Awajimijan Nathaniel Mbaba1, Michael Promise Ogolodom2*, Rufus Abam1 and Nengi Alazigha2
1Department of Radiology, Rivers State University Teaching Hospital, Port Harcourt Rivers State, Nigeria
2Rivers State Hospitals Management Board, Port Harcourt, Nigeria
*Corresponding Author:
Michael Promise Ogolodom
Rivers State Hospitals Management Board
Port Harcourt
Nigeria
Tel: +2348039697393
E-mail: mpos2007@yahoo.com
Received date: 25 March 2019; Accepted date: 16 April 2019; Published date: 23 April 2019
Citation: Mbaba AN, Ogolodom MP, Abam R, Alazigha N (2019) Mesial Temporal Sclerosis and Epilepsy-A Case Report. Health Sci J Vol.13.No.2:645. DOI: 10.36648/1791-809X.1000645
Copyright: © 2019 Mbaba AN, et al. This is an open-access article distributed under the terms of the creative commons attribution license, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Keywords
Epilepsy; Mesial temporal lobe; Magnetic resonance imaging; Sclerosis
Introduction
Mesial temporal lobe sclerosis (MTS), also known as hippocampal sclerosis, is most commonly associated with intractable temporal lobe epilepsy [1-3] and is seen in about 60% to 80% of cases [4]. It is the most common structural abnormality seen in human epilepsy [5] and pathologically, is characterized by neuronal cell loss, gliosis and sclerosis within the mensial temporal lobe [6]. These changes are responsible for the MRI findings.
MTS can be bilateral in up to 20% of cases although symptoms may be unilateral, [3] depending on the side most affected because in about 80% of cases one side is most severely affected than the other [7]. In about 15% of these patients there may be “dual pathology” which in addition to epileptogenic area, there may be either temporal extrahippocampal or even extratemporal lesion [8] and the most common association is cortical dysplasia.
The etiology of MTS is unknown, but there is a relationship with a precipitating injury such as complex febrile seizures, birth trauma, meningitis, or head injury that happens in early life. A latent period of several years may precede dyscognitive seizures (previously known as complex partial seizures) [9].
MRI is the imaging modality of choice for the evaluation of patients with MTS, since it can identify structural abnormalities. Recognition of subtle changes in medial temporal lobe architecture requires good knowledge of its MRI anatomy.
The medial temporal lobe of the brain also known as the mesial temporal lobe is a complex structure which consists of the amygdala, hippocampus, uncus, dentate gyrus and parahippocampal gyrus. The hippocampus is a curved elevation of gray matter in the floor of the temporal horn of the lateral ventricle and its enlarged ridged anterior end is the head. It consists of two interlocking gray matter folds, the cornu ammonis (or hippocampus proper) and the dentate gyrus. The hippocampus also has a thin covering of white matter called the alveus on its ventricular surface. The amygdala forms the roof of the temporal horn of the lateral ventricle. The parahippocampal gyrus of the temporal lobe lies posterior and medial to the hippocampus. The transitional zone between the cortex of the parahippocampal gyrus and that of the hippocampus is the subiculum. The uncus is the inner most part of the anterior parahippocampal gyrus. Human behavioral patterns, emotion and recent memory are functions associated with hippocampus [10-12]. Coronal T1W MR image is used to illustrate this anatomy (Figures 1 and 2).
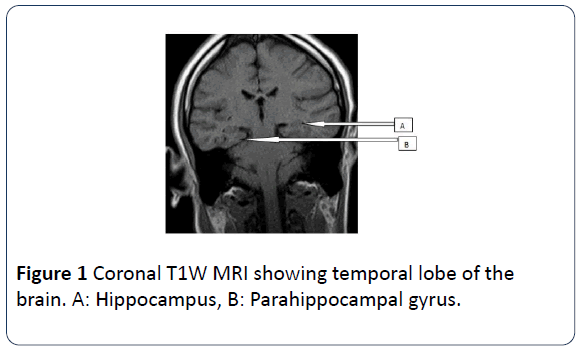
Figure 1: Coronal T1W MRI showing temporal lobe of the brain. A: Hippocampus, B: Parahippocampal gyrus.
