Keywords
Dentate gyrus; Histamine H3 antagonists; Long-term potentiation; Memory disorders
Introduction
Several lines of evidence indicate that the DG encodes the contextual memory engrams that represent discrete environments [1-3]. The granule cells in the dentate gyrus region of the hippocampus are able to induce long-term potentiation (LTP), which is a cellular model of learning and memory [4]. They receive their primary excitatory afferent input from the entorhinal cortex via the so-called perforant pathway [5]. A number of different transmitters which are released in a global manner in the brain act on these synapses, thereby altering the afferent information should be processed and LTP. One example of these transmitters is the biogenic amine histamine, which is released from varicosities of axons originating in the tuberomammillary nucleus of the hypothalamus [6]. The earlier studies have been shown that histamine is involved in the LTP formation in the CA1 region [7-14] and the DG [15,16], and consolidation of memory [17-20].
Among the four histamine receptors, the H3 receptor (H3R) has received particular attention as a target for disease characterized by memory impairment and cognitive decline, such as Alzheimer’s disease [21] and schizophrenia [22]. In the hippocampal formation the dentate gyrus contains the highest density of H3R binding sites in rat brain [23]. These receptors are presynaptically located, are negatively coupled to adenylyl cyclase and mediate presynaptic inhibition of neuronal histamine release [24] as well as other neurotransmitters [25]. H3R -antagonists have been reported to improve spatial recognition memory in animal models [19,20,26]. Other receptor subtype that has been implicated in spatial learning and memory formation is histamine H1 receptor (H1R) [27], which is coupled to Gq protein and phospholipase C (PLC) [28]. The results of behavior studies, however, are controversial: While pharmacological blockade of the H1R improves spatial learning in the Morris water maze [29], it, conversely, impairs spatial learning in the 8-arm radial maze [30].
Binding of histamine to postsynaptic H1 receptors facilitates the induction of LTP in area CA1 [7,10], whereas binding histamine to the H3Rs located on perforant path terminals [31,32] and activation of this receptor by a specific agonist [15] inhibit LTP, by decreasing glutamate release. It would be valuable to study differences in the LTPs in response to direct administration H1R agonist and H3R inverse agonist in the same synaptic terminals because when activated, the H1R has excitatory properties and the H3R has inhibitory properties. Herein, considering above evidences, we hypothesized that thioperamide would give the same outcomes with that of histamine as a result of inverse agonistic effect on histamine release [33], and thereby that of pyrilamine as a result of H1R stimulation. Nevertheless, the action of thioperamide was completely different from both histamine and pyrilamine, suggesting post-synaptic H3R involvement in the dentate gyrus LTP.
Materials and Methods
Experimental animals
Experiments were conducted in adult male Wistar rats between the ages of 5-7 months in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) regarding the protection of animals used for experimental purposes and the guiding principles for the care and use of laboratory animals approved by Erciyes University. Rats were randomly divided into one of the four groups (n=6 in each group) taking into account the drug which will be infused during LTP recording.
Drugs
Drugs used in this study were: histamine (Sigma-aldrich, H7125-5G), and thioperamide maleate (Cayman chem. 148440-81-7) and pyrilamine maleate (Sigma-aldrich, 59-33-6). Drugs were dissolved in saline, titrated to pH 7.4 and stored as stock solutions. Saline, Histamine (10 μM), pyrilamine (10 μM) or thioperamide (10 μM) was infused to the DG in 10 μL volume for 10 minutes using a microinfusion pump (Stoelting Co, Wood Dale, Illinois, USA).
Electrophysiology
Details of the protocols used for electrophysiological experiments are described elsewhere [34]. Briefly, after rats were anesthetized with intraperitoneally injected urethane (1.2 g/kg), a double-barrel glass micropipette (Borosilicate, outer diameter 1.5 mm, length 10 cm length; World Precision Instruments) was inserted into the granule cell layer of the DG in the right hemisphere (in mm, from bregma: anteroposterior: −3.5; mediolateral: 2.15; dorsoventral: 2.5-3 mm below the dura) to record the field potential. A bipolar tungsten electrode (stainless steel, Teflon-coated, 127 μm in diameter, insulated except at its tips) was used to stimulate the medial perforant path (PP, from bregma, in mm: anteroposterior: −8.0; mediolateral: 4.2; dorsoventral: 2-2.5 below the dura) of the right hemisphere. The depth of recording and stimulating electrodes (dorsoventral coordinate) was adjusted to obtain a large positive excitatory postsynaptic potential (EPSP) followed by a negative-going population spike (PS) in response to perforant path stimulation. These positions of both electrodes were previously verified to be in the granule cell layer of the dentate gyrus and in the PP [35,36]. The recording barrel was filled with 3 M NaCl (tip resistance: 2-10 MΩ), and the other was filled with SF or histamine, either alone or together with pyrilamine or thioperamide.
