Keywords
Ketosis; Epilepsy; Ketogenic diet
Ketosis: Background
Therapeutic ketosis has roots as far back as 500 B.C., when fasting and other dietary interventions were used to treat epileptic seizures. Its first modern use was documented by physicians in the early 20th century who prescribed a ketogenic diet (KD) to patients who suffered from seizures in an effort to imitate the effects of fasting [1-18]. In order to properly mimic the benefits of fasting, the ketogenic diet was described in 1925 by Dr. Peterman, a physician at the Mayo Clinic, as “1 gram of protein per kilogram of body weight in children, 10–15 grams of carbohydrates per day, and the remainder of the calories in fat”, a calculation very similar to that used today by nutritionists and physicians [19-25].
With the first breakthrough of anti-epileptic drugs in 1938, much of the medical and research communities lost interest in the ketogenic diet and instead focused on drug therapies. Throughout the rest of the century, the ketogenic diet continued to lose favor with the public, mostly due to the difficulty in adhering to a lowcarbohydrate and high-fat diet, given the high-carbohydrate standard American fare. However, the ketogenic diet remained in medical textbooks as a treatment for epilepsy until the 1980s, including the 1972 textbook by Dr. Livingston of Johns Hopkins Hospital. In it, he described his study of more than 1,000 epileptic children, of whom “52% had complete control of the seizures and an additional 27% had improved control” while following a strict ketogenic diet [26-49].
At the turn of the century, after three decades of having fewer than eight publications per year on PubMed, the ketogenic diet began to catch the attention of the national media, specifically through the coverage of a two-year-old child, Charlie, who had been cured of his seizures through implementation of a ketogenic diet at Johns Hopkins Hospital [50]. This and subsequent success stories have led to a recent surge in the ketogenic diet’s popularity as a potential treatment for neurological disorders, weight loss, Type 2 diabetes, cancer, chronic pain, and other conditions.
Mechanisms
The severe reduction of carbohydrate intake, whether through a low-carbohydrate, high-fat diet or through fasting has several metabolic effects on the body, including reduced levels of insulin and increased levels of glucagon. These changes lead to the activation of phosphoenolpyruvate carboxykinase, fructose 1,6-biphosphatase, and glucose 6-phosphatase and the inhibition of pyruvate kinase, 6-phosphofructo-1-kinase, and glucokinase, all of which favors gluconeogenesis and ketogenesis [22].
Ketogenesis, the process by which ketones are made, produces acetone, acetoacetate, and beta-hydroxybutyrate through the breakdown of free fatty acids from adipose tissue, with betahydroxybutyrate being the most abundant. Under normal conditions, with a diet high in carbohydrates, the acetyl coenzyme A (acetyl CoA) is oxidized via the citric acid cycle (TCA/ Krebs cycle) and then by the mitochondrial electron transport chain to release energy. During times of limited carbohydrate intake, whether through the ketogenic diet or fasting, fatty acids are mobilized and undergo beta-oxidation to become acetyl CoA.
When the amount of acetyl CoA from fatty acid beta-oxidation begins to exceed the processing capacity of the citric acid cycle, the liver (mostly in the mitochondria of hepatocytes) turns acetyl CoA into ketone bodies using acetoacyl CoA and betahydroxy- beta-methylglutaryl CoA [11]. These ketones (betahydroxybutyrate, acetoacetate, and acetone) are then supplied to other tissues, including the brain, and offset most, but not all, of the need for glucose as an energy source.
In these extrahepatic tissues, beta-hydroxybutyrate dehydrogenase converts beta-hydroxybutyrate to acetoacetate. Beta-ketoacyl-CoA transferase reverts acetoacetate to acetoacyl- CoA, using succinyl CoA as the CoA donor. Acetoacyl CoA is then converted to acetyl CoA by thiolase.
Acetyl-CoA enters the citric acid cycle, ultimately producing 23 adenosine triphosphate (ATP) molecules post-oxidative phosphorylation when catabolized from acetoacetate and 26 ATP when catabolized from beta-hydroxybutyrate. The additional 3 ATP are a result of the NADH produced when betahydroxybutyrate is converted to acetoacetate. As a byproduct, acetone is expelled from the body via urination or exhalation, which will be further discussed in the section covering indications of ketosis.
Unfortunately, for the remaining glucose needs, protein is broken down into amino acids for use by the liver to conduct gluconeogenesis for which there is not a 1:1 ratio of grams of amino acid to grams of glucose, but rather 1.6:1, approximately [47]. This leads to a breakdown of close to 200 grams of protein per day to supply the brain with its minimum daily need for glucose [22].
Obviously, this is less than ideal when it comes to muscle preservation. Fortunately, however, ketones are much preferred by the brain for their higher energy density and will be used whenever they are available in the blood stream. 100 grams of glucose yields 8.7 kilograms ATP as opposed to 10.5 and 9.5 kilograms ATP from 100 grams of beta hydroxybutyrate and acetoacetate, respectively [39].
Additionally, while fasting or on a ketogenic diet, the transporters of ketones across the blood-brain barrier (MCT1 and MCT2) propagate, allowing virtually unlimited usage of ketones by the brain for energy whenever they are available, due to the transporter’s high Km value [9]. To reiterate, though, basal glucose needs remain and are met via gluconeogenesis in the liver.
As mentioned previously, this process is induced by increased levels of glucagon, among other hormones, and is inhibited by insulin. Lower levels of insulin, the result of fasting or reduced carbohydrate intake, lead to increased free fatty acids and their increased uptake into mitochondria, and ultimately, increased production of ketones. Ketones can be used as an alternative fuel by most body tissues, except by the liver, surprisingly, due to its lack of the enzyme beta ketoacyl-CoA transferase [7].
Indications
At times, it is necessary to determine whether a patient is in a state of ketosis. Those following a ketogenic diet for purposes of lipolysis, for example, may need to know whether ketones are present in their urine in order to adjust their macronutrient ratios, if necessary.
