Keywords
Mini-tablets; Pellets; Multi-particulate; Pediatric drug delivery
Introduction
The development of pediatric dosage form is challenging even after significant advancement in pharmaceutical technology because most pharmaceutical companies delay pediatric formulation development long after the adult product has been marketed. However, regulatory agencies are now insistent of pharmaceutical companies to communicate a pediatric formulation assessment for new drug products, which often leads to pediatric formulation development activities even prior to application of the adult formulation [1]. Developing a pediatric drug product is often challenging since a single product may not be feasible for all pediatric age groups including neonates, infants, toddlers, young children, and adolescents. The challenge lies in the differences in swallowing abilities, taste preferences, and dosage requirements in children. The traditional solid dosage forms such as tablets and capsules are considered not suitable due to swallowing difficulties of young children. Hence, the acceptable drug products among pediatric patients are typically formulated as powder for suspension, oral granules, oral solutions and most recently mini-tablets. To date the most commonly prescribed dosage form for pediatric patients is a liquid dosage form due to its ease of administration. Liquids are the most frequently used pediatric formulations; however they have major disadvantages such as chemical, physical and microbial instability, palatability of the solution, accuracy of dosing, lack of controlled release, and elevated toxicological risks [2-5]. There are also limitations on which excipients, preservatives and solvents can be used in pediatric formulations [6]. Mini-tablets as shown in Figure 1 are being developed as the pediatric dosage form. In a recent study, it has been reported that mini-tablets are superior to syrups for administration to children including toddlers [7,8] and it represent a flexible drug delivery tool for single unit or composite of multiple units. Hence, mini-tablets present a very promising alternative to liquid formulations administered to children of different age groups [9].

Figure 1: Illustrates mini-tablets and their different packaging configurations.
With the aim to improve drug delivery for pediatric patients, the development of a mini-tablet may prove beneficial. However, there are no strict regulatory guidelines that define mini-tablets. Smaller than typical tablets considered as mini-tablets. The term micro-tablets are sometimes used alternatively to mini-tablets with typical tablet diameters between one to five millimeters (mm). In general, a tablet with a diameter less than three millimeters refers to mini-tablets [10,11]. Mini-tablets can also be considered oral granules or sprinkles because both are similar in diameter (≤ 2.5 mm) and are intended for dosage administration by mixing with soft food or puree [12]. Minitablets combine the advantages of both solid and liquid formulation as it allows exact and individual dosing according to age, body weight, body area, dose titration, and therapeutic demands. They also improve patient compliance due to the ease of swallowing or mixing with foods. Butler et al. [13] compared the Gastrointestinal (GI) transit of 3.8 mm mini-tablets with that of a traditional Multi-Particulate (MP) system under fasted and fed conditions and found that the transit characteristics of the mini-tablets were similar to those of the reference MP formulation, indicating that the mini-tablet dosage form achieved the same relative independence to GI transit characteristics as MP systems [13].
In addition to dosing advantages, mini-tablets have many other benefits such as customized delivery in terms of combinations of release rates and mechanisms to target release to different segments of the GI tract. It is possible in one dosage form to incorporate a number of different mini-tablets, each type formulated individually and programmed to release drug at different sites of the GI tract. These combinations can be Immediate Release (IR), delayed release, and/ or Extended Release (ER) [14,15]. For instance, in one study, Butler et al. [13] filled nine mini-tablets into the final capsule product as a combination of three different intermediate components: IR minitabs, ER mini-tablets targeted to release drug in the upper small intestine and ER mini-tablets targeted to release drug in the lower small intestine. Mini-tablets also allow the opportunity to present a multi-particulate dosage form where more than one drug substance is included in one dosage form to be used as a combination therapy, by incorporating each Active Pharmaceutical Ingredient (API) in a different mini-tablet and then dosing each type in a capsule, sachet, or stick pack dosage form. Additionally, the small size of mini-tablets enables rapid disintegration, which can be formulated as an orally disintegrating dosage form. This allows their dispersion in a liquid prior to administration which is appropriate for young children or infants who can only swallow disintegrated particulates [16]. Despite mini-tablets having all these superiority, there are potential challenges to overcome. The most notable challenge is their small size and hence not easy to handle individually during manufacturing operation or during analytical testing in the lab. Mini-tablets require a the large number of units per batch and the number of units required for testing is significantly higher and thus harder to handle when compared to traditional tablet development. Additionally, small size tablet dosage form might not be appropriate for patients with motor impairment, unless administered in clinical setting or with the help of a caregiver or a dosing device. Few products commercially available as mini-tablets are listed in Table 1. This brief technical review introduces and compiles opportunities and challenges associated with manufacturing of minitablets as a tool for development of pediatric dosage form.
