Jin-Ping Lai1 2*, Chyi-Chia Lee11, Melissa Crocker3 4, Mufaddal Najmuddin2, Eileen Lange3 4,and Maria Merino11 Constantine A Stratakis3 4
Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA
Department of Pathology, Saint Louis University School of Medicine, St Louis, MO 63104, USA
Section on Endocrinology and Genetics, Program on Developmental Endocrinology Genetics (PDEGEN), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), USA
Inter-Institute Pediatric Endocrinology Training Program, National Institutes of Health (NIH), Bethesda, MD 20892, USA
*Corresponding Author:
Jinping Lai
Department of Pathology
Saint Louis University School of Medicine
St Louis, MO 63104
USA
Tel: 001-314-577-8782, 001-314-577-6132
E-mail: jinpinglai@slu.edu
Background: Large cell calcifying sertoli cell tumor (LCCSCT) is an exceedingly rare lesion of the testicle. It is most often seen in patients with Carney complex (CNC) or Peutz-Jeghers syndrome (PJS). We now report the first pediatric patient with what appears to be bilateral LCCSCT and no other conditions or a genetic syndrome, such as PJS or CNC, has been associated with it.
Methods: A 10-year-old boy was found to have a right testicular mass during a routine pediatric examination; he underwent right orchiectomy. He was then evaluated clinically for PJS or CNC and underwent genetic testing. His tumor was studied by immunohistochemistry for the expression of calretinin, NY-ESO-1, inhibin, CD99, S100, PLAP, AE1/AE3, Bcl-2, p53, and Mib1.
Results: Patient did not have clinical features or genetic abnormalities of CNC and PJS. Microscopic features showed large, round or cubical intratubular and aggregated tumor cells with prominent nuclear atypia, large and prominent nucleoli and extensive calcification. In the Immunohistochemical studies, calretinin and inhibin alpha were up regulated in LCCSCT as compared to the adjacent benign Sertoli cells. Meanwhile, NY-ESO and CD99 were down-regulated in LCCSCT. Focally and weakly positive S100 was found in the tumor tissue, but no S100 expression was present in the adjacent Sertoli cells. There was no expression of PLAP, P53, Bcl-2, Mib1 and AE1/AE3 in LCCSCT and adjacent Sertoli cells. Micro-calcifications were found in the other gonad by ultrasonography, suggesting LCCSCT.
Conclusion: LCCSCT is a rare testicular neoplasm, and may present in isolated rather than in more typical association with syndromes such as CNC and PJS.
Key words
Large cell calcifying sertoli cell tumor; Carney complex
Introduction
Sertoli cell tumors account for approximately 4% of all testicular tumors in childhood [1,2]. Based on the distinct pathological features and clinical presentation, large cell calcifying sertoli cell tumor (LCCSCT) has been recognized as a rare variant of sertoli cell tumors. It is most often reported in patients with the familial lentiginoses, Carney complex (CNC) along with cardiac myxomas, pituitary, and adrenal tumors [3], or Peutz-Jeghers syndrome (PJS) in association with gastrointestinal hamartomatous polyps and other neoplasms [4,5]. Rarely, LCCSCT is seen in the context of tuberous sclerosis or Bourneville syndrome [6].
We report the case of a young boy with LCCSCT without any of the above syndromes, pointing to the possibility that this tumor can in fact occur in a sporadic, isolated setting. We also performed immunohistochemistry for NY-ESO-1, calretinin, inhibin alpha, S100, CD99, PLAP, P53, Bcl-2, Mib1 and AE1/AE3 in the LCCSCT and adjacent benign Sertoli cells.
Case Report
The patient initially presented to the urologist at the age of 10 years and 3 months after a mass was discovered in his right testis during a routine examination. He had no additional symptoms.