The acetoacetate that is excreted in urine during ketogenesis can be measured for this purpose using a semi-quantitative test. Urinalysis reagent strips, such as Ketostix, are a quick and convenient way to measure the rough amount of ketogenic byproducts using a strip nitroprusside test, with greater than 160 mmol/dL indicating high levels of acetoacetate and a state of deep ketosis [45]. Studies have shown that ketonuria, the presence of this ketone body in the urine, is best detected during early morning and post-dinner urinations [44]. Urinalysis is usually sufficient for the purposes of diet modification, however, for higher accuracy, blood serum levels of ketone bodies can be tested to indicate a state of ketogenesis. This tests for the presence of beta-hydroxybutyrate in the blood, which is indicative of ketogenesis at certain levels with the highest levels being detected in the early morning.
Another indicator of ketogenesis can be detected orally. The byproduct, acetone, is exhaled from the body, leading to breath that can be described as smelling “fruity” or “like nail polish”. While this is not a strictly qualitative test, it can be used in conjunction with urine or blood analysis to confirm results. It is interesting to note, however, that high levels of acetone in the breath strongly correlate with increased fat loss [1]. Other common symptoms of early ketosis include light-headedness, lethargy, headache, and weakness, which are collectively referred to as the “keto flu”, due to the unpleasant transition into ketosis, especially immediately following a diet high in carbohydrates. However, these symptoms should subside within a few days and are followed with feelings of mental clarity, abundant energy, and appetite suppression, a result of a sustained and steady state of ketosis and the body’s successful transition to using ketones for much of its energy needs.
Controversy
As one can imagine, the ketogenic diet, a radical departure from the conventional dietary wisdom of the food pyramid lauded by the United States Department of Agriculture (USDA) for decades, is quite controversial among physicians, researchers, food scientists, and dieticians. In fact, the macronutrient ratios of the ketogenic diet resemble those of the Food Pyramid if it were flipped completely upside down, with most daily calories coming from fat and minimal daily calories coming from carbohydrates.
In addition to the countercurrent dietary advice of the ketogenic diet, certain studies have suggested that there may be adverse effects of adopting a ketogenic lifestyle. One such study found that the levels of beta-hydroxybutyrate in the blood from lowcarbohydrate diets may lead to enhanced “fatigability and can reduce the desire to exercise in free-living individuals” [49-52]. Furthermore, a different study involving both men and women indicates that a short-term, low-carbohydrate diet may have a detrimental effect on “exercise performance in activities that are heavily dependent on anaerobic energy systems”. This would mean that high-intensity athletes, like sprinters and powerlifters may not benefit from a ketogenic diet in their sport [53]. Considering the “slow-release”, blood-glucose-leveling, and enduring metabolic effects of ketogenesis, it certainly makes sense that exercise requiring fast, short, depleting bursts of energy may not be enhanced by the use of ketones for energy.
Another concern is the suitability of a ketogenic lifestyle for pregnant women or women of childbearing age. A growth study conducted with mouse embryos concluded that a ketogenic diet may lead to alterations in fetal organ development, which may lead to post-natal organ dysfunction, and potentially, behavioral changes [42]. At this time, the common advice of most medical professionals is that women who are pregnant should avoid extremely restrictive and selective diets and instead opt for a diet rich in diverse, nutrient-dense whole foods.
Additionally, one study of children and adults with epilepsy suggests that the ketogenic diet may increase arterial stiffness, although its authors acknowledge that it is far too early to determine if there is any real effect of diet on blood vessels. What studies like this warn, however, is that as dietetic therapies are used more frequently, any potential vitamin deficiencies should be noted and addressed, and that further research ought to be conducted in this area [19].
It should be noted that many of the controversy surrounding the ketogenic diet is due to its novelty and some key misconceptions. It is also true that the dietary restrictions and the social effects of a ketogenic make it difficult to maintain in today’s society, which may add to the controversial nature. In certain cultures and countries, the availability of certain foods also play an impactful role.
Although there are studies that suggest there may be adverse effects of a ketogenic lifestyle, it is important to remember that, especially for those adhering to a low-carbohydrate diet for treatment of serious conditions, “most complications of the KD are transient and can be managed easily with various conservative treatments” [8].
Ketoacidosis is one of the most commonly referenced arguments amidst the controversy of ketosis. It is important to clarify that, although commonly mistaken for one another, dietary ketosis and diabetic ketoacidosis are very different, one being a very natural metabolic response to low levels of blood glucose and the other being a very dangerous condition which can result in death if not treated promptly.
The crux of the difference between dietary ketosis and diabetic ketoacidosis is insulin. As previously mentioned, insulin is a hormone that is released in response to the presence of glucose in the blood. It also has an inhibitory effect on the enzyme lipase, which aids in the breaking down of triglycerides into free fatty acids. In a normal, healthy person in a state of ketosis, insulin levels remain low but are still present in just the right amounts to keep lipolysis in check, so as to prevent the liver from overproducing ketones.
In a person with type-1 diabetes, a condition where the body is unable to produce insulin, ketogenesis would remain uninhibited without insulin injections and the acidic nature of the excessive ketone bodies in the blood would bring the blood to a dangerously low pH, causing it to become acidic, thus the word ‘acid’ in ketoacidosis. This can become very dangerous very quickly and is usually caused by someone with type-1 diabetes forgetting to take an insulin injection or giving themselves an insufficient dose.
Induction
The ketogenic diet and variations
The ketogenic diet comes in many different variations, but ultimately, it is most broadly defined as a diet that is high in fat, moderate in protein, and low in carbohydrates. The ratios of these macronutrients are very important to the proper implementation of the ketogenic diet and the efficient induction of ketosis. The standard ketogenic diet prescribes macronutrient ratios of 65-70% daily calories from fat, 25-30% from protein, and 5-10% from carbohydrates [49-51].
While there is some flexibility in the exact macronutrient ratios that should be implemented, it has been shown that, especially at the onset of a ketogenic diet, a higher ratio of fat to carbohydrates yields quicker and more effective results. However, this ratio can be tapered over time with little to no decrease in efficacy [52].
Additionally, fluids should not be restricted, and electrolyte consumption should be increased. Frequent urination, one of the markers or ketosis, can cause dehydration and should be remedied with increased water and electrolyte intake.
