Mohamed Z Sayed-Ahmed1,2*, Hafiz A Makeen2, Mohsen M Elsherbini3,4, Nabeel K Syed2 and Sherif M Shoeib5
1Department of Internal Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
2Department of Clinical Pharmacy, College of Pharmacy, Jazan University, Jizan, Saudi Arabia
3Department of Histology and Cell Biology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
4Department of Health Informatics, Faculty of Public health and Tropical Medicine, Jazan University, Jizan, Saudi Arabia
5Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Mansoura University, Mansoura , Egypt
*Corresponding Author:
Mohamed Z Sayed-Ahmed
Department of Internal Medicine and Infectious Diseases
Faculty of Veterinary Medicine
Mansoura University, Mansoura 35516, Egypt
Tel: 00966-594-886878
Fax: 00966-17-3216837
E-mail: drzakaria-infect@hotmail.com
Received Date: 08 July 2018; Accepted Date: 28 July 2018; Published Date: 08 August 2018
Citation: Sayed-Ahmed MZ, Makeen HA, Elsherbini MM, Syed NK, Shoeib SM (2018) Oncolytic Viruses: A gene Therapy for Treatment of Cancer in Companion Animals. Health Sci J Vol.12.No.4:579.
Copyright: © 2018 Sayed-Ahmed MZ, et al. This is an open-access article distributed under the terms of the creative commons attribution license, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Keywords
Cancer; Canine; Oncolytic virus; Oncolysis; Companion animals
Abbreviations
OVs: Oncolytic Viruses; CDV: Canine Distemper Virus; RGD: Arginine- Glycine-Aspartic Acid; CAR: Coxackie-and Adenovirus Receptor; Ad: Adenoviruses; CAV-2: Canine Adenovirus 2; MV: Measles Virus; MCT: Mast Cell Tumors; VACV: Vaccinia Virus; VEGF: Vascular Endothelial Growth Factor
Introduction
Cancer is one of the most common causes of natural death in dogs in both developed and developing countries. It is among the top deadly diseases in dogs and cats [1-3]. The incidence of canine cancer ranges from 1% to 2% and currently accounts half of the death in the canine population older than 10 years [1,4,5]. In contrast to the progress of human oncolytic virotherapy, there are very few clinical trials using OVs for canine or feline cancer patients [6,7]. The most common forms of cancer in dogs and cats are skin, lymphoma, mammary, bone, connective tissue, and oral cancers [8,9]. Treatment of pet cancer with advanced disease stages is very poor prognosis by major traditional options for canine cancers treatment includes surgery, chemotherapy, radiation therapy, and hyperthermia. Therefore, developing unique cancer therapies is essential for work synergistically in combination with the conventional treatment options. Since many forms of canine or feline neoplasms resemble their human counterparts in histological appearance, tumor genetics, biologic behavior, pathologic expression, recognized risk factors and response to therapy [10,11], it is reasonable to expect that the human clinical protocols will transfer directly to the treatment of pet cancer patients. In human, Several Oncolytic viruses (OVs) including adenovirus, vaccinia virus, herpes simplex virus, Seneca Valley virus and reovirus are currently entering Phase III human clinical trials [12,13]. In contrast to the progress of human oncolytic virotherapy, there are very few clinical trials using OVs for canine cancer patients [14,15]. A mouse was considered the first animal model to demonstrate full regression through viral oncolysis, Alice Moore was the first scientist tested Russian Far East encephalitis virus in some cases of mouse sarcoma 180 [16]. However, the use of oncolytic virotherapy in veterinary medicine is far from reality and present are also the challenges and the major obstacles to the optimal practice of oncolytic virotherapy in canine cancer patients. This review describes the most common classes of oncolytic viruses for canine cancer therapy and focuses on ways of their progress in preclinical studies with canine cancers.
Cancer gene therapy
Gene therapy is a field of research aiming to treat diseases caused by defective genes by altering the genomes of cells and tissues [17]. Disease entities for which gene therapy is being developed include for example cancer, cardiovascular disease, neurological diseases, hematological diseases, monogenic inherited diseases and infectious diseases. By June 2014, a total of 2076 clinical gene therapy trials had been initiated. The vast majority of these (64%) were aimed at cancer gene therapy.