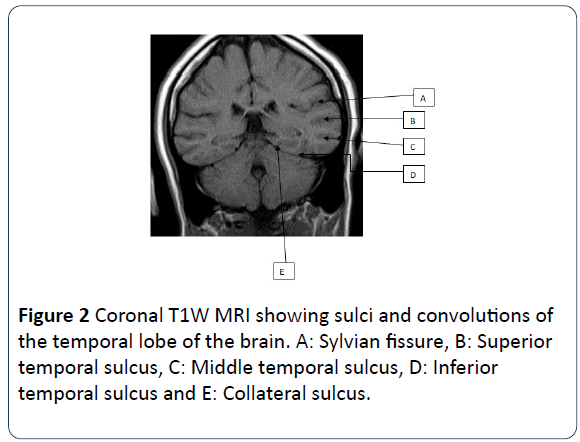
Figure 2: Coronal T1W MRI showing sulci and convolutions of the temporal lobe of the brain. A: Sylvian fissure, B: Superior temporal sulcus, C: Middle temporal sulcus, D: Inferior temporal sulcus and E: Collateral sulcus.
Radiological diagnosis of structural changes in mesial temporal lobe epilepsy can be challenging without an organized reference that could facilitate identification of diseased structures especially in our setting where dedicated epilepsy protocols are not often employed. We intend to highlight the pertinent normal radiological anatomy of the mesial temporal lobe so as to recognize structural changes as in mesial temporal lobe sclerosis and emphasize the need for diligence when dealing with MR images of these structures because subtle changes in the architecture of the mesial temporal lobe can cause significant symptoms.
Case Report
Brain MRI scans was performed on a 22 years old male patient who presented with history of recurrent seizure. The scan showed slight reduction in volume of the right hippocampus with increase in T2 signal intensity. There is also slight dilatation of the ipsilateral temporal horn of the lateral ventricle due to loss in volume. The findings are indicative of right sided Mesial Temporal Sclerosis (Figures 3 and 4).
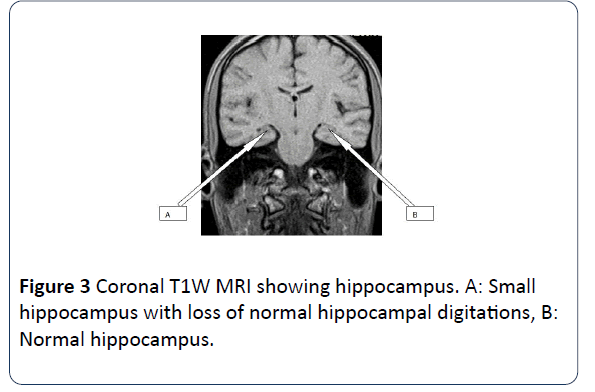
Figure 3: Coronal T1W MRI showing hippocampus. A: Small hippocampus with loss of normal hippocampal digitations, B: Normal hippocampus.
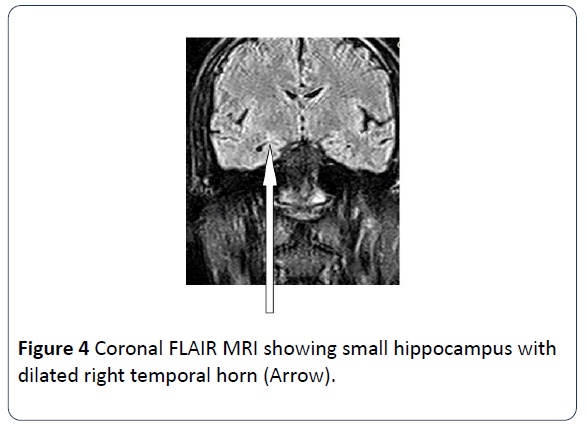
Figure 4: Coronal FLAIR MRI showing small hippocampus with dilated right temporal horn (Arrow).