A common misconception about the ketogenic diet is that it requires conscious calorie restriction due to the high amounts of dietary fat and the caloric density of fats, but one of the key benefits of maintaining a steady state of ketosis is appetite suppression. A lack of spikes and drops in blood glucose creates a feeling of satiety and allows for a steady supply of energy due to the lipolytic properties of ketosis and the regular supply of adipose tissue and thus, free fatty acids for consumption.
The variations of the ketogenic diet extend beyond the ratios. Another popular dietary lifestyle community that seems to have some overlap with the ketogenic community is that of the “Paleo Diet”, which is essentially a diet that restricts food groups to those that could be hunted or gathered by early humans in the Paleolithic era. Because this precedes the Agricultural era, grains, legumes, dairy, and any processed foods are restricted. Although not strictly ketogenic due to the unrestricted consumption of fruits and starchy root vegetables, the Paleo Diet inherently has a natural reduction of carbohydrates due to the elimination of breads, grains, cereals, etc.
Because of the similarities in carbohydrate restriction, it is not uncommon for someone to adopt a ketogenic Paleo diet. As shown in Figure 1 below, this creates further restriction of both diets: a ketogenic diet sans dairy and a Paleo diet sans fruits or starchy tubers.
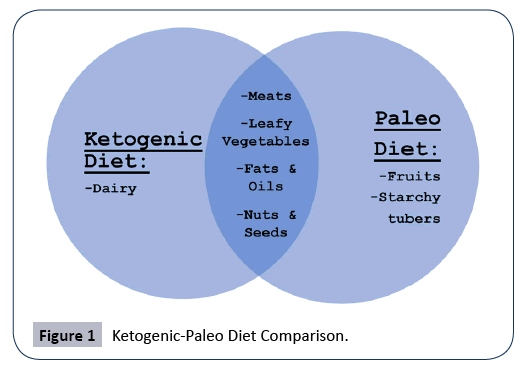
Figure 1: Ketogenic-Paleo Diet Comparison.
Regardless of the ketogenic diet variation adopted, the most important aspect of inducing ketosis is maintaining a sufficiently low carbohydrate intake and a sufficiently high intake of fats. Some of the most common mistakes made by those attempting to induce a state of ketosis is not consuming enough fats (usually due to a socially engrained fear of fat), consuming too much protein (which can be converted to glucose via gluconeogenesis), and inadvertently consuming “hidden” carbohydrates in foods that appear to be low-carbohydrate, but contain added sugars or sugar alcohols that can interrupt ketosis if consumed in large quantities.
Adherence to the ketogenic diet can be improved by finding foods that are low in carbohydrates but mimic carbohydrate-rich foods, such as substituting minced cauliflower for rice [31]. The ketogenic diet is typically prescribed for a minimum period of two to three weeks to a maximum period of six to twelve months, with a strong emphasis on the transition back to a standard diet in a gradual and well-controlled manner [30].
Fasting protocols
The state of ketosis is not only induced by a low-carbohydrate, high-fat diet, but also may be induced through various fasting protocols. As previously mentioned in this paper, the ketogenic diet was initially designed for the purpose of mimicking the effects of fasting and reaping the metabolic and restorative benefits of fasting without having to abstain from eating [48]. For some, fasting remains the easiest, quickest, and simplest method of entering and maintaining a ketogenic state.
The definition of fasting is simple and uncomplicated: the consumption of zero (or negligible) calories from food or drink over time. While fasting, the continued, and even increased, consumption of water is not only allowed, but is also advised as medically prudent due to the increased loss of electrolytes through urination and to prevent the dangers of dehydration. Although the consumption of stimulants, such as caffeine, is not considered pure fasting, for the purposes of inducing ketosis, other liquids are typically allowed at the fasters’ discretion, such as: black coffee, herbal tea, and other unsweetened, noncaloric beverages. However, the consumption of calories, even inadvertently, will technically break the fast and, depending on the macronutrient composition of the food or drink consumed, bring the faster into a state more appropriately defined in dietary terms.
It is important to mention that fasting, whether intentional, is not a new concept to humans, as many groups of people throughout history have independently used fasting as a religious or spiritual practice [17]. For example, fasting for Ramadan involves eating no food or drink during daylight hours for the period of thirty days. Conversely, the general population technically fasts for a period of 8 to 10 hours from the time they go to sleep at night to when they break their fast the next morning by eating breakfast [25]. In fact, the mechanism of fasting-induced ketosis has long been relied upon by the human body to meet its energy needs during times of seasonal shortages and absence of food by utilizing its own energy reserves [43].
For those seeking to reap the metabolic and therapeutic benefits of ketosis through fasting, there are several different protocols which can be implemented:
A 24-hour to 48-hour fast may be used to speed up the induction of ketosis prior to beginning a ketogenic diet. This ‘priming’ fasting protocol allows the body to exhaust its glycogen stores more rapidly to begin mobilizing fatty acids for energy and is then typically followed by a low-carbohydrate, high-fat diet, as described in the previous section. A study of children with epilepsy showed, however, that fasting prior to initiating a ketogenic diet does not significantly improve the benefits of ketosis [51].
Fasts that last for longer than 72 hours are considered prolonged fasts. This method of fasting induces a deeply ketogenic state. While prolonged fasting allows for maximum mobilization of free fatty acids and enhanced lipolysis, fasters are also more prone to the side effects of fasting, which may include lethargy, headaches, and a general sense of exhaustion as the body focuses its energy on the most basic of bodily functions [20].
Additionally, the proper reintroduction of food is most important following a prolonged period of fasting. The digestive system will have been at rest and the abundance and diversity of the gut flora may be reduced, so reintroducing fermented foods and foods high in probiotics may help to reestablish a thriving microbiome and prevent any unintended digestive issues when resuming an eating regimen [18].
Another fasting protocol that has gained much momentum in nutrition and fitness culture recently is intermittent fasting, which gained significant notoriety following a documentary released in 2012 that highlighted the 5:2 intermittent fasting approach [14]. Intermittent fasting is the intermittent use of fasting in various scheduling patterns. For example, the aforementioned 5:2 fast involves five days of regular eating patterns, followed by two days of fasting.