After a stable EPSP was obtained, the PP was stimulated by pulses at an intensity that ranged from 0.1 to 1.5 mA at 0.05Hz three times and by increasing the intensities from a 0.1 mA to a 1.5 mA step by 0.2 mA per step to create an input-output curve, which was stored for off-line analysis. The stimulus intensity produced by half of the maximum PS amplitude was determined (test stimulus) and then used throughout the experiment. LTP was induced using high-frequency stimulation (HFS; 100 Hz, 1 sec, 4 times), with the test stimulus, after 15 min of baseline recording. Before LTP induction protocol, a 10 μl volume of drugs or saline was infused into the dentate gyrus at a rate of 1 μl/min using a Hamilton syringe (25 μl) driven by a syringe pump (Stoelting Co., Wood Dale, Illinois, USA). Following the delivery of HFS, the test stimulus was repeated every 30 s for up to 60 min.
Data analysis and statistics
The slope of the EPSP was calculated as the amplitude change at 20-80% of the voltage difference between the start and the peak of the waveform, whereas the amplitude of PS was calculated as the difference from the first positive peak to the negative peak for each current value. The raw values of the EPSP slope and PS amplitude during the I/O experiment were analyzed using a separate two-way repeated measures ANOVA, with drug as between-subjects variables, and stimulus intensity (8 levels of intensity) as a within-subjects variable. The mean value of the EPSP slope and PS amplitude during 5- min baseline recording was evaluated as 100 percent. Each EPSP and PS was expressed as the percentage of this value. The average between 15-20 minutes of recording was defined as the magnitudes of short-term potentiation. The averages between 85-90 minutes of recording were defined as the magnitudes of LTP.
Possible differences in the magnitudes of PTP and LTP were assessed by one-way ANOVA with drug as between-subjects factors, followed by post hoc comparisons (Tukey’s test) between the groups. Significance was set at p<0.05 (twotailed). All statistical analyses were performed using SPSS software (SPSS, Chicago, IL).
Results
Pyrilamine or thioperamide has no effect on baseline transmission in the synapses between perforant pathway and the dentate gyrus
The input/output curve represents a global relationship between stimulus intensity and the synaptic (EPSP slope) and spike (PS amplitude) components of the compound field potential, namely the baseline synaptic transmission. Figure 1 shows the I/O curves taken before and after a 10-minute infusion of saline or drugs alone. The absence of a treatment effect and significant treatment × intensity interaction (p>0.05) indicates inefficacy of the drug used on baseline synaptic transmission. Moreover, there was no significant difference in both EPSP slopes and PS amplitudes during infusion period between groups (Figure 1).
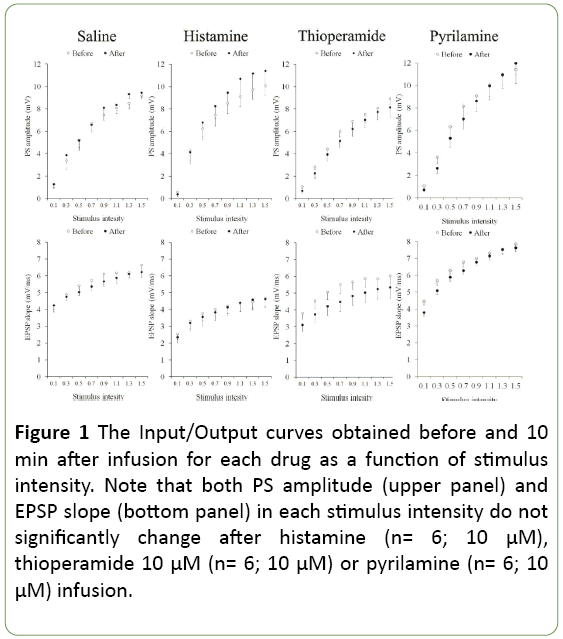
Figure 1: The Input/Output curves obtained before and 10 min after infusion for each drug as a function of stimulus intensity. Note that both PS amplitude (upper panel) and EPSP slope (bottom panel) in each stimulus intensity do not significantly change after histamine (n= 6; 10 μM), thioperamide 10 μM (n= 6; 10 μM) or pyrilamine (n= 6; 10 μM) infusion.