He had been previously healthy except for frequent episodes of otitis media as an infant; his only surgeries were placement of myringotomy tubes and an adenoidectomy. He had a clavicular fracture at the age of three. He was not on any medications. His growth records were available from age 5 and showed consistent height at the 50th to 75th percentiles and weight at the 25th percentile. At the time of presentation to the urologist, the right testis was described as firm and hard and larger in size than the left testis. Ultrasound revealed a calcified mass occupying 40% of the volume of the testis with additional micro-calcifications within the normal tissue. Tumor markers including CA-125, CEA, AFP and beta-HCG were normal. A right orchiectomy was performed. Additional investigations included an echocardiogram and an electrocardiogram, both of which were normal. A CAT scan of the abdomen and pelvis revealed no metastasis. Gonadotropins and testosterone were known to be in the pre-pubertal range, and other pituitary functions were normal. Serial measurements of Inhibin B were 109 pg/mL, eight months after surgery, and 180 pg/mL, 15 months after surgery. A repeat testicular ultrasound noted micro-calcifications in the left testis. Bone age had remained normal with no advancement. Growth had remained stable, and the left testis had enlarged to 8 milliliters over the last 15 months following surgery with development of Tanner II stage pubic hair. The remainder of the examination remained normal to date.
Immunohistochemistry
Paraffin embedded tissue sections (5 μm) were deparaffinized through xylene and graded alcohols. Immunohistochemical stains for NY-ESO-1 (Invitrogen, 1:100 dilution), Calretinin (Zymed 1:50 dilution), Inhibin alpha (Serotec, 1:10 dilution), S100 (BioGenex, 1:8000), PLAP (BioGenex, 1:100), AE1/AE3 (Dako, 1;100 dilution), CD99 (12E7, Dako, 1:100 dilution), Bcl-2 (Dako, 1:20 dilution), Ki-67(Mib1, Dako, 1:200) and P53 (DO-7; DAKO, 1;1000 dilution) were done after antigen retrieval using target retrieval solution, low pH (DAKO) [7]. Slides were incubated in Tris goat (3%) for 15 min and then incubated for 1 to 2 h at room temperature with primary antibodies. Detection was carried out on an automated system (Autostainer; DAKO) using a horseradish peroxidase/3,3′- diaminobenzidine polymer-based detection system (Envision+; DAKO) according to the manufacturer's recommendations. Images were taken using an Olympus Bx41 microscope, objective UPlanFI 40×/0.75 ∞/0.17, with an adaptor U-TV0.5×C using a digital camera Q-imaging Micropublisher 5.0RTV. The images were captured using “Q-Capture Version 3.1” and imported into Adobe Photoshop 7.0 [7].
Genetic analysis of carney syndome and PJS
DNA was extracted from peripheral blood and sequenced for PRKAR1A and STK11/LKB1, the genes for CNC and PJS, respectively, by previously reported standard methods [8,9].
Results
Histological findings
Grossly, his right testicular tumor consisted of a tan, welldemarcated and firm mass with calcification measuring 2.0 cm in diameter. Microscopically, it was composed of large, round or cubical tumor cells with abundant eosinophilic cytoplasm, prominent nuclear atypia, and some with prominent nucleoli. Tumor cells were arranged in cords, trabeculae, clusters and small sheets. There were several foci of intra-tubular growth patterns. Calcification was present. Some areas showed abundant eosinophilic stroma and lymphocyte infiltrate (Figure 1).