Alternate-day fasting is another popular approach which consists of fasting every other day. However, this method may not be suited for inducing ketosis due to a fasting period of only 24 hours. Intermittent fasting methods which promote shorter periods of fasting, such as the alternate-day and one-meal-a-day methods, may be beneficial, not through ketogenesis, but through the inherent ease of compliance that intermittent fasting provides for calorie restriction [2].
Of course, the exception would be combining intermittent fasting with a ketogenic diet by adhering to a low-carbohydrate, high-fat diet during non-fasting periods, which would encourage a state of ketosis at all times. Regardless of the method used, intermittent fasting has surged in popularity due to the simplicity of such a binary ‘feed-or-fast’ approach to dietary decision-making.
Applications
Effects of ketosis on fat loss and muscle preservation
One of the most sought-after applications of ketosis is the effect it has on increased lipolysis and enhancement of body composition. In a study comparing a low-fat, high carbohydrate diet to a lowcarbohydrate, high-fat diet, the latter was shown to yield not only greater participant retention, but also greater weight loss. In addition, the blood serum triglycerides were improved on the low-carbohydrate diet, further supporting the claim that dietary fat intake has little to do with triglyceride levels [55].
Additional evidence suggests that obese patients on a ketogenic diet may experience a reduction in appetite and an increase in satiety, as well as an increase in lipolysis and a decrease in lipogenesis. Additionally, with an increase in the metabolism of consumed fats, the energy required for gluconeogenesis, and the thermic effect of protein consumed, all would work in concert to reduce the body fat percentage and body mass index of patients adhering to ketogenic protocols [32].
Naturally, the fat burning mechanisms of ketosis not only decrease overall body fat but have been shown to specifically decrease visceral adipose tissue, the fat that surrounds vital organs, thus reducing the individual burden of obesity-related disease [27].
Body fat is not the only factor considered when analyzing the effects of ketosis on body composition. A primary concern in many seeking to reduce their body fat percentage is simultaneously maximizing the preservation of lean muscle mass. While it is true that proteolysis occurs in a state of ketosis to support gluconeogenesis in the liver, there are studies that have shown that a diet very low in carbohydrates may actually induce preservation of muscle mass [23].
Different muscle-preserving mechanisms have been proposed, two of which I find the most compelling. The first of these mechanisms involves adrenergic stimulation by levels of glucose in the blood. When blood glucose levels are low, there is an increase in the secretion of adrenaline. A study on the hind-quarters of mice suggested that proteolysis is inhibited by the presence of adrenaline [16]. This adrenergic effect may counteract the proteolytic nature of ketosis and spare muscle tissue.
It has also been suggested that ketone bodies themselves may have a suppressive effect on the breakdown of muscle. As long as there are sufficient ketone bodies and fatty acids, studies have shown that beta-hydroxybutyrate actually decreases leucine oxidation and enhances the synthesis of new proteins [28]. Although not yet conclusive, current scientific literature has shown that a ketogenic diet may indeed provide protection against muscle protein catabolism.
Ketogenic enhancement of athletic and physical performance
In addition to fat loss and muscle preservation, research suggests that athletic and physical performance may be markedly enhanced during a state of ketosis. Specific to weight-class athletics, one study of Taekwondo athletes highlights how a ketogenic diet not only improved their aerobic capacity, but also increased their ability to resist fatigue. It was suggested by this study that these performance enhancements may have been linked to a decrease in tumor necrosis factor-alpha in the athletes following a ketogenic diet, which may have reduced their inflammatory response to vigorous exercise [37].
The benefit of ketosis on aerobic performance is further supported by a study involving eight experienced and trained off-road cyclists who were prescribed a ketogenic diet for one month and a standard diet for month during training season to measure any athletic enhancements from dietary intervention [53-55]. The findings suggest that with highly aerobic sports, a ketogenic diet may be beneficial during the preparatory season when athletes are training for long hours at low to moderate intensity due to a significant increase in maximal oxygen uptake and oxygen uptake at lactate threshold [56].
This combination of a low-carbohydrate, high-fat diet and lowintensity endurance exercise has been shown to decrease body mass and body fat percentage, as well as decrease the extent of post-exercise damage to muscles, allowing for speedy recovery. It was suggested, however, that a ketogenic diet may not be as beneficial for high-intensity exercise or during the competitive season of certain sports, like cycling, when fast-acting glycolysis is necessary for maximum work output.
The often-misunderstood metabolic state of ketosis enhances physical endurance by making changes to the body’s fuel consumption for oxidative phosphorylation. This decreased reliance on glycolysis in favor of ketolysis leads to a decrease in plasma lactate concentrations and provides an alternative source of substrate for oxidative phosphorylation, leading to decreased muscle fatigue and making the ketogenic diet an attractive diet protocol for endurance athletes for at least part of their training regimen [4].
It must also be noted that the increases in athletic performance and physical capacity from ketosis, while promising, may only be the indirect result of ketosis through improved body composition and not necessarily a benefit of ketogenesis itself. The interaction of ketosis and athletic performance is still poorly understood in its mechanisms. Future studies involving both professional and amateur athletes should hopefully be able to shed more light on this important potential benefit of ketosis.
Applications of ketosis in treating epilepsy
The application of a ketogenic diet as an alternative or adjuvant therapy against neurological disorders is one of the hallmarks of the dietary intervention. One of the earliest documented uses of a low-carb, high-fat lifestyle was to help reduce the frequency and intensity of seizures in people with epilepsy, mostly children, with the majority of refractory and generalized epilepsy syndromes being improved [46].
Due to their naïveté with carbohydrate intake and high risk of seizure relapse, young children are often brought into a state of ketosis during a three to four-day hospital, under medical and nutritional supervision [38]. Parents often must receive tailored nutritional education to fully understand the requirements and importance of maintaining a state of dietary ketosis in their young children. Due to the occasional need for medication in these young patients, pharmacists are also able to recommend ketogenic-friendly options, with chewables and tablets typically having less carbohydrates than liquid forms of the same medications [38].