| Brand Name |
Drug Name |
Indication |
Manufacturer |
Dosage Form |
| Rythmol® SR |
Propafenone HCl |
Antiarrhythmic |
GlaxoSmithKline |
Capsule |
| Enzym-Lefax® |
Pancreatin |
Indigestion |
Bayer |
Capsule |
| Lamisil® Oral Granules |
Terbinafine HCl |
Antifungal |
Novartis |
Sachet |
| Orfiril® long |
Sodium Valproate |
Epilepsy |
Desitin |
Capsule, Sachet |
| Pankreatan® |
Pancreatin |
Pancreatic Insufficiency |
Novartis |
Capsule |
| Trilipix® |
Fenofibric acid |
Cholesterol |
Abbott |
Capsule |
| Kalydeco® |
Ivacaftor |
Cystic Fibrosis (CF) |
Vertex |
Stick Pack |
Table 1: Example of Commercially Available Mini-tab Products.
Manufacturing of Mini-tablets
Despite the increasing importance of mini-tablets for its advantages as pediatric formulations and in modified-release applications, its popularity is limited due to the lack of formulation and processing knowledge in developing such dosage forms. The development of minitablets as a Drug Product (DP) is mainly dictated by the type of dosage form required, physico-chemical properties of the active ingredient, and other excipients and factors related to the manufacturing process. Depending on the nature of the active ingredient, the most common manufacturing process for mini-tablets includes blending operations, compression of the final blend into mini-tablet cores, coating minitablet cores and finally packaging of the coated mini-tablets into appropriate packaging configuration.
Physical properties of blend
The current drug development pipeline has many molecules with poorly aqueous solubility. These drug candidates are typically developed into their different salt forms or processed as Amorphous Solid Dispersion (ASD). Some challenges observed during pre-formulation with such salt and crystalline forms include poor flow properties, electrostatic effects and low densities due to very fine particle size. When a high drug load is required, challenges related to flow is magnified. When a drug molecule is electro static in nature, sticking to the wall of the blender which can lead to low assay and blend uniformity issue is observed. In some cases, additional granulation (dry or wet) is required to improve flow properties by controlling particle size of the generated granules. It is important to avoid very large granules that could also cause inadequate flow by blocking the space within the die during compression. The ideal flow should be such that it supports consistent die filing. Sticking is another problem due to drug molecule. This can be avoided by adequate lubrication during a pre-mixing step. Early development work related to excipient screening based on particles size and selection of processing parameters for granulation determines the quality of the final blend which ultimately controls blend uniformity. So, proper characterization and optimization of powder blends should include densities (bulk density/BD and tapped density/TD), flow properties, and segregation potential. The selection of different grades of excipients is also important to evaluate critical material properties required for successful development of mini-tablets with specifically small size (≤ 2.0 mm).
Compression of mini-tablets
The main difference in tableting between regular size tablets and mini-tablets is type of tooling required. Generally, tablet press to manufacture mini-tablets are using standard reciprocating or a rotary tablet press with single or multi-tip tooling [11,17]. However, certain modifications to the press or/and tooling might be necessary [18,19] depending on tableting requirements. Compression with a single-tip die or tooling with a low number of tips is not practical commercially due to slow output of mini-tablets and thus a long run time. The multitip tooling must meet certain requirements regarding precision and mechanical stability. It is typically more expensive compared to the standard tooling of normal size tablets. Depending on the type of the press, tooling, and size of the mini-tablets, up to 26 tips or even more tips can be accommodated on a punch (Figure 2). Multiple-tip tooling has a key similar to the tooling designed for non-round tablets for orientation. Mini-tablets compressed with deeply concaved tooling can have larger tablet density gradients compared to standard or shallow concave designs. Such gradients can lead to tablet attrition during coating, packaging, or other handling steps. The use of tapered die designs can decrease residual stresses, resulting in smaller and fewer micro defects resulting in more robust mini-tablets. Recessed dies are also advisable to protect the punch tips. Effective control over the feed frame is critical as tablet ejection can be challenging. This requires an optimized setup with precise adjustment of specially designed scraper blades to limiting tablet jumping. Multiple-tip punches are available as multiple-part assemblies or as monoblocks from tool suppliers. Monoblock punches offer faster tooling installation and easier cleaning, can be manufactured to tighter tolerances, as well as are more resistant to tip breakage than assembled multiple part tooling. However, multiple-part punches allow the replacement of damaged punch parts [20].