Discussion
About fifty million people worldwide have epilepsy, and the World Health Organization estimates a related morbidity of nearly 7.5 million disability-adjusted life years (health years lost) by 2015 [13]. The socio-economic and health care burdens of epilepsy on the individual, family and the society are numerous. The disease is associated with considerable morbidity and has three-fold mortality rates [14-16]. In Africa, the burden of epilepsy is numerous and its prevalence in sub-Saharan Africa is 1-1.5% [17].
About 30% of patients with epilepsy do not respond to adequate medical treatment [18]. They are then said to have medically refractory or intractable epilepsy and the most effective treatment options for them is surgical resection of epileptogenic lesion.
Mesial temporal lobe sclerosis is not only the most common cause of complex partial seizures but also the most common structural abnormality in human epilepsy [4]. Accurate diagnosis of Mesial Temporal Lobe sclerosis contributes greatly to the management of epilepsy, whether medical or surgical. MRI is the imaging modality of choice for the evaluation of patients with seizures, especially in mesial temporal lobe epilepsy. The role of MRI in the evaluation of patients with epilepsy is to pinpoint underlying brain pathologies that relate to the patients symptoms and that helps to characterize the clinical syndrome [19].
Interpretations of MR images in mesial temporal lobe sclerosis require in-depth knowledge of the MRI anatomy and pattern of structural changes within the hippocampus. The morphological changes in mesial temporal lobe sclerosis can be grouped into primary and secondary.
The primary changes include:
• A small atrophic unilateral hippocampus
• Hyperintensity on both T2 W and FLAIR images (Fluid attenuated inversion recovery images)
• Loss of the hippocampal internal architecture and that of normal digitations of the head
• Previous studies documented that visual MRI evaluation of hippocampal size, architecture and signal intensity changes have sensitivities of 87-100% [20-22] with the eye being able to detect asymmetry of 14% or more [22,23].
The secondary changes on the other hand include:
• Unilateral atrophy of the mamillary body, fornix columns (circuit of paper), and the amygdala.
• Increased T2 W signal in the anterior temporal lobe white matter with loss of grey-white demarcation in the ipsilateral anterior temporal lobe.
• Unilateral dilatation of the temporal horn (a less reliable secondary sign)
• Unilateral atrophy of the collateral white matter bundle.
These secondary changes are seen in about 40-60% of patients with MTS. The secondary changes alone are unreliable, but there is increased diagnostic accuracy when considered with the primary findings [24]. In cases of bilateral hippocampal abnormalities, secondary findings can determine the more important side to resect. They also serve as a pointer in subtle changes and thereby improving sensitivity.
Coronal T2W and FLAIR images are the most sensitive for detecting MTS as it shows the anatomical details of the temporal lobe structures and especially of the hippocampus as well as the cortex, where most of the epileptogenic lesions are seen. An important aspect of neurological assessment in epilepsy is the search for asymmetries between both cerebral hemispheres. It should be noted that in temporal lobe asymmetry, the right side is usually larger than the left side. Consequently a smaller right temporal lobe demands proper scrutiny in symptomatic patients [25]. Asymmetry of collateral white matter is infrequent but might be encountered in normal individuals and should be regarded as a supportive finding rather than primary sign of hippocampal sclerosis [26]. On axial slices Mesial Temporal Sclerosis is usually ignored.
Routine anatomic MRI has contributed immensely to identifying and characterizing culprit lesions such as Mesial Temporal Sclerosis, which constitutes over 70% of cases of TLE, and cortical dysplasias [27-31]. In the case under review conventional MRI protocols were used for the demonstration of morphological changes within the temporal lobe. The findings confirmed hippocampal sclerosis (HS) as the cause of the patient’s intractable epilepsy.