Long-term potentiation
Comparable pre-LTP input/output curves showed that no gross differences in baseline function between groups that might confound the interpretation of other measures of synaptic function. Multivariate analysis with the independent factor of "group" (controls, histamine, thioperamide, pyrilamine) showed a significant group effect on both shortterm potentiation (F3,22=6.16; p=0.003) and long-term potentiation (F3,22=4.37; p=0.015) of EPSP as well as those of PS amplitude (F3,22=4.10; p=0.019 and F3,22=3.99; p=0.021, respectively). The short-term and long-term potentiation of EPSP, not of PS, in the thioperamide group were significantly higher in comparison with controls (Tukey test, Ps<0.030). This indicates that potentiation of neural output does not significantly alter, even if induction and maintenance of synaptic LTP could be further enhanced by H3R antagonism. There were also significant differences in short-term potentiation of EPSP between the thioperamide and pyrilamine groups (p=0.030), and in short-term and long-term potentiation of PS between the histamine and pyrilamine groups (p=0.013 and p=0.048, respectively). Other group comparisons did not reach significance (p>0.05) (Figure 2).
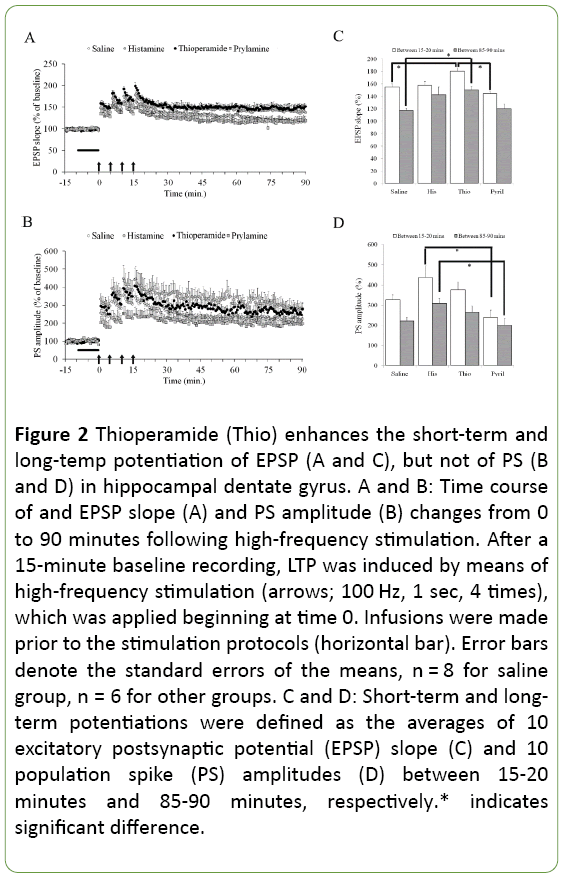
Figure 2: Thioperamide (Thio) enhances the short-term and long-temp potentiation of EPSP (A and C), but not of PS (B and D) in hippocampal dentate gyrus. A and B: Time course of and EPSP slope (A) and PS amplitude (B) changes from 0 to 90 minutes following high-frequency stimulation. After a 15-minute baseline recording, LTP was induced by means of high-frequency stimulation (arrows; 100 Hz, 1 sec, 4 times), which was applied beginning at time 0. Infusions were made prior to the stimulation protocols (horizontal bar). Error bars denote the standard errors of the means, n = 8 for saline group, n = 6 for other groups. C and D: Short-term and longterm potentiations were defined as the averages of 10 excitatory postsynaptic potential (EPSP) slope (C) and 10 population spike (PS) amplitudes (D) between 15-20 minutes and 85-90 minutes, respectively.* indicates significant difference.