Although this non-pharmacological treatment has been prescribed in the United States since the 1920s, the exact anti-convulsant mechanism of the ketogenic diet in refractory epilepsy is still not fully understood and the biochemical pathways involved are not entirely clear. Recent studies have proposed possible mechanisms and several viable hypotheses have been posited. The most supported effects of ketosis on refractory epilepsy are its role in modulating neurotransmitters, impacting levels of biogenic monoamines, and inducing neuronal antioxidant protection [21].
GABA and glutamate, the major inhibitory and excitatory neurotransmitters in the brain, are the key players in the proposed mechanism involving neurotransmitter modulation. Studies have shown that ketone bodies inhibit glutamate decarboxylase and increase the production of GABA, which contributes to a significant reduction in seizure activity [29]. Additionally, neurons are hyperpolarized by the increased amounts of GABA.
High levels of GABA stimulate receptors of chlorine channels and allow an influx of negatively charged ions. This hyperpolarization of neurons may further contribute to seizure control by inhibiting calcium and sodium channels, which are essential for the excitation of neurons. The effect of ketone bodies on VGLUT, which plays a role in glutamate release, may also be a contributing factor. An in vitro study suggests that ketone bodies may act as a competitive inhibitor on VGLUT, thus reducing vesicular glutamate uptake in neurons [15].
The anti-convulsant mechanism involving biogenic monoamines is supported by some studies, but the specificities of how it works remain unclear. A clinical study showed that serotonin and dopamine levels dropped from 410 to 342 and from 158 to 137 nmol/L, respectively, in the cerebrospinal fluid of children following a ketogenic diet [6]. It was suggested by these results that changes in monoamine levels dictated how well the children would respond to the ketogenic diet symptomatically.
Another study suggests that ketone bodies increase the level of adenosine and that adenosine may be a key player in the control of seizures. This upregulation of adenosine by ketone bodies may exert a suppressive influence on seizures, although more research needs to be conducted to confirm this hypothesis [21]. Additional hypotheses have been formed suggesting that calorie restriction and a ketogenic diet may increase mitochondrial biogenesis by as much as 46% in neurons, promoting protection from oxidative stress and mitochondrial dysfunction, which both contribute to cell death (Figure 2).
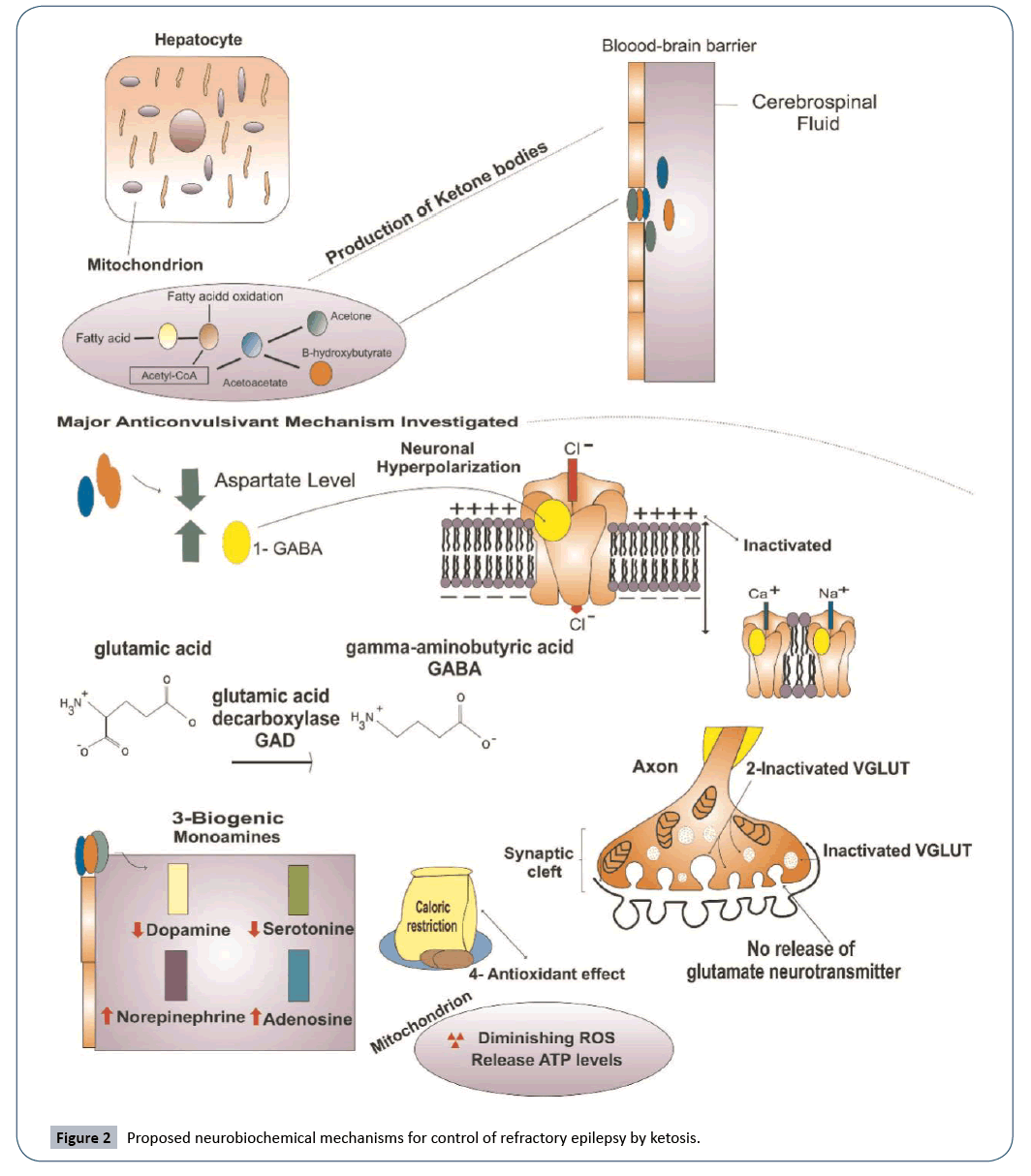
Figure 2: Proposed neurobiochemical mechanisms for control of refractory epilepsy by ketosis.
