID-19 and its complications. A Chinese team published results of a study demonstrating that chloroquine and hydroxychloroquine inhibit SARS-CoV-2 in vitro with hydroxychloroquine being more potent than chloroquine [44,45]. Another team located in Marseille reported that hydroxychloroquine combined with azithromycin has the potential to clear viral nasopharyngeal carriage of SARS-CoV-2 in just three to six days [46]. Other drugs showing potential to inhibit SARS-CoV-2 in vitro and subjected to observational or randomized trials against COVID-19 worldwide are remdesivir, lopinavir, ritonavir and interferon beta [45]. There is however, not enough data to definitively approve or defy any of the aforementioned medications.
Current published guidelines, as discussed above, mostly recommend the use of hydroxychloroquine. There are few who propagate strongly against it but unfortunately some of them are highly influential. For example WHO recommends in its Clinical management of COVID-19: interim guidance that chloroquine and hydroxychloroquine should not be administered as treatment or prophylaxis for COVID-19 outside of the context of clinical trials [47]. A document issued by the European Medicine Agency also emphasizes that chloroquine and hydroxychloroquine should be used by health professionals for their authorized uses or as part of clinical trials or national emergency use national emergency use programmes for the treatment of COVID-19 [48]. These statements have apparently managed to swerve public opinion and more and more clinicians worldwide seem afraid to use hydroxychloroquine despite its potential benefits.
The Association of American Physicians & Surgeons seems to have recognized the dangerous influence of strong recommendations issued by international authorities and institutions. They have filed complaints against the federal Department of Health & Human Services (“HHS”), two of its constituent agencies – the Food and Drug Administration (“FDA”) and the Biomedical Advanced Research & Development Authority (“BARDA”) with allegations for irrational interference with timely access to hydroxychloroquine although it has been approved as safe by the FDA for sixty-five years and is safer than numerous medications that are widely available over the counter (“OTC”) without requiring a prescription, including anti-depressants (St John’s Wort), sleeping pills (diphenhydramine), bronchodilators (ephedrine), many pain medications including ibuprofen, acetaminophen and even aspirin [49].
Their claim is backed by a 57 page memorandum which extensively reviews hydroxychloroquine policies and compares them to mortality rates in numerous countries worldwide. Multiple foreign governments, including China, India, South Korea, Costa Rica, United Arab Emirates, and Turkey, successfully recommend use of HCQ for effective early treatment of COVID-19, and for use as a prophylaxis for the disease. There is a dramatic difference in saving lives in countries allowing early and prophylactic use of hydroxychloroquine compared with the United States [50]. For example, in UK, France and Italy, the use of hydroxychloroquine is discouraged and case fatality rate from COVID-19 in these countries is 14%, 18.5% and 14.5% respectively. At the same time Russia, India and Turkey have case fatality rates of 1.4%, 3.2% and 2.6% respectively and their national policies actively support hydroxychloroquine use [50].
Our analysis suggests that a possible correlation exists between the changes in the use of hydroxychloroquine and the mortality rate from COVID-19. It seems to be non-linear with a high eta coefficient but unfortunately available data is not enough to fully characterize it. A retrospective multicenter cohort study of patients from a random sample of all admitted patients with laboratory-confirmed COVID-19 aims to investigate changes in mortality in patients with coronavirus infection treated with hydroxychloroquine, azithromycin or neither. Contrary to our results Rosenberg et al, find no significantly associated with differences in in-hospital mortality. However, they point out that this result could be due to the study design and a number of other limitations [51].
Other clinicians seem to find a connection between hydroxychloroquine use and beneficial changes in mortality. For example, the working group of Marco Rizzi from Pope John XXIII Hospital in Bergamo, Lombardia, prepared a document enlisting all relevant instructions for the treatment of coronavirus patients based on the vast experience of Lombardia. According to them hydroxychloroquine should be considered as one of the first treatment options both in patients who have been hospitalized and in those who are treated at home [52].