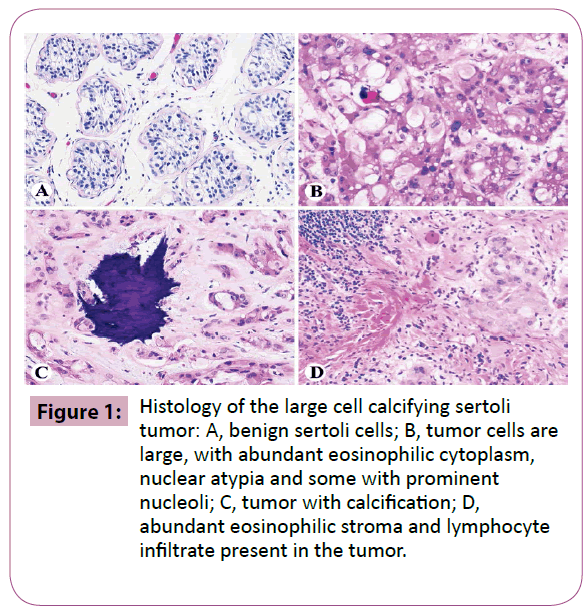
Figure 1: Histology of the large cell calcifying sertoli tumor: A, benign sertoli cells; B, tumor cells are large, with abundant eosinophilic cytoplasm, nuclear atypia and some with prominent nucleoli; C, tumor with calcification; D, abundant eosinophilic stroma and lymphocyte infiltrate present in the tumor.
Immunohistochemistry
In immunohistochemical studies, calretinin and inhibin alpha were up-regulated in LCCSCT as compared to adjacent benign sertoli cells, while, NY-ESO-1 and CD99 were down-regulated in LCCSCT (Figure 2). S100 was focally and weakly positive in LCCSCT but not in the adjacent sertoli cells. There was no expression of PLAP, P53, Bcl-2, Mib1 and AE1/AE3 in both LCCSCT and adjacent sertoli cells (Figure 2).
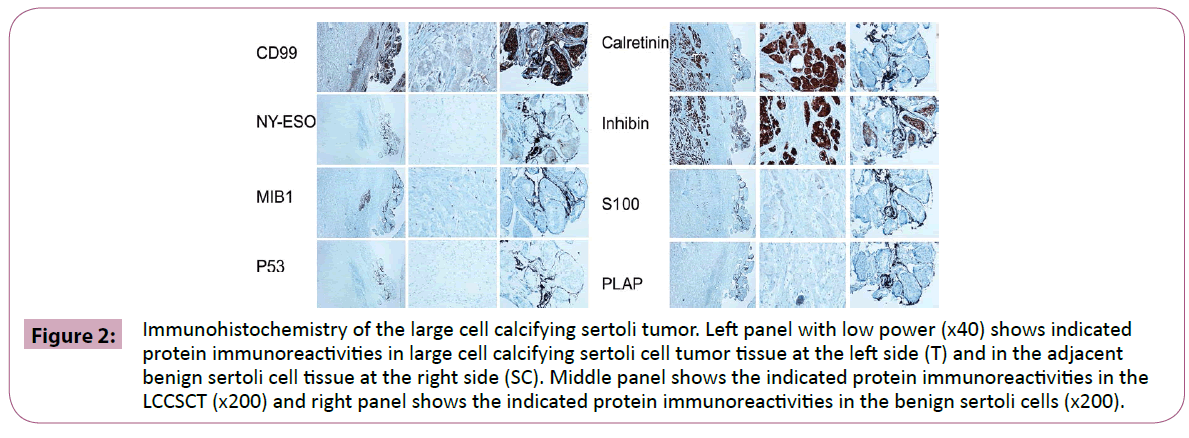
Figure 2: Immunohistochemistry of the large cell calcifying sertoli tumor. Left panel with low power (x40) shows indicated protein immunoreactivities in large cell calcifying sertoli cell tumor tissue at the left side (T) and in the adjacent benign sertoli cell tissue at the right side (SC). Middle panel shows the indicated protein immunoreactivities in the LCCSCT (x200) and right panel shows the indicated protein immunoreactivities in the benign sertoli cells (x200).
Clinical follow up and genetic analysis
He had been followed up for 17 months after right orchiectomy. A calcification was found in his left testicle by ultrasound examination (Figure 3). During his most recent visit at the National Cancer Institute, given the association of LCCSCTs with Carney complex and Peutz-Jaghers syndrome, he underwent a cardiology consult, EKG and CT of the abdomen and pelvis, all of which were normal, and was genetically tested for Carney complex and PJS. All the tests returned negative and he had not demonstrated any complications of either syndrome. All his hormone levels were normal in range (Table 1).