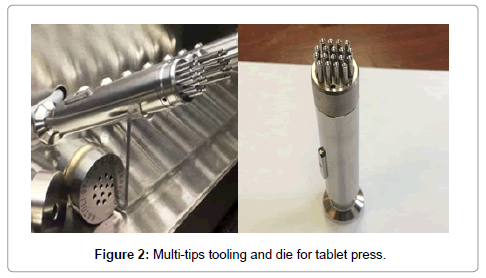
Figure 2: Multi-tips tooling and die for tablet press.
A certain degree of care should be applied when handling and using mini-tablet tooling as it is easy to damage. Multi-tip tooling must meet tighter requirements for machining and mechanical stability compared to larger tablet tooling. This labor-intensive manufacturing leads to higher production costs. Any excessive force applied to the tooling can lead to damage of punches. Due to the small diameter of the punches, they are easy to deform and break (Figure 2). Therefore, careful handling and following the manufacturer recommendations for the maximum compression force is essential. If multiple tip tooling is used, then the maximum compression force value will be higher and is typically recommended by the tooling manufacturer. The surface area/ weight ratio of mini-tablets is significantly higher than that of normal size tablets. This results in higher ejection forces during compression and may potentially result into “sticking” issues. Therefore, during development, higher levels of lubricant needs to add to offset any “sticking” issues. Shallow convex mini tablets have improved uniform density distribution throughout the core, which can lead to a more robust compression process. Deep convex mini-tablets roll more effectively, and are more suitable for tablet counting technologies commonly used by sachet filling and encapsulation machine providers.
The weight uniformity of mini-tablets is crucial because it impacts content uniformity and dosing accuracy. In cases where the blend is containing relatively small particles, extra processing steps such as fluid bed granulation, high-shear granulation, or roller compaction may be required in the manufacturing process to improve flow properties. It is also important to control or minimize friability of mini-tablets by optimizing tablet press speed. It has been noticed that higher press speeds can contribute to higher friability or fragmented mini-tablets. Typically, the optimum ratio of particle-to die diameter to minimize weight variability in tablet compression is between 1:20 to 1:30. A large proportion of particles larger than the optimum ratio in mini-tablet manufacturing can lead to increased tablet weight variability. The tolerance for weight variability in mini-tablets is smaller than for larger tablets. Small absolute weight variations will lead to more significant relative variations in potency. The weight checks of mini-tablets need to be assessed using high precision analytical balances with high accuracy (≤ 0.001 mg). Additionally, hardness and thickness needs to be measured as in-process controls during compression process. This may require a hardness/thickness tester which is capable of handling mini-tablets. The tablet press has to be equipped with force feeder as compared to gravity feeder to reduce weight variability. These challenges to monitor and control physico-mechanical attributes, sensitive characterization techniques and thus the specialized equipment needed for these measurements results in an increases manufacturing cost.
Coating of mini-tablets
Film coatings are frequently applied when it comes to solid oral dosage pharmaceutical drug delivery. The purpose for coating can vary including altering properties range from cosmetic considerations (color, gloss), improving the stability (light protection and moisture), taste masking. Moreover, functional coatings can be used to modify the drug release behavior of the dosage form. It is possible to delay the release of the drug (such as in enteric coatings) or sustain the release of the drug over an extended periods of time, depending on the polymers used. The drug can also be layered as functional coating material to produce immediate release effect of the same drug or a different drug for a combination of drugs. The equipment used to employ coating on the surface of the tablet cores are pan coaters and fluid beds. Many believe that pan coating is mainly designed to coat regular-sized tablets because of the large opening in pans which are not suitable for minitablets. However mini-tablet pans are available with smaller openings.
Thus, both pan coating and fluidized bed coating are suitable methods.