Cancer gene therapy approaches fall into four main strategies
Insertion of a normal gene into cancer cells to replace a mutated gene: For example, a mutation in a p53 protein, which interferes with the ability of tumor cells to destruct themselves by apoptosis, is found in most of the cancers [18].
Silence a mutated gene which is activated or overexpressed in cancer cells: Such oncogenes can for example drive tumor growth, blood vessel formation, induce metastasis to other tissues, and allow for resistance to chemotherapy. Silencing can be accomplished by using e.g., small interfering RNA (siRNA) silencing technology, which has been used to specifically target, for example, tumor suppressor p53 molecules containing a single point mutation, leaving the wild-type suppressor intact [19].
Introducing genes that make cancer cells more sensitive to standard chemotherapy or for radiation treatments: Drug convertases (“suicide genes”) which can turn an inactive prodrug into an active drug which can be introduced to tumor cells to cause cell-specific toxicity. For example, the herpes virus thymidine kinase can phosphorylate and convert nontoxic drug ganciclovir into toxic metabolites [20].
Direct cell killing with targeted viruses: After genetic engineering, oncolytic viruses selectively replicate in cancer cells leading to tumor cell destruction and oncolysis [21].
Oncolytic viruses as a gene therapy for cancer
Different approaches utilizing viruses have been used for cancer treatment for several decades. Non-replicating or replicating viruses can be used as a gene transfer vector to introduce for example a therapeutic gene, co-stimulatory molecule or cytokine into cancer cells or to prime lymphocytes with tumor antigens in cancer vaccine approaches [21]. The initial reports of virus-induced oncolysis date back to 1904 (DOCK) and 1912 (DE PACE), respectively, and referred tumor regression of human cervical carcinomas after rabies vaccination. In the 1920s animal experiments confirmed that viruses were capable of infecting and lysing experimental murine tumors and several studies followed in the 1950s demonstrating potent oncolysis of murine tumors by Newcastle disease virus and Influenza virus [22]. Studies of human cancer were initiated in the 1950s. Perhaps the most recognized of these investigations was one report from the National Cancer Institute in 1956, in which wild-type adenoviruses of different serotypes were injected in patients with cervical carcinoma [23]. There are two important aspects to oncolytic virotherapy; there is a direct treatment of tumors with replicating oncolytic viral vectors alone or in combination with therapeutic transgene delivery, chemotherapy, or radiation therapy. On the other hand, there is indirect increase of antitumor immunity through a modulation of the immune response, as with viral oncolysate vaccine, and tumorprotective monoclonal antibodies [24].
By June 2014, viruses were being used as vector systems in approximately two-thirds of all gene therapy trials. Out of different virus vectors, adenoviruses (23%) and retroviruses (19%) have been reported as the most commonly used vectors (Figure 1) [21].
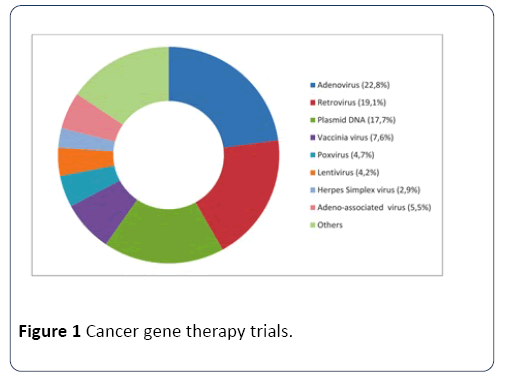
Figure 1: Cancer gene therapy trials.
By February 2018, a gene therapy trial that recently led to dramatic benefits for babies born with a fatal neuromuscular condition has raised hopes for using a similar approach to treat other diseases. But a new animal study suggests that the high doses (2x10E14 genome copies per kilogram body weight) of gene-carrying viruses used in such treatments may not always be as safe as the human clinical trial indicated. In the new research, the disclosure of which briefly sent the stock prices of several gene therapy companies plummeting yesterday, researchers injected a handful of young monkeys and pigs with many copies of adeno-associated virus 9 (AAV9), a normally harmless virus that infects neurons and is increasingly being used to ferry therapeutic genes into cells to treat neuromuscular diseases. Within days, some of the animals developed severe liver and neuron damage.