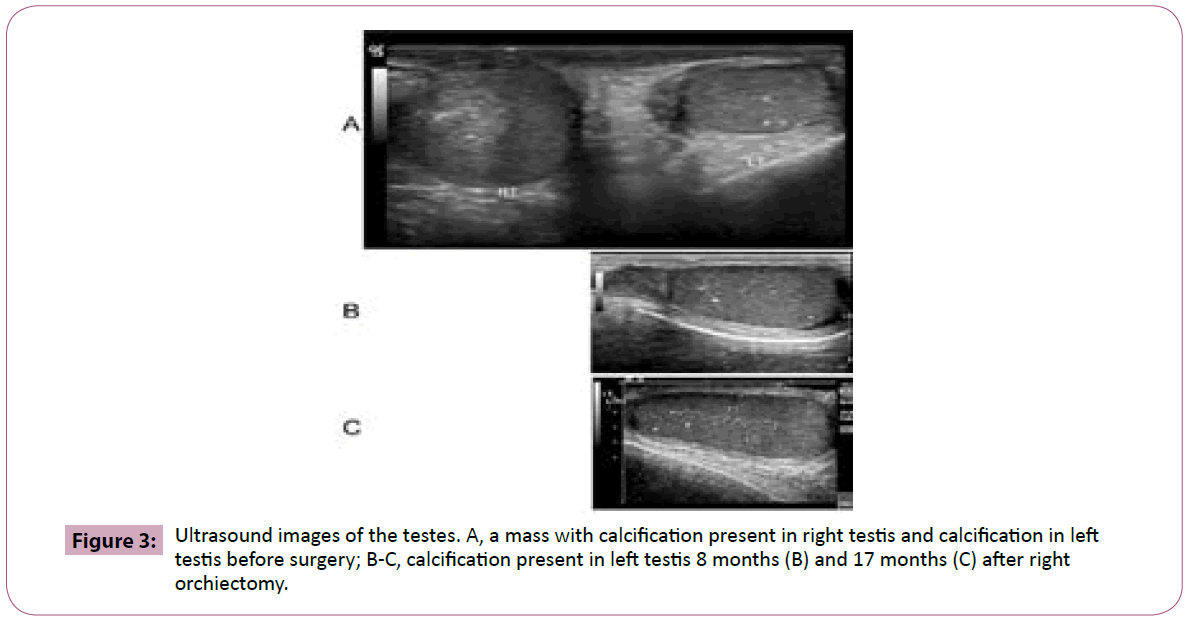
Figure 3: Ultrasound images of the testes. A, a mass with calcification present in right testis and calcification in left testis before surgery; B-C, calcification present in left testis 8 months (B) and 17 months (C) after right orchiectomy.
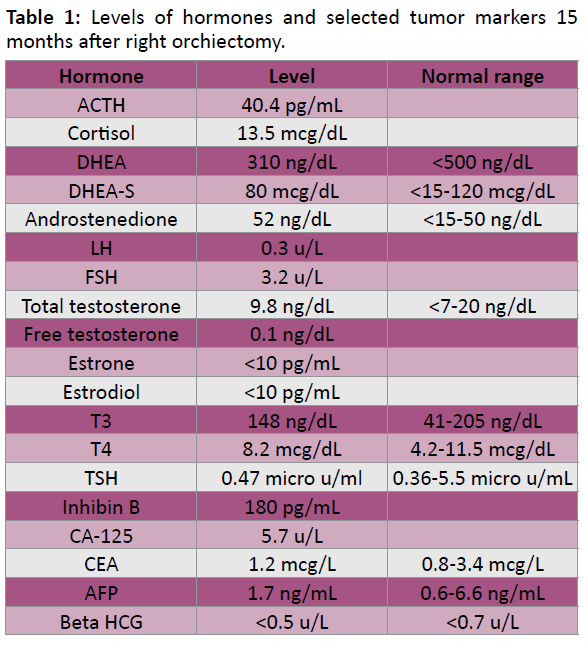
Table 1: Levels of hormones and selected tumor markers 15 months after right orchiectomy.