Fluid-bed coating of mini-tablets: The coating of mini-tablets is typically carried out by fluid-bed technology where materials are in fluidized state and spraying gun position dictates different ways of coating. In bottom spray set up also known as Würster coating, the mini-tablets are circulated in a vertical expansion chamber equipped with the column, while the coating solution is sprayed from the bottom of the bed near the distributor plate through upward-pointing gun. A number of design modifications on the conventional fluidized bed have been adopted to improve the coating process for mini-tablets. In order to coat mini-tablets using Glatt fluid beds, a specially designed “D” plate is required (Figure 3). A careful evaluation of an appropriate fluid bed critical processing parameters such as fluidizing air flow, bed temperature, spray rate, atomization pressure and filter bag mesh size used are imperative to the mini-tablet coating process. The processing parameter values will differ depending on the equipment, batch size, and the type of coating formulation used. Fluid bed technology is used as an alternative approach to pan coatings as it helps in achieving a fast and uniform coating using air to mix, coat, and dry the substrate at the same time. A major concern during fluid bed mini-tablet coating is high friability and compromise in core tablet appearance due to physical stress including frequent collisions, high friction, increased moisture and high temperature which occurs during the fluidization process. Pan coating is a much gentler process. Hence, mini-tablet coating by fluidized-bed technology requires higher strength for minitablet cores, and a high standard for tablet formulation design. Neha et al. recently observed that keeping high fluidization, with low bed, led to fines formation and mini-tablet breaking. Moreover, lower fluidization with respect to bed load, led to non-uniform coating and formation of doublets during process [21]. Vuong et al. [22] investigated the enteric coating efficiency of mini-tablets using either a perforated pan or a fluid-bed coating machine. For both types of equipment, good enteric coating efficiency was obtained and the results were comparable [22]. Despite the processing challenges, Würster coating is a preferred method for coating of mini-tablets. However, the main benefit of using the perforated pan instead of a fluid-bed was a shorter process time [23,24].
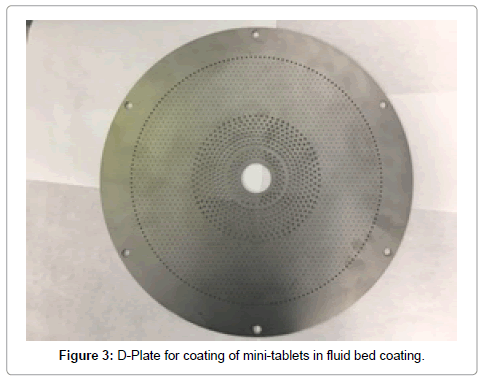
Figure 3: D-Plate for coating of mini-tablets in fluid bed coating.
Pan coating of mini-tablets: The main challenge for perforated pan coating of mini-tablets is fabrication of the pan to prevent minitablets falling through the perforations in the coating pan. A simple and economical way to pan coat mini-tablets is to use a mesh insert [24]. It was also noticed that due to the small size of mini-tablets they tend to jump out of the coating pan increasing residual loss. A Perspex shield plate can be placed at the front of the pan to prevent jumping. The coating of mini-tablets was found to be faster and more reproducible due to uniform shape, size, smooth surface, low porosity, and high attainable strength. It was found that one third reductions of functional coating of mini-tablets was needed compared to coating of granules because of lower surface to volume ratio [25]. Mini-tablets has also been successfully coated in a solid wall pan machine, GS (IMA) coating equipment where drying air was delivered to the core tablet bed by means of two immersed perforated swords. From a commercial stand point, coating of mini-tablets in a perforated pan can be more efficient due to higher production volumes, lower waste of coating material, and faster equipment cleaning time compared to the conducting the process in a fluid bed [26].
Other coating technology for mini-tablets: Even though pan coating and fluid bed coating technology for mini-tablets are better understood, these conventional coating methods are considered laborious, expensive, and require technical expertise for material selection and process development. Thus, formulation scientists are continuously searching for new, effective, inexpensive, and simple coating approaches. Recently, Atomic Layer Deposition (ALD) was investigated as a novel technique for thin coating of mini-tablets, and for masking the bitter taste of the drug substances. ALD is a surface controlled, self-limiting layer-by-layer proceeding coating method for depositing ultra-thin, high quality, and conformal thin films. The concept of ALD seems attractive, as ALD coatings are not only continuous, ultra-thin, dense, and smooth, but also most importantly pinhole-free, very conformal to the substrate, and provide good diffusion barriers with low gas and moisture permeability. As compared to the conventional pan or fluid bed coating, ALD process is very different and it does not involve certain limitations as tablet cores are stationary, and are coated during separate surface saturation (deposition) cycles involving chemical interactions in reactor [27,28].