Oncolytic viruses are distinguished by their property to either inherently or after genetic modification replicates selectively in cancer cells. These viruses have multiple mechanisms to harm the host cells including direct lysis, induction of apoptosis and autophagy, expression of toxic proteins and shut-down of protein synthesis. At the end of the replication cycle, cells are destroyed and infective viral progeny is released into remaining tumor tissue. In addition to local amplifying antitumor effect, infective viral particles are able to enter the systemic circulation and infect distant metastasis (Figure 2) [21,25]. In addition to naturally occurring oncolytic viruses such as reovirus [26]. Several human DNA and RNA viruses such as measles virus (MV), vesicular stomatitis virus (VSV), adenovirus, vaccinia virus (VV) and herpes simplex virus (HSV) have been genetically modified to selectively replicate in tumor cells, while their activity in normal cells is attenuated [25,27].
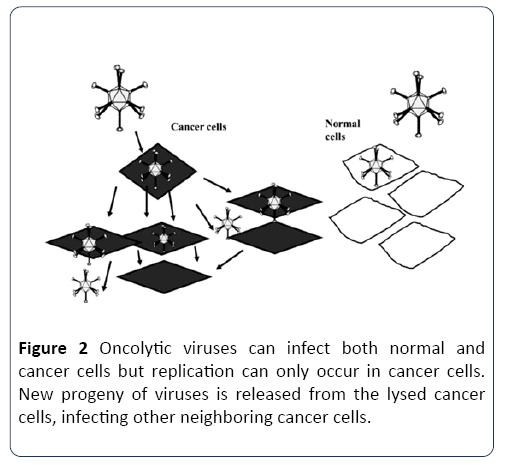
Figure 2: Oncolytic viruses can infect both normal and cancer cells but replication can only occur in cancer cells. New progeny of viruses is released from the lysed cancer cells, infecting other neighboring cancer cells.
Adenoviruses
Adenoviruses are non-enveloped, double-stranded DNA viruses of approximately 90 nm in diameter. The virus is protected by an icosahedral protein capsid consisting of Penton and hexon proteins, knobbed fiber proteins extended from the twelve vertices. Each penton protein has flexible loops on its surface, featuring an arginine-glycine- aspartic acid (RGD) motif which is involved in cellular binding and internalization [28]. Adenovirus enters the cells by binding to a high-affinity cell surface receptor with its fiber knob. Most adenovirus species have been shown to bind to coxsackie and adenovirus receptor (CAR), which triggers secondary interaction with RGD motif and cellular αvβ-integrins leading to endocytosis via clathrin-coated pits [29,30]. The adenovirus life cycle can be divided into two phases separated by the onset of viral DNA replication.
Adenoviruses (Ad) are one of the most commonly used vectors for cancer gene therapy. Adenoviruses were first identified in the 1950s and ever since they have been intensively studied as gene therapy vectors [31]. Adenoviridae family can be divided into 4 genera and 6 species [32] and so far 59 serotypes of human adenoviruses have been identified [33]. The various serotypes have been further classified into subgroups A-G, depending on their ability to agglutinate erythrocytes [34]. In general, adenoviruses are endemic in most parts of the world and have low pathogenicity in humans. Different serotypes have been shown to have different pathological effects but typically adenoviruses infect the epithelial cells in the respiratory and gastrointestinal tract or the eyes causing mild flu, conjunctivitis and infantile gastroenteritis [35,36].
Canine adenovirus 2 (CAV-2), by inserting osteocalcin promoter showed restricted replication in canine osteosarcoma cells. Osteocalcin promoter is active only in osteosarcoma cells and not active in other canine nonneoplastic cells. This promoter was tested as a therapeutic agent for canine osteosarcoma [37]. In addition, administration of this modified canine adenovirus to normal dogs showed only moderate virus-associated toxicity and showed therapeutic benefits in the xenograft model in killed canine osteosarcoma cells in cell culture [37,38]. The expression of heterologous genes by canine adenoviruses can lead to enhanced therapeutic activity. IntratumoralInjections of this adenoviral vector AdCD40L exhibited complete tumor regression in 5 of 19 canine melanoma patients [39].