The epileptogenic lesion may be revealed using routine MRI protocols. However, some authors are of the opinion that routine MRIs quite often miss smaller or subtle lesions and the findings of such investigations may be considered normal. Consequently, they suggested that an optimized epilepsy protocols with adequate spatial resolution and multiplanar reformatting should be used to improve identification of pathologies [32,33]. This may partially be responsible for predominantly MRI negative findings we observed when investigating people with epilepsy in our setting. Dedicated epilepsy protocols are yet to be made part of the epilepsy investigations. Insufficient experience of the radiologists and knowledge of MRI anatomy of the Mesial Temporal Lobe may also be contributing factors, although some studies revealed that about 20%-30% of patients with temporal lobe epilepsies and 20%-40% of patients with extratemporal epilepsies had no identifiable MRI features [7,34,35].
CT does not detect HS at all [36]. The overall percentage of success of CT in detecting lesions in focal epilepsies is low, approximately 30% [5]. CT is however, recommended in emergency situation.
There is a wide range of complimentary imaging techniques available for diagnosing and locating Mesial Temporal Lobe Sclerosis. These include MR hippocampal volumetrics, MR hippocampal T2 relaxometry, MR Spectroscopy, Simple Photon Emission Computed Tomography (SPECT), Ictal SPECT and Positron Emission Tomography (PET). These methods all have high sensitivities but are either not available or not in common usage in our setting.
It has been documented that MR using simple visual inspection is the most extensively used imaging technique. It has a greater sensitivity for lesional detection in patients with intractable epilepsy amounting to about 85% [19,22]. It is widely available, and trained observers readily detect MTS or other causes of epilepsy [5,37] MRI technique using visual inspection is the standard practice in our setting, hence there is need for our radiologists to master the nitty-gritty of this available technique.
Conclusion
MRI is the imaging modality of choice for the investigation of people with epilepsy (PWE). Mesial Temporal Lobe Sclerosis is the major culprit in intractable temporal lobe epilepsy and is best demonstrated on coronal T2W and FLAIR MR images. Experience, diligence and a good knowledge of the MRI anatomy of the Mesial Temporal Lobe are prerequisite to accurate diagnosis as well as mastering the fundamentals of simple visual inspection technique although sensitivity and specificity can be improved with dedicated TLE protocols.
24360
References
- Shinnar S (2003) Febrile seizures and mesial temporal sclerosis. Epilepsy Curr 3: 115-118.
- Tarkka R, Paakko E, Pyhtinen J, Uhari M, Rantala H (2003) Febrile seizures and mesial temporal sclerosis: No association in a long-term follow-up study. Neurol 60: 215-218.
- Camacho DL, Castillo M (2007) MR imaging of temporal lobe epilepsy. Semin Ultrasound CT MR 28: 424-436.
- Engel J (1992) Recent advances in surgical treatment of temporal lobe epilepsy. Acta Neurolscan 86: 7180.
- Bronen RA, Fulbright RK, Spencer DD, Spencer SS, Kim JH, et al. (1996) Refractory epilepsy: comparison of MR imaging, CT, and histopathologic findings in 117 patients. Radiol 201: 97-105.
- Gupta RG (2002) Magnetic resonance imaging of temporal lobe epilepsy. Appl Radiol 31: 12.
- Arora V, Nijjar IBS, Mahajan DS, Sandhu PS, Singh JP, et al. (2005) MRI in seizure disorder - A pictorial essay. Ind J Radiol Imag 15: 331-340.
- Salanova V, Markand O, Worth R (2004) Temporal lobe epilepsy: analysis of patients with dual pathology. Acta Neurol Scand 109: 126-131.
- Cendes F, Sakamoto AC, Spreafico R, et al. (2014) Epilepsies associated with hippocampal sclerosis. Acta Neuropathol 128: 21-37.
- Ryan S, McNicholas M, Eustace S (2010) Anatomy for diagnostic imaging. (3nd Edn.). pp: 60-61.