Packaging of Mini-Tablets
The packaging configuration of mini-tablets is mainly dictated by the target product profile or product design requirements. The selection of correct packaging configuration also depends on drug product performance in a particular packaging configuration during long term storage. There are number of ways to deliver mini-tablets to the patients, these include encapsulation in capsule shells, packaged into unit-dose packaging such as stick-packs or sachets, or prefilling a container for disintegration (Figure 1).
Encapsulation of mini-tablets
Among the possible packaging configurations, encapsulation of mini-tablets is preferred option. The encapsulation machines are capable to fill mini-tablets, pellets, powder, and granules with direct or indirect filling operation mechanism. In the case of direct filling operation, the mini-tablets are fed into the body until it is completely full. An encapsulator such as the Qualifill TM Pellet filler works on direct filling operation mechanism. For indirect filling, operation the encapsulates have modified d osators that use either suction to hold the material in the tube during transfer or are pushed through the material bed as seen in Zanasi 40 E encapsulator. However, most advanced encapsulation equipment currently on the market is units such as Bosch GKF 2500 (Figure 4). These machines offer filling of minitablets based on number of individual mini-tablets per capsule. Table 2 represents number of mini-tablets of 2 mm size can be filled in different capsule size. Custom designed dosing discs with cavities deliberate to be filled with mini-tablets are used. Each dosing disc is designed to accommodate the specific size of the mini-tablets and count required per capsule. A variable thickness dosing disc can slide underneath to hold the material prior to transfer to allow only one mini-tablet per cavity. The mini-tablets are held in position on the wheel by vacuum, and this is electronically monitored by a webcam sensor which checks the disc for the presence of mini-tablets. All insufficiently filled capsules are automatically rejected in the finished product discharge chute. The dosing discs are intended to count mini-tablets in an accurate manner across a wide range of target fills.

Figure 4: Encapsulation of mini-tablets by Bosch GKF 2500.
A pilot scale encapsulator such as the Zanasi 40 E is capable of filling capsules at relatively moderate speed of around 40,000 capsules per hour whereas the Bosch GKF 2500 can be used for commercial scale and is capable of filling capsules at speeds of 150,000 capsules or more per hour. Moreover, modern encapsulators are capable of filling combination products, such as mini-tablets with different release profiles (ER component and IR component) of same drug, or different types of mini-tablets, or mini-tablets combined with pellets or powder.
Lopes et al. [28] has described such system where a capsule is filled with powder as immediate release component and coated mini-tablets as extended release part of the combination therapy [29]. A recent study by Mitra et al. concluded that the number of mini-tablets dispensed is critical when more than one mini-tablet is needed to achieve a target dose within a 15% content uniformity limit. Hence, the ability to accurately dispense varying mini-tablet quantities using different encapsulators can cover a wide dose range and such dosing flexibility is beneficial in clinical trials [30].
Unit-dose packing of mini-tablets
Unit-dose packaging of mini-tablets has been receiving attention recently, especially for pediatric formulations. The unit-dose packaging can be referred to as stick-packs or sachets depending on the fill volume: stick-packs have smaller fill volumes, whereas sachets have larger fill volumes. The main advantage of unit-dose packaging is that it is suitable for packaging relatively large numbers of mini-tablets which can be beneficial for high dose drugs. With increasing complexity, more options can be included, such as an increased number of minitablets per dose, or the possibility of dispensing two or more products simultaneously.
Stick packing requires specific equipment and there are a number of stick packing machines available. They usually work on same vertical intermittent-motion principle. Specifically, a packaging machine such as the SBL-50 from Merz System is a vertically operating, fully automatic forming-, filling- and sealing machine for the production of very small tubular bags, referred to here as “Stickpacks” (Figure 5). During stick packing, the machine processes flexible composite films (often including foil) from the flat laminate reel, cut lengthwise, formed to a tube during transportation and sealed lengthwise. It is then filled, sealed transversally, and cut. At the same time, photocell control assures the exact positioning of the print. The filling of stickpacks is dependent on multiple factors including the size of the dose, type of laminate/ sachet material, and the type of adhesive/polymer used to seal the sachet. In early process optimization, supplier recommended sealing criteria of the laminate/sachet material can be used but eventually critical process parameters needs to be identified and then the range has to be established through development work. The critical process parameters related to stick packing are sealing temperature, sealing pressure; sealing dwell time and size of the stick pack which depends on fill volume. There is change parts required depending on the selection of products meaning powder dosing vs. mini-tablets dosing. In case of mini-tablets dosing, specifically fabricated dosing disk selected based on number of count of mini-tablets per stick packs. By means of air pressure or vacuum, mini-tablets are removed from the dosing disk. The physical characteristics of mini-tablets such as thickness, length, width, diameter, diagonal length are very critical while ordering a dosing disk. With increasing complexity, more options can be included, such as an increased number of mini-tablets per dose, or the possibility of dispensing two or even more products simultaneously.