Discussion
To our knowledge this is the first electrophysiological study showing action of thioperamide, histamine H3R antagonists/ inverse agonist, in the DG-LTP. The present study shows that thioperamide, but not histamine enhances LTP in the PP–DG synapses, without causing any changes in baseline synaptic transmission. These findings agree with the result of a previous study showing that intraperitoneal injection of 1 mg/kg methimepip, a histamine H3R receptor agonist, decreased LTP in the dentate gyrus of control rats [15]. The enhancement in LTP conforms to the findings that blockade of H3R augments glutamate release, which is required for induction of LTP, within the DG, whereas its activation inhibits glutamate release and LTP [31,32,37], and may be underlying mechanism of improved performance in diverse cognitive tasks in rats [38]. In addition, the present study indicates effectiveness of histamine on the DG as has been previously reported when LTP was induced by one time 60-Hz tetanic stimulation [16]. Nevertheless, histamine effect might change across different hippocampal regions. In the CA1 region, histamine (l0-100 μM) was found to produce a statistically significant LTP of fEPSPs when combined with a weak tetanus (0.25 sec, 50 Hz) [7]. In the CA3 region of the hippocampus, histamine promotes synchronized bursts of action potentials [39], an activity pattern that is known to be a physiological stimulus for the occurrence of LTP.
The functional role of postsynaptic H3R subtypes is yet to be explored. It has been described at least three functional rat H3 receptor isoforms (H3A, H3B, and H3C) that couple to the Gi protein-dependent inhibition of adenylate cyclase or stimulation of p44/p42 mitogen activated protein kinase (MAPK) [40]. H3A isoform was more effective in the stimulation of p44/p42 MAPK and the expression of this isoform in the dorsal part of the dentate gyrus, in proportion to overall expression, was more prominent than that of the other isoforms [40]. The MAPK pathway is believed to be important in neuronal plasticity and is activated in hippocampal long-term potentiation [41]. Interestingly, in the caudate putamen and nucleus accumbens H3R is mainly expressed at the post-synaptic site on the MSNs of both the direct and indirect movement pathways [42]. If the MAPK pathway mediated thioperamide effect on the LTP, then possible existence of postsynaptic H3Rs would be speculated. Indeed, there is fragmentary evidence pointing to possible existence of postsynaptic H3Rs. Such conclusion is arrived at when agonist stimulation of the H3R produces the same outcome as a parallel experiment, in which the targeted effectors (histamine or otherwise) are exogenously administered or suppressed [43]. Suggesting this conclusion, when we infused histamine we observed an increase in EPSPLTP, in a similar way as thioperamide, although the difference between the control and histamine groups remained trend level.
Histamine effect on the LTP did not reach statistical significance compared to control group, whereas PS-LTP, not EPSP-LTP, was attenuated by H1R blocker, pyrilamine, compared to histamine group. This finding indicates an effect on the number of granule cells that discharge in synchrony because the changes peculiar to PS amplitude following induction protocols can be result from voltage-sensitive changes of intrinsic excitability and/or shift in net inhibition [44-46]. Although the H1-receptor antagonist mepyramine (1 μM), in CA1 pyramidal cells, blocked the initial depression of firing and attenuated the long-lasting histamine-mediated excitation [12], in the striatum [47], septal neurons [48], and dorsal lateral geniculate [49] activation of H1R may increase neuronal excitability by blocking a leak K conductance. Together, these data suggest that a functional H1R could involve a distinctive mechanism operating in synaptic plasticity.
Receptor binding studies showed that granule cells in the DG synthesize the histamine H1Rs and H2Rs in their dendrites [40,50,51], which are coupled positively to phospholipase C and to adenylyl cyclase, respectively [52]. The phospholipase C – PKC signalling cascade have been shown to be important in the induction and early stages of synaptic plasticity [4], whereas the cAMP – PKA signalling cascade in the late phase of NMDA receptor dependent LTP [53]. These evidences point to H1R and H2R as the effectors of histamine in LTP induction. In agreement with this fact, LTP in the CA1 area of hippocampus was significantly reduced in both H1R and H2R gene knockout mice when compared with their respective mice [14,54]. Nevertheless, contrary results have also been reported: Histamine facilitates the induction of LTP [7] via activating phospholipase C [24], whereas depressed medial perforant path EPSCs more strongly, with an effect which was blocked by thioperamide, but not by the H1R and H2R antagonists [37]. The most conservative explanation of the results presented here is that the effect of histamine is dosedependent and is affected by extracellular pH. A dosedependent effect of histamine was reported in baseline PS (an increase in lover doses) and EPSP (a decrease in higher doses) activity of the CA1 synapses in vitro [7]. Histamine increases by up to tenfold synaptic transmission that was mediated by NMDA receptors at lowered pH, but there was no significant action of histamine at a pH of 7.4 [55]. Decreased activation of H1Rs and H2Rs due to decreased synthesis and its release of histamine, however cannot be excluded, because an activation of H3Rs by infusion of histamine at the PP-DG synapses [25].