There are a number of limitations intrinsic to the analysis. First of all, data about tablets used and mortality rates for each country are changing daily. Although we have tried to use most recent data, there is still a chance of significant changes occurring after our access dates. Secondly, hydroxychloroquine is habitually used for malaria, rheumatoid arthritis and lupus erythematosus on an yearly basis. However, we have accepted that any large differences in use detected in each country for the period January 2019 – April 2020 are most likely due to its use in the treatment of COVID-19. Recommendations for management of coronavirus infections published worldwide are almost daily updated due to the dynamic investigation of the proffered treatments.
Conclusion
Our analysis suggests that a possible correlation exists between the changes in the use of hydroxychloroquine and the mortality rate from COVID-19. We attempted to compare the mortality rate in two independent groups of countries: those with >50% increase in use of hydroxychloroquine and these with ≤ 50 % increase in use of hydroxychloroquine. The difference between the two groups is 1.26% and the p=0.255 means it is statistically non-significant. The ANOVA model and the correlation analysis suggest that the relation between the changes in the use of hydroxychloroquine and the mortality rate from COVID-19 seems to be non-linear with a high eta coefficient but unfortunately available data is not enough to fully characterize it.
Declarations
Ethical Approval and Consent to participate Not applicable. Competing interests EF, EG, KU, VP and SH are employees of Tchaikapharma High Quality Medicines Inc. The other authors report no competing interests.
Authors' contributions EF, EG, KU, VP, SH and TV were involved in literature search guideline interpretation. KK performed quality assessment of studies, data extraction and statistical analysis.
EF, EG, KU, VP, SH, KK and TV were involved in interpretation of results.
Acknowledgements The authors wish to thank Assya Petrova for providing support as a language editor.
32674
References
- World Health Organization (2020) Clinical management of COVID-19: interim guidance.
- Liu J, Cao R, Xu M, Wang X, Zhang H, et al. (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6: 16.
- Yao X, Ye F, Zhang M, Cui C, Huang B, et al. (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 71: 732-739.
- Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D (2020) Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents 55: 105932.
- Gautret P, Lagier JC, Parola P, Brouqui P, Raoult D, et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020: 105949.
- Chen Z, Hu J, Zhang Z, Jiang S, Han S, et al. (2020) Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv.
- (2020) COVID-19 and good drug use. Department of Health, Government of the Grand Duchy of Luxembourg.
- Kluge S, Janssens U, Welte T, Weber-Carstens S, peelings G, et al. (2020) Recommendations for intensive care therapy for patients with COVID-19-3rd version. The anesthesiologist 69: 653-664.
- Bubenek S, Săndesc D, Filipescu D, Tomescu D, Grințescu I, et al. (2020) Management Guide: Covid-19 Infection in ati Sections.
- The Roscongress Foundation (2020) Guidelines for the prevention and treatment of the novel coronavirus infection COVID-19.
- van Ierssel S, Dauby N, Bottieau E (2020) Interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium.
- Spanish Agency for Medicines and Health Products (2020) Available treatments subject to special access conditions for the management of respiratory infection by SARS-CoV-2.
- High Council for Public Health (2020) Coronavirus SARS-CoV-2 treatment recommendations.
- The Norwegian Medical Association (2020) Antiviral and immunomodulatory agents in COVID-19 - Overview of current treatment options for patients admitted to Norwegian hospitals.
- (2020) Overview of evaluated drugs for COVID-19 disease. Press releases on the activities of SÚKL.
- (2020) COVID-19 (SARS-CoV-2 Infection) Child Patient Management and Treatment.
- Rapid Evidence Review (2020) Clinical evidence for hydroxychloroquine and azithromycin combination therapy for COVID-19. National Medicines Information Centre, Ireland.
- NHS (2020) Clinical management of persons admitted to hospital with suspected COVID-19 infection. Specialty guides for patient management during the coronavirus pandemic.