Discussion
Large cell calcifying Sertoli cell tumor (LCCSCT) is an exceedingly rare variation of sertoli cell tumor of the testis. This 10 year old boy with an isolated LCCSCT was diagnosed based on the characteristic histologic features and the immonohistochemical profile of the lesional tumor cells. As LCCSCT is often seen in patients with Carney Complex (CNC), Peutz-Jegher syndrome (PJS), and other syndromes. In the present case, we carefully examined the patient, performed genetic analysis for CNC and PJS. We did not find evidence of above syndromes or presence of other pathology. We first compared the tumor NY-ESO-1, CD99, Inhibin alpha, and Calretinin levels to that in the adjacent benign sertoli cells and found an upregulation of calretinin and inhibin alpha, and a down-regulation of NY-ESO-1 and CD99 in the LCCSCT cells.
LCCSCT usually presents in a benign behavior with low malignant potential in children [1,10,11]. Classification of LCCSCT is divided into two subgroups. “Early-onset LCCSCT” has a mean age of onset of 17 years and is usually bilateral, multifocal, hereditary and associated with other clinical signs of the dysplastic syndromes. “Late-onset LCCSCT” presents with a mean age of onset of 39 years and is monofocal, monolateral and asymptomatic [5,11]. Only 5.5% of early-onset LCCSCT were found to metastasize, while 23.5% of late-onset LCCSCT developed metastases [12]. Malignant features include large sized solid tumor (>4 cm in diameter) and demonstrate extratesticular extension, necrosis, cellular atypia and high mitotic rate (>3 mitotic figures in 10 high power fields). In the present case, although the tumor size was less than 4 cm in diameter, we found 2 mitotic figures in 10 high power fields and there was prominent cellular atypia. During the frozen section, pathology was interpreted as malignant tumor versus sertoli cell tumor, therefore a complete orchiectomy was performed. The patient had been followed up for 17 months, and no metastasis was found; however, calcification was noted in the other testis.
In immunohistochemistry of LCCSCT, positive expression of Inhibin alpha, calretinin, S100 and CD99 have been reported in a few cases [13]. However, based on our study, they may be expressed in both benign sertoli cells and LCCSCT tumor cells. Therefore, we compared them in tumor cells with adjacent sertoli cells. We found that calretinin and Inhibin alpha are up-regulated in LCCSCT as compared to adjacent benign sertoli cells. S100 is focally and weakly positive in LCCSCT but not in the adjacent sertoli cells. CD99 has been reported to function as an apoptotic promoter [14-16]. In the present study we found CD99 is down-regulated in LCCSCT as compared to the adjacent sertoli cells. Consistent with most of the literature, we found in this case that PLAP, P53, Bcl-2, Mib1 and AE1/AE3 are negative in LCCSCT.
NY-ESO-1 is a cancer-testis antigen that is (physiologically) expressed in the testis as well as (aberrantly) in a growing yet limited subset of malignancies [17,18] and is expected to be expressed in sertoli cells. To date, there are no reports regarding NY-ESO-1 expression in sertoli cell tumors. In this case we first report that NY-ESO-1 is expressed in the cytoplasm of sertoli cells of the testis but not in LCCSCT. To further characterize the role of NY-ESO-1 in LCCSCT tumorigenesis, NY-ESO-1 level should be included in future studies of immunohistochemistry in LCCSCT.