There was no significant difference between before and after histamine or thioperamide infusion anywhere along the input-output curve. Input-output curves for fEPSPs and PS can vary for a number of reasons, such as differences in synaptic density, threshold for synaptic transmission, excitability or altered strength of individual synapses within the population [56]. A lack of difference between before and after infusion indicates that synaptic organization or baseline excitatory transmission in the synapses between perforant pathway and granule cell does not affected by histamine or its antagonists. Thus, there appear to be no gross differences in baseline function between before and after infusion that might confound interpretation of other measures of synaptic function.
Our study supports that H3R antagonists are involved in the regulation of memory consolidation [25] and may have cognition improving properties in animal models of narcolepsy, Alzheimer’s disease, attention deficit/ hyperactivity disorder and schizophrenia, which all characterized with the brain histaminergic system is defective [57]. H3R antagonists enhanced behavioral performance in a variety of rodent learning paradigms [58-61] and reversed contextual fear conditioning and spatial navigation deficits induced by fetal ethanol [62] or scopolamine [61].
Strong and consistent evidence exist to suggest that administration of H3R antagonists produces strong wakefulness decreases slow wave sleep and REM sleep in a dose-dependent fashion [63-66]. Although, the exact neurophysiological mechanisms underlying memory consolidation processes during sleep are still a matter of debate, recent data indicate that sleep contributes to memory formation by consolidating new information and by integrating it with previously stored contents [67]. We have speculated that Thioperamide- enhanced LTP probably lead to an increase in synchronous activation between amygdala and hippocampus, underlying sleep-dependent memory consolidation. Supporting this speculation, H3Rs modulated the hippocampal theta oscillation [68], which greatly facilitates the induction of LTP in the hippocampus [69].
Because previous studies have reported a dose dependent effect of thioperamide on LTP and behavior of animals in animal models of various CNS diseases, it would be worthwhile to explain why the used dose of the drugs was selected. A study using extracellular and whole-cell patch-clamp recording techniques showed that the dose of 10 μM thioperamide is reported to be enough for blockage of 10 μM histamine – induced depression in the excitatory synaptic transmission in the dentate gyrus for 1 h [31]. Histamine induced reduction of intracellularly recorded excitatory postsynaptic currents were similar magnitude at the concentrations of 7 μM and 70 μM [32]. Surprisingly, an earlier study reported dose-dependent effect of histamine on extracellularly recorded PS amplitude, but not EPSP slope at CA1 region [7]. A dose of 10 μM was used based on the findings of the above-mentioned studies that histamine had no dose-dependent effect on basal transmission, as shown by EPSP slope.
Conclusion
In conclusion, although more detailed studies are needed, histamine seems not to play a significant functional role in the perforant pathway-dentate gyrus synapses, but the antagonism of H3R should be considered in treatment of cognitive dysfunctions.
Acknowledgement
We are grateful to Dr. Sami Aydo an for gift of drugs and Dr. Nazan Dolu for her help in doing of experiments.
Conflict of Interest
There is no conflict of interest.
23558
References
- Matsuo N, Reijmers L, Mayford M (2008) Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science 319: 1104-1107.
- Reijmers LG, Perkins BL, Matsuo N, Mayford M (2007) Localization of a stable neural correlate of associative memory. Science 317: 1230-1233.
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, et al. (2012) Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484: 381-385.
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31-39.
- Amaral DG, Witter MP (1989) The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 31: 571-591.
- Weiler HT, Hasenöhrl RU, Van Landeghem AA, Van Landeghem M, Brankack J, et al. (1998) Differential modulation of hippocampal signal transfer by tuberomammillary nucleus stimulation in freely moving rats dependent on behavioral state. Synapse 28: 294-301.