- Minister of health of the republic of lithuania (2020) On order of the minister of health of the republic of lithuania of 16 march 2020 no. Amendment v-383 " approval of the description of the diagnosis and treatment procedure for covid-19 disease (coronavirus infection) in adults".
- SNS (2020) Covid-19 | Hydroxychloroquine. Infarmed and DGS recommend discontinuing the use of the drug.
- Hellenic Chest Diseases Society (2020) Therapeutic algorithm of a patient with covid-19 infection in the hospital.
- (2020) COVID-19 CORONAVIRUS Statement of the Lebanese Pulmonary Society, The Lebanese Society of Critical Care Medicine, The Lebanese Society of Anesthesiology.
- Kim SB, Huh K, Heo JY, Joo EJ, Kim YJ, et al. (2020) Interim Guidelines on Antiviral Therapy for COVID-19. Infect Chemother 52: 281-304.
- Naciri A, Sine H, Baba MA, Bouchriti Y, Kharbach A, et al. (2020) National Guidelines on Management of Coronavirus Disease COVID-19 in Morocco. European Journal of Medical and Educational Technologies 13: em2003.
- Corona Virus Disease (COVID-19) (2020) Guidelines for Health Care and Public Health Workers. Department of Disease Control, Thailand.
- Clinical Management Protocol: Covid-19 (2020) Government of India. Ministry of Health and Family Welfare. Directorate General of Health Services (EMR Division).
- Ministry of Health (2020) Supportive care and antiviral treatment of suspected or confirmed COVID-19 infection.
- National Health Commission & State Administration of Traditional Chinese Medicine (2020) Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7).
- Ministry of Health and Welfare Taiwan Centers for Disease Control (2020) Interim Guidelines for Clinical Management of SARS-CoV-2 Infection (5th edition).
- COVID-19 and Chloroquine/hydroxychloroquine (2020) A production of the National Institute of Excellence in Health and Social Services.
- 34.COVID-19 Treatment Guidelines (2020) Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health.
- Grupo de Trabajo Mexicano COVID-19/COMMEC (2020) Guide COVID-19 for the care of the critical patient with SARS-coV-2 infection Mexican College of Critical Care Medicine. Med Crit 34: 7-42.
- Cordoba VB (2020) Drug repositioning for COVID-19. Colombia Médica 51: Coronavirus disease 2019.
- SMIU (2020) Update of the evidence of the specific treatment of COVI-1930 March 2020.
- Institute of Public Health of Chile (2020) On the use of Hydroxychloroquine, Chloroquine and Azithromycin in the disease caused by the SARS-CoV-2 virus: COVID-19.
- United Argentina (2020) Conditional recommendations for the therapeutic approach of COVID-19 - Version 3.0.
- Ojeda A, Cedillo AJO, Cedillo POO, Cedillo AEO, León A (2020) New Alternative for Treatment for Covid 19 in Ecuador. InterAm J Med Health 3: e202003013.
- IFF (2020) Guidelines for the Diagnosis and Treatment of COVID-19 (MS). Portal of Good Practices in Women, Children and Adolescents' Health.
- Ocha (2020) Venezuela: Intersectoral COVID-19 Preparedness and Response Plan (Second iteration: 10 April 2020).
- National COVID-19 Clinical Evidence Taskforce (2020) Australian guidelines for the clinical care of people with COVID-19 .
- Yao X, Ye F, Zhang M, Cui C, Huang B, et al. (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 71: 732-739.
- Schlagenhauf P, Grobusch MP, Maier JD, Gautret P (2020) Repurposing antimalarials and other drugs for COVID-19. Travel Med Infect Dis 34: 101658.
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 56: 105949.
- European Medicines Agency (2020) COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes.
- Association of American Physicians & Surgeons (2020) Complaint for declaratory and injunctive relief.
- Gold Kist (2020) Memorandum of law in support of plaintiff’s motion for a preliminary injunction.
- Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, et al. (2020) Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA 323: 2493-2502.