Alleviation of thermal and neuropathic pain by ketosis
Chronic pain is suffered by many people and, in some countries, has been shown to be up to three times as costly as all types of cancer [34]. It is difficult to assess and treat due to the subjectivity of patients’ perception of pain. Furthermore, opioid abuse complicates the issue and compounds the concern of using pharmacological methods to treat pain, creating a huge opportunity for alternative treatments, such as the ketogenic diet.
The anti-convulsant effects of a ketogenic diet have been successfully documented, but the overlap of certain mechanisms may allow the ketogenic diet to be used to reduce inflammation, as well as thermal and neuropathic pain. Recent studies have explored this possible benefit of ketosis.
Similar to epilepsy, neuropathic pain involves the increased excitability of both peripheral and central neurons [36]. As with the effect of ketosis on neurotransmitters in epilepsy, this may be a reason that a ketogenic diet can alleviate chronic pain. In addition, the analgesic properties of 2-deoxyglucose [10], which inhibits glycolysis, suggests that a ketogenic diet may also be analgesic, or pain-relieving. Another possible overlap in anticonvulsant and analgesic mechanisms may be linked to adenosine levels, which, as previously stated, are thought to be increased by ketogenesis. Studies have also shown that adenosine may have anti-nociceptive properties [40], further supporting the use of a ketosis to alleviate pain.
A study using rats on a hot plate showed delayed signs of thermal pain, such as increased movement or the licking of paws, in rats prescribed a ketogenic diet. However, artificially manipulated levels of blood ketones and blood glucose in the subjects did not produce the same results, suggesting that ketones themselves do not directly affect “molecular targets underlying thermal pain sensitivity” [24].
Continued research into the possible benefits of pain management from ketosis is necessary and ongoing. Overlapping neurobiochemical mechanisms between epilepsy and pain may even lead to improved treatment options of not only these conditions, but some of their comorbidities, like anxiety, depression, and insomnia.
Ketosis as an alternative or adjuvant cancer therapy
In recent research, the ketogenic has been proposed as an alternative or adjuvant cancer therapy. Results from preclinical studies have suggested that a ketogenic diet may slow down the growth of tumors, reduce tumor size, and even increase the rate of survival in malignant glioma and cancers of the prostate and gastrointestinal tract [3]. Other potential target cancers would most likely be those whose malignancy is correlated with metabolic status. Additionally, ketosis, whether induced by fasting or a ketogenic diet, has been shown to increase the efficacy of chemotherapy against cancer cells and even reduce the side effects of such treatment [3].
Although there is some concern with the ketogenic effect of weight loss on cancer patients, there has not been any significant findings to suggest that a ketogenic diet is not a perfectly safe and healthy treatment option for patients with certain cancers. In fact, a German study of 16 patients with advanced stages of cancer found that a ketogenic diet caused a reduction in tumor size and an improvement in the quality of life. The study also concluded that there are no significant adverse side effects of the ketogenic diet, even for patients in the advanced stages of cancer [41].
One of the mechanisms by which the ketogenic diet is suggested to have anti-carcinogenic effects as a metabolic therapy can be defined by glucose and lactate shortages during ketosis, both of which tumor cells need to proliferate. Unlike healthy, differentiated cells, cancer cells rely on aerobic glycolysis for their energy needs, not oxidative phosphorylation in the mitochondria. This is known as the “Warburg effect” and is what makes cancer cells particularly susceptible to metabolic therapy, like the ketogenic diet.
A study at the Dana-Farber Cancer Institute in Boston, MA has observed that cancer cells in tumors are voracious consumers of glucose compared to other cells [12]. Glucose limitation may inhibit tumor proliferation by interrupting the supply of preferred energy substrate, effectively “starving” the cancer cells of the glucose they crave [13].
It is further suggested that this “starvation” of cancer cells with a ketogenic diet would force them to use mitochondrial oxidative phosphorylation for their energy needs and cause them metabolic oxidative stress. It is thought that this stress would cause the cancer cells to become hypersensitized and more susceptible to cytotoxins and radiation, making a ketogenic diet a safe and practical adjuvant therapy to these conventional therapies. A study of malignant gliomas highlights the necessity for alternatives to conventional cancer therapies. Cancers of the brain are particularly difficult to treat long-term due to the ineffective targeting of tumor cells and the negative implications that has for the surrounding, healthy brain cells. The study of the efficacy of KetoCal®, a ready-to-drink ketogenic nutritional shake often used as a meal replacement when strict adherence to a ketogenic diet is necessary, on malignant mouse astrocytoma (CT-2A) and human malignant glioma (U87-MG) showed that the dietary protocol decreased the growth of CT-2A and U87-MG tumors by 65% and 35%, respectively [57]. The anti-tumor and anti-angiogenic effects of KetoCal® are promising, with the overall decrease in the patients’ blood glucose levels being responsible for these therapeutic effects. As previously mentioned, this can be achieved by a strict ketogenic diet, as in the study, or by fasting, although the latter seems to be impractical for long-term malignant brain cancer management.
The use of ketosis in the treatment of type-2 diabetes
Type-2 diabetes is typically a result of an excessively highcarbohydrate diet and obesity. It is a condition characterized by excessive glycemia and resistance to insulin, making treatment of type-2 diabetes by a low-carb, high-fat ketogenic diet one of the most straight-forward therapeutic applications of ketosis. Evidence suggests that a ketogenic diet is both safe and extremely effective in bringing blood glucose back down to normal levels and restoring insulin sensitivity in patients with type-2 diabetes. Ketosis may even be able to reverse certain complications of type-2 diabetes in humans [26].
The potential reversal of diabetic nephropathy by ketosis in humans has been demonstrated by a study involving mice. While insulin therapy has been shown to “slow the development of diabetic complications” [5], there has been little evidence to suggest that insulin therapy actually reverses diabetic complications. This makes the prospect of reversal by ketosis a very exciting possible breakthrough in the treatment of type-2 diabetes. After two months on the ketogenic diet, albumincreatinine ratios in the mice indicated complete reversal of diabetes-associated nephropathy, with partial reversal evident in tissue samples [35].