- Brown RE, Fedorov NB, Haas HL, Reymann KG (1995) Histaminergic modulation of synaptic plasticity in area CA1 of rat hippocampal slices. Neuropharmacology 34: 181-190.
- Femenía T, Magara S, DuPont CM, Lindskog M (2015) Hippocampal-dependent antidepressant action of the H3 receptor antagonist clobenpropit in a rat model of depression. Int J Neuropsychopharmacol 18: PYV032.
- Li WW, Cheng LZ, Zou Z, Tian ML, Zhang H, et al. (2014) (R)-alpha-methylhistamine suppresses inhibitory neurotransmission in hippocampal CA1 pyramidal neurons counteracting propofol-induced amnesia in rats. CNS Neuroscience & Therapeutics 20: 851-859.
- Luo T, Leung LS (2010) Endogenous histamine facilitates long-term potentiation in the hippocampus during walking. J Neurosci 30: 7845-7852.
- Ono Y, Kataoka T, Miyake S, Sasaguri K, Sato S, et al. (2009) Chewing rescues stress-suppressed hippocampal long-term potentiation via activation of histamine H1 receptor. Neurosci Res 64: 385-390.
- Selbach O, Brown RE, Haas HL (1997) Long-term increase of hippocampal excitability by histamine and cyclic AMP. Neuropharmacology 36: 1539-1548.
- Chepkova A, Yanovsky E, Parmentier R, Ohtsu H, Haas HL, et al. (2012) Histamine receptor expression, hippocampal plasticity and ammonia in histidine decarboxylase knockout mice. Cell Mol Neurobiol 32: 17-25.
- Dai H, Kaneko K, Kato H, Fujii S, Jing Y, et al. (2007) Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci Res 57: 306-313.
- Varaschin RK, Rosenberg MJ, Hamilton DA, Savage DD (2014) Differential effects of the histamine H-3 receptor agonist methimepip on dentate granule cell excitability, paired-pulse plasticity and long-term potentiation in prenatal alcohol-exposed rats. Alcohol Clin Exp Res 38: 1902-1911.
- Chang M, Saito H, Abe K (1997) Cimetidine inhibits the induction of long-term potentiation in the dentate gyrus of rats in vivo. Jpn J Pharmacol 74: 281-283.
- Kruk M, Miszkiel J, McCreary AC, Przegaliński E, Filip M, et al. (2012) Effects of the histamine H-3 receptor antagonist ABT-239 on cognition and nicotine-induced memory enhancement in mice. Pharmacol Rep 64: 1316-1325.
- Benetti F, Baldi E, Bucherelli C, Blandina P, Passani MB (2013) Histaminergic ligands injected into the nucleus basalis magnocellularis differentially affect fear conditioning consolidation. Int J Neuropsychopharmacol 16: 575-582.
- Bernaerts P, Lamberty Y, Tirelli E (2004) Histamine H3 antagonist thioperamide dose-dependently enhances memory consolidation and reverses amnesia induced by dizocilpine or scopolamine in a one-trial inhibitory avoidance task in mice. Behavioural Brain Research 154: 211-219.
- Orsetti M, Ferretti C, Gamalero R, Ghi P (2002) Histamine H3-receptor blockade in the rat nucleus basalis magnocellularis improves place recognition memory. Psychopharmacology (Berl) 159: 133-137.
- Kubo M, Kishi T, Matsunaga S, Iwata N (2015) Histamine H3 receptor antagonists for Alzheimer's disease: A systematic review and meta-analysis of randomized placebo-controlled trials. J Alzheimers Dis 48: 667-671.
- Passani MB, Blandina P (2011) Histamine receptors in the CNS as targets for therapeutic intervention. Trends in Pharmacological Sciences 32: 242-249.
- Pollard H, Moreau J, Arrang JM, Schwartz JC (1993) A detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience 52: 169-189.
- Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M (1991) Histaminergic transmission in the mammalian brain. Physiol Rev 71: 1-51.
- Haas HL, Sergeeva OA, Selbach O (2008) Histamine in the nervous system. Physiol Rev 88: 1183-1241.
- Molinengo L, Di Carlo G, Ghi P (1999) Combined action of thioperamide plus scopolamine, diphenhydramine, or methysergide on memory in mice. Pharmacol Biochem Behav 63: 221-227.