In conclusion, LCCSCT is a rare testicular neoplasm, and may present in isolated rather than in more typical association with syndromes such as CNC and PJS. More case reports and the related protein expression profiling, particularly NY-ESO-1, Calretinin, Ihibin alpha, S100 and CD99, are needed to further characterize this disease.
3790
References
- Thomas JC, Ross JH, Kay R (2001) Stromal testis tumors in children: a report from the prepubertal testis tumor registry. J Urol 166: 2334- 2338.
- Charoniti I, Kavazarakis E, Kontaxaki C, Bonou-Boukouvalea I, Fretzayas A, et al. (2006) Large cell calcifying Sertoli cell tumor of the testis in a boy with brucellosis. PediatrInt 48: 501-503.
- Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL (1985) The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 64: 270-83.
- Young S, Gooneratne S, Straus FH, Zeller WP, Bulun SE, et al. (1995) Feminizing Sertoli cell tumors in boys with Peutz-Jeghers syndrome. Am J Surg Pathol19: 50-58.
- Giglio M, Medica M, Rose AF, Germinale F, Ravetti JL, et al. (2003) Testicular sertoli cell tumours and relative sub-types. Analysis of clinical and prognostic features.Urol 70: 205-210.
- Perez-Atayde AR, Nunez AE, Carroll WL, Murthy AS, Vaitukaitis JL, et al. (1983) Large-cell calcifying sertoli cell tumor of the testis. An ultrastructural, immunocytochemical, and biochemical study. Cancer 51: 2287-2292.
- Tabe Y, Sebasigari D, Jin L, Rudelius M, Davies-Hill T, et al. (2009) MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma. Clin Cancer Res 15(3): 933-942.
- Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, et al. (2009) Mutations in regulatory subunit type 1A of cyclic adenosine 5'-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J ClinEndocrinolMetab 94: 2085-2091.
- Boardman LA, Couch FJ, Burgart LJ, Schwartz D, Berry R, et al. (2000) Genetic heterogeneity in Peutz-Jeghers syndrome. Hum Mutat 16: 23-30.
- Chang B, Borer JG, Tan PE, Diamond DA(1998) Large-cell calcifying Sertoli cell tumor of the testis: case report and review of the literature. Urology 52: 520-523.
- Plata C, Algaba F, Andújar M, Nistal M, Stocks P, et al. (1995) Large cell calcifying Sertoli cell tumour of the testis. Histopathology 26: 255-259.
- Kratzer SS, Ulbright TM, Talerman A, Srigley JR, Roth LM, et al. (1997) Large cell calcifying Sertoli cell tumor of the testis: contrasting features of six malignant and six benign tumors and a review of the literature. Am J SurgPathol 21: 1271-1280.
- Sato K, Ueda Y, Sakurai A, Ishikawa Y, Okamoto SY, et al. (2005) Large cell calcifying Sertoli cell tumor of the testis: comparative immunohistochemical study with Leydig cell tumor. PatholInt 55: 366-71.
- Sohn HW, Choi EY, Kim SH, Lee IS, Chung DH, et al. (1998) Engagement of CD99 induces apoptosis through a calcineurin-independent pathway in Ewing's sarcoma cells. Am J Pathol 153: 1937-1945.
- Pettersen RD, Bernard G, Olafsen MK, Pourtein M, Lie SO (2001) CD99 signals caspase-independent T cell death. J Immunol 166: 4931-42.
- Scotlandi K, Baldini N, Cerisano V, Manara MC, Benini S, et al. (2000) CD99 engagement: an effective therapeutic strategy for Ewing tumors. Cancer Res 60: 5134-42.
- Lai JP, Rosenberg AZ, Miettinen MM, Lee CC (2012) NY-ESO-1 expression in sarcomas: A diagnostic marker and immunotherapy target. Oncoimmunology 1: 1409-1410.
- Lai JP, Robbins PF, Raffeld M, Aung PP, Tsokos M, et al. (2012) NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol 25: 854-858.