The efficacy of the ketogenic diet in treating type-2 diabetes is further highlighted by a study involving 28 overweight subjects who were prescribed a 16-week ketogenic dietary protocol. The results were favorable with most of the participants significantly reducing or completely stopping their use of type-2 diabetes medication [54]. The success of ketosis in treating type-2 diabetes is strongly supported by scientific literature, but further research is needed to determine the extent to which reversal of complications can be achieved.
Conclusion
The analysis of literature suggests that a low-carb, high-fat, ketogenic diet is a safe and effective dietary protocol for entering ketosis. While fasting is also a great tool for inducing a state of ketosis, it is not always the most practical method due to the severity of caloric restriction and the fact that the most beneficial effects of ketosis are experienced after prolonged maintenance of a ketogenic state.
It is also important to note that the safety and efficacy of ketosis are not guaranteed in individuals with certain autoimmune conditions, especially type-1, or insulin-dependent, diabetes. Dietary therapies, like the ketogenic diet, should always be approved by a physician to avoid any unintended complications. The marked improvement of body composition is strongly supported by research as one of the hallmark benefits of ketosis. Reduction of body fat alone has many direct and indirect health benefits for most people and has been shown to be catalyzed by a ketogenic diet. Additionally, ketosis has many therapeutic applications and is a natural, non-invasive alternative to many medications and treatments in the fight against a range of ailments, from chronic to life-threatening.
Individuals suffering from epilepsy, neuropathic pain, cancer, obesity, and type-1 diabetes all stand to potentially benefit from ketosis and should consider a ketogenic lifestyle as an alternative or adjuvant therapy. Furthermore, research suggests that athletic and physical performance can also be improved by ketosis, broadening the array of benefits ketosis has to offer from realm of treatment to enhancement.
In conclusion, when properly induced and maintained, a state of ketosis is not only safe, but beneficial for most people. Future studies will continue to shed more light on this often misunderstood and certainly underutilized metabolic therapy and perhaps discover additional clinical and personal applications of ketosis.
26461
References
- Anderson JC (2015) Measuring breath acetone for monitoring fat loss: Review. Obesity 23: 2327-2334.
- Azevedo FR, Ikeoka D, Caramelli B (2013) Effects of intermittent fasting on metabolism in men. Revista Da Associação Médica Brasileira 59: 167-173.
- Chung HY, Park YK (2017) Rationale, Feasibility and Acceptability of Ketogenic Diet for Cancer Treatment. Journal of Cancer Prevention 22: 127-134.
- Cox P, Kirk T, Ashmore T, Willerton K, Evans R, et al. (2016) Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metabolism 24: 256-268.
- Crofford OB (1995) Diabetes control and complications. Annual Review of Medicine 46: 267-279.
- Dahlin M, Mansson JE, Amark P (2012) CSF levels of dopamine and serotonin, but not norepinephrine, metabolites are influenced by the ketogenic diet in children with epilepsy. Epilepsy Research 99: 132-138.
- Duchowny MS (2005) Food for Thought: The Ketogenic Diet and Adverse Effects in Children. Epilepsy Curr 5: 152-154.
- Fernstrom JD, Fernstrom MH (2003) Nutrition and the brain. Nutrition & Metabolism pp: 145-167.
- Fisher MC, Bodnar RJ (1992) 2-Deoxy-D-glucose antinociception and serotonin receptor subtype antagonists: test-specific effects in rats. Pharmacol Biochem Behav 43: 1241-1246.
- Goodridge AG, Sul HS (2000) Lipid metabolism – Synthesis and oxidation. Biochemical and Physiological Aspects of Human Nutrition pp: 305-350.
- Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 324: 1029-1033.
- Huebner J, Marienfeld S, Abbenhardt C, Ulrich C, Muenstedt K, et al. (2014) Counseling patients on cancer diets: a review of the literature and recommendations for clinical practice. Anticancer Res 34: 39-48.
- Jane L, Atkinson G, Jaime V, Hamilton S, Waller G, et al. (2015) Intermittent fasting interventions for the treatment of overweight and obesity in adults aged 18 years and over: A systematic review protocol. JBI Database System Rev Implement Rep 13: 60-68.
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, et al. (2010) Metabolic control of vesicular glutmate transport and release. Neuron 68: 99-112.
- Kadowaki M, Kamata T, Noguchi T (1996) Acute effect of epinephrine on muscle proteolysis in perfused rat hindquarters. American Journal of Physiology-Endocrinology and Metabolism 270.
- Kerndt PR, Naughton JL, Driscoll CE, Loxterkamp DA (1982) Fasting: The history, pathophysiology and complications. West J Med 137: 379-399.
- Kohl KD, Amaya J, Passement CA, Dearing MD, Mccue MD (2014) Unique and shared responses of the gut microbiota to prolonged fasting: A comparative study across five classes of vertebrate hosts. FEMS Microbiol Ecol 90: 883-894.
- Kossoff E (2014) Danger in the Pipeline for the Ketogenic Diet? Epilepsy Curr 14: 343-344.
- Li C, Sadraie B, Steckhan N, Kessler C, Stange R, et al. (2017) Effects of A One-week Fasting Therapy in Patients with Type-2 Diabetes Mellitus and Metabolic Syndrome – A Randomized Controlled Explorative Study. Exp Clin Endocrinol Diabetes 125: 618-624.
- Lima P, Sampaio L, Damasceno N (2014) Neurobiochemical mechanisms of a ketogenic diet in refractory epilepsy. Clinics 69: 699-705.
- Manninen AH (2004) Metabolic Effects of the Very-Low-Carbohydrate Diets: Misunderstood "Villains" of Human Metabolism. Journal of the International Society of Sports Nutrition 1: 7.
- Manninen AH (2006) Very-low-carbohydrate diets and lean body mass. Obesity Reviews 7: 297.