- Zlomuzica A, Ruocco LA, Sadile AG, Huston JP, Dere E (2009) Histamine H1 receptor knockout mice exhibit impaired spatial memory in the eight-arm radial maze. Br J Pharmacol 157: 86-91.
- Li B, Zhu JN, Wang JJ (2014) Histaminergic afferent system in the cerebellum: Structure and function. Cerebellum Ataxias 1: 5.
- Hasenohrl RU, Weth K, Huston JP (1999) Intraventricular infusion of the histamine H-1 receptor antagonist chlorpheniramine improves maze performance and has anxiolytic-like effects in aged hybrid Fischer 344x Brown Norway rats. Experimental Brain Research 128: 435-440.
- Masuoka T, Kamei C (2007) Role of hippocampal H1 receptors in radial maze performance and hippocampal theta activity in rats. Brain Research Bulletin 73: 231-237.
- Brown RE, Haas HL (1999) On the mechanism of histaminergic inhibition of glutamate release in the rat dentate gyrus. J Physiol 515: 777-786.
- Brown RE, Reymann KG (1996) Histamine H3 receptor-mediated depression of synaptic transmission in the dentate gyrus of the rat in vitro. J Physiol 496: 175-184.
- Itoh Y, Oishi R, Nishibori M, Saeki K (1991) Characterization of histamine release from the rat hypothalamus as measured by in vivo microdialysis. Journal of Neurochemistry 56: 769-774.
- Bitiktas S, Tan B, Kavraal , Yousef M, Bayar Y (2017) The effects of intra-hippocampal L-thyroxine infusion on long-term potentiation and long-term depression: A possible role for the alphavbeta3 integrin receptor. J Neurosci Res 95: 1621-1632.
- Artis A, Bitiktas S, Ta Dolu N, Liman N, et al. (2012) Experimental hypothyroidism delays field excitatory postÃÂsynaptic potentials and disrupts hippocampal longÃÂterm potentiation in the dentate gyrus of hippocampal formation and ÂÃÂmaze performance in adult rats. Journal of Neuroendocrinology 24: 422-433.
- Suer C, Dolu N, Artis AS, Sahin L, Aydogan S (2011) Electrophysiological evidence of biphasic action of carnosine on long-term potentiation in urethane-anesthetized rats. Neuropeptides 45: 77-81.
- Chang M, Saito H, Abe K (1998) Histamine H3 receptor-mediated inhibition of excitatory synaptic transmission in the rat dentate gyrus in vivo. Jpn J Pharmacol 77: 251-255.
- Medhurst AD, Atkins AR, Beresford IJ, Brackenborough K, Briggs MA, et al. (2007) GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer's disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther 321: 1032-1045.
- Yanovsky Y, Haas HL (1998) Histamine increases the bursting activity of pyramidal cells in the CA3 region of mouse hippocampus. Neurosci Lett 240: 110-112.
- Drutel G, Peitsaro N, Karlstedt K, Wieland K, Smit MJ, et al. (2001) Identification of rat H3 receptor isoforms with different brain expression and signaling properties. Mol Pharmacol 59: 1-8.
- English JD, Sweatt JD (1996) Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem 271: 24329-24332.
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, et al. (2002) A detailed mapping of the histamine H 3 receptor and its gene transcripts in rat brain. Neuroscience 114: 173-193.
- Navarro CE, Otoya R, Donoso AO (1993) Stimulation of H3-Histamine Receptors Increases the Release of Prolactin in Male-Rats. Neuroendocrinology 57: 654-657.
- Zhang W, Linden DJ (2003) The other side of the engram: Experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci 4: 885-900.
- Taube JS, Schwartzkroin PA (1988) Mechanisms of long-term potentiation: EPSP/spike dissociation, intradendritic recordings, and glutamate sensitivity. J Neurosci 8: 1632-1644.
- Chavez-Noriega LE, Halliwell JV, Bliss TV (1990) A decrease in firing threshold observed after induction of the EPSP-spike (E-S) component of long-term potentiation in rat hippocampal slices. Exp Brain Res 79: 633-641.
- Bell M, Richardson P, Lee K (2000) Histamine depolarizes cholinergic interneurones in the rat striatum via a H1ÃÂreceptor mediated action. British Journal of Pharmacology 131: 1135-1142.