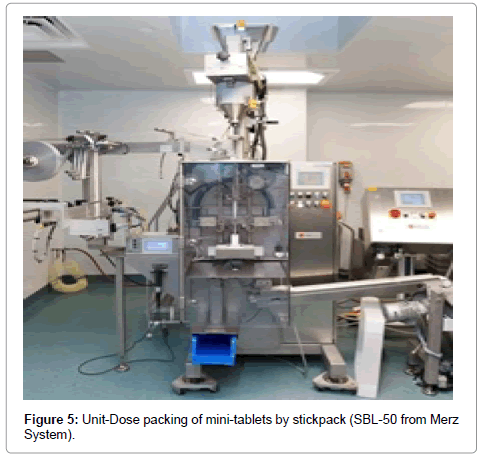
Figure 5: Unit-Dose packing of mini-tablets by stickpack (SBL-50 from Merz System).
Conclusion
Mini-tablets present a promising alternative to liquid formulations administered to children of different age groups. Additionally, minitablets offer the advantage of combination therapy that it is not easily achievable with conventional tablets or capsules. In contrast, difficulty may also be encountered when designing a mini-tablet based dosage form because mini-tablets can be more easily dropped or lost relative to larger tablets but these risks can be mitigated through the appropriate choice of packaging configurations. Due to their unique size, some adjustments to the manufacturing process steps may be needed. The earlier development work related to flow property assessment can provide useful insights to help guide the development efforts. The manufacturing process typically involves unit operations such as dry or wet granulation to improve flow properties, compression using multi tip tooling, Würster or pan coating, and encapsulation or stick packing. These manufacturing processes have a number of technological challenges when producing mini-tablets when compared to conventional tablets but careful evaluation of each unit operation can produce a better suited and robust mini-tablets based dosage form. As such, mini-tablets seem best implemented for small volume, high value products, particularly for pediatric patient populations that would benefit by this unique dosage form.
Acknowledgements
The authors would like to thank Joel Herrera and Lee Crawford for their technical input and discussions in preparing this review paper.
23480
References
- Aleksovski A, Dreu R, Gasperlin M, Planinsek O (2015) Mini-tablets: A contemporary system for oral drug delivery in targeted patient groups. Expert Opin Drug Deliv 12: 65-84.
- Breitkreutz J, Wessel T, Boos J (1999) Dosage forms for peroral administration to children. Paediatr Perinat Drug Ther 3: 25-33.
- Breitkreutz J, Boos J (2007) Paediatric and geriatric drug delivery. Expert Opin Drug Deliv 4: 37-45.
- Cram A, Breitkreutz J, Desset-Brethes S, Nun T, Tuleu C (2009) Challenges of developing palatable oral paediatric formulations. Int J Pharm 365: 1-3.
- Bowles A, Keane J, Ernest T, Clapham D, Tuleu C (2010) Specific aspects of gastro-intestinal transit in children for drug delivery design. Int J Pharm 395: 37-43.
- Ernest TB, Elder DP, Martini LG, Roberts M, Ford JL (2007) Developing pediatric medicines: identifying the needs and recognizing the challenges. J Pharm Pharmacol 59: 1043-1055.
- Thomson SA, Tuleu C, Wong IC, Keady S, Pitt KG, et al. (2009) Minitablets: new modality to deliver medicines to preschool-aged children. Pediatrics 123: 235-238.
- Spomer N, Klingmann V, Stoltenberg I, Lerch C, Meissner T, et al. (2012) Acceptance of uncoated mini-tablets in young children: results from a prospective exploratory cross-over study. Arch Dis Child 97: 283-286.