- Chummy SS (2011) LAST’S Anatomy-Regional and Applied. (11th Edn). pp: 485-490.
- Duvernoy HM (2005) The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Berlin: Springer Verlag.
- WHO (2005) Atlas: Epilepsy care in the world. Geneva: World Health Organization. pp: 8-16.
- Sanya EO, Wahab KW, Desalu OO, Bello HA, Ademiluyi BA, et al. (2015) A 3 year audit of adult epilepsy care in a Nigerian tertiary hospital (2011-2013). Ann Afr Med 14: 97-102.
- Hart YM, Shorvon SD (1995) The nature of epilepsy in the general population. I. Characteristics of patients receiving medication for epilepsy. Epilepsy Res 21: 43-49.
- Sander JW, Shorvon SD (1996) Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry 61: 433-443.
- Radhakrishnan K (2009) Challenges in the management of epilepsy in resource-poor countries. Nat Rev Neurol 5: 323-330.
- Grainger and Allison’s Diagnostic Radiology-A textbook of medical imaging. (6th Edn.). 2: 1551-1554.
- Osborn AG, Cooper JA, Castillo M (2004) Diagnostic Imaging–Brain. (3rd Edn.). St Louis: WB Saunders.
- Connor EJ, Jarosz JM (2001) Magnetic resonance imaging of patients with epilepsy. Clin Radiol 56: 787-801.
- Arora V, Nijjarr IBS, Mahajah DS, Sandhu PS, Singh JP, et al. (2005) MRI in seizure disorder: A pictorial Essay. Ind J Radiol Image 15: 331-340.
- Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GC, et al. (1990) Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology 40: 1869-1875.
- Connor EJ, Jarosz JM (2001) Magnetic resonance imaging of patients with epilepsy. Clin Radiol 56: 787-801.
- Szabo CA, Xiong J, Lancaster JL, Rainey L, Fox P, et al. (2001) Amygdalar and hippocampal volumetry in control participants; differences regarding handedness. Am J Neuroradiol 22: 1342-1345.
- Bronen RA, Cheung G (1991) MRI of the temporal lobe: normal variations, with special reference toward epilepsy. Magn Reson Imaging 9: 501-507.
- Banati RB, Goerres GW, Myers R, Gunn RN, Turkheimer FE, et al. (1999) [11C] (R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology 53: 2199-2203.
- Barba C, Di Giuda D, Policicchio D, Bruno I, Papacci F, et al. (2007) Correlation between provoked ictal SPECT and depth recordings in adult drug-resistant epilepsy patients. Epilepsia 48: 278-285.
- Barkovich AJ, Truwit CL (1990) Brain damage from perinatal asphyxia: correlation of MR findings with gestational age. AJNR 11: 1087-1096.
- Barkovich AJ, Rowley HA, Andermann F (1995) MR in partial epilepsy: value of high-resolution volumetric techniques. AJNR 16: 339-343.
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB, et al. (2012) A developmental and genetic classification for malformations of cortical development: update 2012. Brain 135: 1348-1369.
- Gaillard WD, Cross JH, Duncan JS, Stefan H, Theodore WH, et al. (2011) Epilepsy imaging study guideline criteria: commentary on diagnostic testing study guidelines and practice parameters. Epilepsia 52: 1750-1756.
- Bastos AC, Comeau R, Andermann F, Melanson D, Cendes F, et al. (1999) Diagnosis of subtle focal dysplastic lesions: curvilinear multiplanar reformatting from three dimensional magnetic resonance imaging. Ann Neurol 46: 88-94.
- Tellez-Zenteno JF, Ronquillo HL, Moien-Afshari F, Wiebe S (2010) Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 89: 310-318.
- Jack CRJ, Sharbrough FW, Cascino GD, Hirschorn KA, O'Brien PC, et al. (1992) Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol 31: 138-146.
- Bronen RA (1992) Epilepsy: The role of MR imaging. AJR Am J Roentgenol 159: 1165-1174.