- Masino SA, Ruskin DN (2013) Ketogenic Diets and Pain. J Child Neurol 28: 993-1001.
- Maughan RJ, Fallah J, Coyle EF (2010) The effects of fasting on metabolism and performance. Br J Sports Med 44: 490-494.
- Mobbs CV, Mastaitis J, Isoda F, Poplawski M (2013) Treatment of Diabetes and Diabetic Complications With a Ketogenic Diet. J Child Neurol 28: 1009-1014.
- Moreno B, Crujeiras AB, Bellido D, Sajoux I, Casanueva FF (2016) Obesity treatment by very low-calorie-ketogenic diet at two years: Reduction in visceral fat and on the burden of disease. Endocrine 54: 681-690.
- Nair KS, Welle SL, Halliday D, Campbell RG (1988) Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J Clin Invest 82: 198-205.
- Neal E, Cross J (2010) Efficacy of dietary treatments for epilepsy. J Hum Nutr Diet 23: 113-119.
- Paoli A, Bianco A, Grimaldi KA, Lodi A, Bosco G (2013) Long term successful weight loss with a combination biphasic ketogenic Mediterranean diet and Mediterranean diet maintenance protocol. Nutrients 5: 5205-5217.
- Paoli A, Cenci L, Grimaldi KA (2011) Effect of ketogenic Mediterranean diet with phytoextracts and low carbohydrates/high-protein meals on weight, cardiovascular risk factors, body composition and diet compliance in Italian council employees. Nutr J 10: 112.
- Paoli A (2014) Ketogenic Diet for Obesity: Friend or Foe? Int J Environ Res Public Health 11: 2092-2107.
- Peterman MG (1925) The Ketogenic Diet In Epilepsy. JAMA: The Journal of the American Medical Association 84: 1979.
- Phillips CJ (2006) Economic burden of chronic pain. Expert Review of Pharmacoeconomics & Outcomes Research 6: 591-601.
- Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, et al. (2011) Reversal of Diabetic Nephropathy by a Ketogenic Diet. PLoS ONE 6: e18604.
- Raja SN, Meyer RA, Campbell JN (1988) Peripheral mechanisms of somatic pain. Anesthesiology 68: 571-590.
- Rhyu H, Cho S (2014) The effect of weight loss by ketogenic diet on the body composition, performance-related physical fitness factors and cytokines of Taekwondo athletes. J Exerc Rehabil 10: 326-331.
- Runyon AM, So T (2012) The Use of Ketogenic Diet in Pediatric Patients with Epilepsy. ISRN Pediatrics pp: 1-10.
- Salway JG (1999) Metabolism at a Glance (3rdedn). Wiley-Blackwell, pp: 128.
- Schmidt AP, Böhmer AE, Antunes C, Schallenberger C, Porciúncula LO, et al. (2009) Anti-nociceptive properties of the xanthine oxidase inhibitor allopurinol in mice: role of A1 adenosine receptors. Br J Pharmacol 156: 163–172.
- Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U (2011) Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab (Lond) 8: 54.
- Sussman D, Eede MV, Wong MD, Adamson SL, Henkelman M (2013) Effects of a ketogenic diet during pregnancy on embryonic growth in the mouse. BMC Pregnancy Childbirth 13.
- Toledo FW, Buchinger A, Burggrabe H, Hölz G, Kuhn C, et al. (2013) Fasting Therapy - an Expert Panel Update of the 2002 Consensus Guidelines. Forsch Komplementmed 20: 434-443.
- Urbain P, Bertz H (2016) Monitoring for compliance with a ketogenic diet: What is the best time of day to test for urinary ketosis? Nutr Metab (Lond) 13: 77.
- Vining EPG, Freeman JM, Ballaban-Gil K, Camfield CS, Camfield PR, et al. (1998) A Multicenter Study of the Efficacy of the Ketogenic Diet. Arch Neurol 55: 1433-1437.
- Wang HS, Lin KL (2012) Ketogenic diet: An early option for epilepsy treatment, instead of a last choice only. Biomed J 36: 16.
- Watford M, Goodridge AG (2000) Regulation of fuel utilization. Biochemical and Physiological Aspects of Human Nutrition, pp: 384-407.
- Wheless JW (2004) History and Origin of the Ketogenic Diet. Epilepsy and the Ketogenic Diet pp: 31-50.
- White AM, Johnston CS, Swan PD, Tjonn SL, Sears B (2007) Blood Ketones Are Directly Related to Fatigue and Perceived Effort during Exercise in Overweight Adults Adhering to Low-Carbohydrate Diets for Weight Loss: A Pilot Study. J Am Diet Assoc 107: 1792-1796.
- Williams E, Abrahams J, Maguire A, Harris G (2012) A parents perspective on dietary treatments for epilepsy. Epilepsy Res 100: 338-343.
- Wirrell EC, Darwish HZ, Williams-Dyjur C, Blackman M, Lange V (2002) Is a Fast Necessary When Initiating the Ketogenic Diet? Journal of Child Neurology 17: 179-182.
- Wirrell EC (2008) Ketogenic Ratio, Calories, and Fluids: Do They Matter? Epilepsia 49: 17-19.
- Wroble KA, Trott MN, Schweitzer GG, Rahman RS, Kelly PV, et al. (2018) Low-carbohydrate, ketogenic diet impairs anaerobic exercise performance in exercise-trained women and men: a randomized-sequence crossover trial. J Sports Med Phys Fitness 59: 600-607.
- Yancy WS, Foy M, Chalecki AM, Vernon MC, Westman EC (2005) A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond) 2: 34.
- Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC (2004) A Low-Carbohydrate, Ketogenic Diet Versus a Low-Fat Diet to Treat Obesity and Hyperlipidemia: A Randomized, Controlled Trial. Ann Intern Med 140: 769-777.
- Zajac A, Poprzecki S, Maszczyk A, Czuba M, Michalczyk M, et al. (2014) The Effects of a Ketogenic Diet on Exercise Metabolism and Physical Performance in Off-Road Cyclists. Nutrients 6: 2493-2508.
- Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, et al. (2007) The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 4: 5.