- Gorelova N, Reiner PB (1996) Histamine depolarizes cholinergic septal neurons. J Neurophysiol 75: 707-714.
- Mccormick DA, Williamson A (1991) Modulation of neuronal firing mode in cat and guinea-Pig ligand by histamine-possible cellular mechanisms of histaminergic control of arousal. Journal of Neuroscience 11: 3188-3199.
- Lintunen M, Sallmen T, Karlstedt K, Fukui H, Eriksson KS, et al. (1998) Postnatal expression of H1-receptor mRNA in the rat brain: correlation to L-histidine decarboxylase expression and local upregulation in limbic seizures. Eur J Neurosci 10: 2287-2301.
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, et al. (2002) A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience 114: 173-193.
- Brown RE, Stevens DR, Haas HL (2001) The physiology of brain histamine. Prog Neurobiol 63: 637-672.
- Frey U, Huang YY, Kandel ER (1993) Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260: 1661-1664.
- Payne GW, Neuman RS (1997) Effects of hypomagnesia on histamine H1 receptor-mediated facilitation of NMDA responses. Br J Pharmacol 121: 199-204.
- Saybasili H, Stevens DR, Haas HL (1995) pH-dependent modulation of N-methyl-D-aspartate receptor-mediated synaptic currents by histamine in rat hippocampus in vitro. Neurosci Lett 199: 225-227.
- Fleck MW, Hirotsune S, Gambello MJ, Phillips-Tansey E, Suares G, et al. (2000) Hippocampal abnormalities and enhanced excitability in a murine model of human lissencephaly. J Neurosci 20: 2439-2450.
- Vohora D, Bhowmik M (2012) Histamine H3 receptor antagonists/inverse agonists on cognitive and motor processes: relevance to Alzheimer's disease, ADHD, schizophrenia, and drug abuse. Front Syst Neurosci 6: 72.
- Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, et al. (2005) Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther 313: 176-190.
- Fox GB, Pan JB, Faghih R, Esbenshade TA, Lewis A, et al. (2003) Identification of novel H3 receptor (H3R) antagonists with cognition enhancing properties in rats. Inflamm Res 52: S31-S32.
- Foley AG, Prendergast A, Barry C, Scully D, Upton N, et al. (2009) H3 receptor antagonism enhances NCAM PSA-mediated plasticity and improves memory consolidation in odor discrimination and delayed match-to-position paradigms. Neuropsychopharmacology 34: 2585-2600.
- Orsetti M, Ghi P, Di Carlo G (2001) Histamine H(3)-receptor antagonism improves memory retention and reverses the cognitive deficit induced by scopolamine in a two-trial place recognition task. Behav Brain Res 124: 235-242.
- Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, et al. (2010) Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcohol Clin Exp Res 34: 1793-1802.
- Monti JM, Jantos H, Boussard M, Altier H, Orellana C, et al. (1991) Effects of selective activation or blockade of the histamine H3 receptor on sleep and wakefulness. Eur J Pharmacol 205: 283-287.
- Guo RX, Anaclet C, Roberts JC, Parmentier R, Zhang M, et al. (2009) Differential effects of acute and repeat dosing with the H3 antagonist GSK189254 on the sleep-wake cycle and narcoleptic episodes in Ox-/- mice. Br J Pharmacol 157: 104-117.
- Vanni-Mercier G, Gigout S, Debilly G, Lin JS (2004) Waking selective neurons in the posterior hypothalamus and their response to histamine H3-receptor ligands: an electrophsiological study in freely moving cats. Behavioural brain research 152: 163.
- Barbier AJ, Berridge C, Dugovic C, Laposky AD, Wilson SJ, et al. (2004) Acute wake-promoting actions of JNJ-5207852, a novel, diamine-based H3 antagonist. Br J Pharmacol 143: 649-661.
- Diekelmann S, Born J (2010) The memory function of sleep. Nature Reviews Neuroscience 11: 114.
- Hajos M, Siok CJ, Hoffmann WE, Li S, Kocsis B (2008) Modulation of hippocampal theta oscillation by histamine H3 receptors. J Pharmacol Exp Ther 324: 391-398.
- Pavlides C, Greenstein YJ, Grudman M, Winson J (1988) Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res 439: 383-387.