- Stoltenberg I, Winzenburg G, Breitkreutz J (2011) Solid oral dosage forms for children-formulations, excipients and acceptance issues. Eur Ind Pharm 8: 4-7.
- Lennartz P, Mielck JB (1998) Minitabletting: improving the compactibility of paracetamol powder mixtures. Int J Pharm 173: 75-85.
- Tissen C, Woertz K, Breitkreutz J, Kleinebudde P (2011) Development of mini-tablets with 1 mm and 2 mm diameter. Int J Pharm 416: 164-170.
- Guidance for Industry: Size of Beads in Drug Products Labeled for Sprinkle (2012) US Department of Health and Human Services FDA, Center for Drug Evaluation and Research.
- Butler J, Cumming I, Brown J, Wilding I, Devane JG (1998) A novel multiunit controlled-release system. Pharm Tech, pp: 122-138.
- Li YH, Zhu JB (2004) Modulation of combined-release behaviours from a novel “tablets in capsule systemâ€ÂÂÂÃÂ. J Contr Rel 95: 381-389.
- Ishida M, Abe K, Hashizume M, Kawamura M (2008) A novel approach to sustained pseudoephedrine release: differentially coated mini-tablets in HPMC capsules. Int J Pharm 359: 46-52.
- Stoltenberg I, Breitkreutz J (2011) Orally disintegrating mini-tablets (ODMTs) - A novel solid oral dosage form for paediatric use. Eur J Pharm Biopharm 78: 462-469.
- Riis T, Bauer-Brandl A, Wagner T, Kranz H (2007) pH-independent drug release of an extremely poorly soluble weakly acidic drug from multiparticulate extended release formulations. Eur J Pharm Biopharm 65: 78-84.
- Flemming J, Mielck JB (1995) Requirements for the production of micro tablets: Suitability of direct-compression excipients estimated from powder characteristics and flow rates. Drug Dev Ind Pharm 21: 2239-2251.
- Flemming J, Mielck J (1996) Experimental micro tabletting: construction of an instrumented punch holder for an eccentric tabletting machine. Eur J Biopharm 42: 212-214.
- Shah N, Mehta T, Aware R, Shetty V (2017) Investigation on influence of Wurster coating process parameters for development of delayed release minitablets of Naproxen. Drug Development and Industrial Pharmacy.
- Vuong H, Levina M, Rajabi-Siahboomi AR (2008) Investigation of enteric coating of mini-tabs using a perforated pan or a fluid-bed machine. 35th Annual Meeting and Exposition of the Controlled Release Society, New York, NY, USA.
- Porter SC, Bubb G, Mehra DK, Sedlock R (2002) Modified-release matrix tablets coated with a modified-release film coating: Comparison of regular tablets with mini-tablets as an alternative to pellets. AAPS Annual Meeting and Exposition, Toronto, Canada.
- Vuong H, Levina M, Rajabi-Siahboomi AR (2008) Evaluation of the Enteric Performance of Lansoprazole Mini-Tablets Coated in a Perforated Pan. AAPS Annual Meeting and Exposition, Atlanta, GA, USA.
- Munday DL (1994) A comparison of the dissolution characteristics of theophylline from film coated granules and mini-tablets. Drug Dev Ind Pharm 20: 2369-2379.
- Funaro C, Mondelli G, Passerini N, Albertini B (2010) Minitablets coated in a solid-wall pan for theophylline sustained-release capsules. Pharm Tech, pp: 38-42.
- George S (2010) Atomic layer deposition: an overview. Chem Rev 110: 111-131.
- Hautala J, Kinen T, Hoppu P, Kemell M, Hein et al. (2017) Atomic Layer Deposition - A Novel Method for the Ultrathin Coating of Minitablets. International Journal of Pharmaceutics 531: 47-58.
- Lopes CM, Sousa Lobo JM, Pinto JF, Costa P (2006) Compressed mini-tablets as a biphasic delivery system. Int J Pharm 323: 93-100.
- Mitra B, Chang J, Wu SJ, Wolfe CN, Ternik RL, et al. (2017) Feasibility of mini-tablets as a flexible drug delivery tool. International Journal of Pharmaceutics 525: 149-159.
- Alfred CFR, David H, Francis F, Varsha B, Mary AJ, et al. (2016) Minitablets: Manufacturing, Characterization Methods, and Future Opportunities. American Pharmaceutical Review.