Vaccinia viruses
Vaccinia is a genetically complex double-stranded DNA virus, characterized as brick-shaped particles with a size of approximately 300 × 240 × 120 nm [40]. Infectious vaccinia virus particles have a lipoprotein envelope surrounding a complex core of linear double-stranded DNA (191 636 bp, encodes for ~250 genes) [41]. Vaccinia encodes all the proteins it needs for its replication in its genome, some of which have immune evading properties allowing the virus to establish infection [42]. Vaccinia virus enters the cell via fusion of viral and cellular membranes, which is mediated by entry-fusion complex [43,44]. No specific receptor to facilitate entry of the virus into the cell has yet been discovered. After the entry, viral particles are uncoated, and transcription of early genes by the viral RNA polymerase starts followed by the expression of intermediate and late genes [45].
In 1798, Edward Jenner noticed that milkmaids exposed to cowpox developed protection against smallpox [46]. Smallpox was caused by variola, a member of the poxvirus family. This finding eventually leads to the development of a laboratory strain of poxvirus, vaccinia virus, used as a vaccine in the Smallpox Eradication Program led by the World Health Organization [47,48].
Vaccinia is a member of the Orthopoxvirus genus and is its most extensively studied member. It was the first mammalian virus to be visualized microscopically, successfully grown in tissue culture, titrated accurately, purified physically and analyzed biochemically [40]. Due to this historical role, vaccinia virus has the longest and most extensive history of use in humans of any virus and has had a major impact on the development of vaccines. Wild-type vaccinia virus has been used in hundreds of millions of humans as a vaccine for the eradication of smallpox and has shown a good safety profile as only rare serious side effects have been reported during the vaccination program [49]. Although smallpox has been completely eradicated from the 1980s onwards, vaccinia virus has been studied as a viral vector for the development of cancer virotherapy, immunotherapies, as well as the development of next generation smallpox vaccines due to its strong safety profile and high immunogenicity [50].
Morbilliviruses
Canine distemper virus (CDV) is an enveloped, negativesense single stranded RNA virus of the family Paramyxoviridae, closely related to human measles and rinderpest virus, that infects different cell types, including epithelial, mesenchymal, neuroendocrine and hematopoietic cells of various organs and tissues [51,52]. It is a close relative of measles virus (MV). CDV is capable of infecting canine lymphoid cell lines, histiocytic sarcoma cell lines, such as DH82 cells, and neoplastic lymphocytes in vitro, commonly inducing apoptosis of tumour cells [53,54]. Historically, children with Hodgkin’s disease were observed to experience regression after concurrent MV infection [55]. These observations prompted the consideration of attenuated MV for the treatment of human lymphoma and, consequently, measles virus has shown promising anti-tumor activity against a variety of malignant neoplasms in both preclinical and clinical studies [56]. Because of its similarity to MV, CDV was considered for treatment of canine lymphoma. CDV binds to the cellular receptor, the signaling lymphocyte activation molecule (SLAM or CD150) [57], which is overexpressed on malignant canine B and T lymphocytes [58]. Attenuated CDV was able to infect canine lymphoma cells in cell culture via binding to CD150 and to induce apoptosis in these cells [58]. While preliminary, these results support the continued evaluation of CDV for the treatment of canine lymphoma.
Coxsackie virus
Coxsackie virus is an enterovirus belonging to the Picornaviridæ family of nonenveloped viruses containing a linear, positive sense, single-stranded RNA genome. Because RNA viruses replicate in the host cytosol without a DNA phase, insertional mutagenesis is not possible. Coxsackie viruses are divided into two subgroups, A and B, based on pathogenicity in mice. At least 23 serotypes of group A and six serotypes of group B have been described. Coxsackie viruses are considered to be a minor human pathogen. Young children, aged five years and under, are more susceptible to coxsackie virus A disease, often produced by serotype A16. Infection of individuals occurs mainly via entry through exposed areas, such as the skin and mucosal surfaces (i.e., hands, feet, mouth, throat, and eyes). However, in most cases, infection is asymptomatic or elicits only mild disease associated with “common cold-like” symptoms [59-61]. Various nonengineered strains of coxsackie virus from both groups are currently being tested as single oncolytic therapeutics or in combination with conventional chemotherapy drugs.
In preclinical models, coxsackie virus B3 (CVB3) exhibited potent tumor cell lysis in a number of non-small cell lung cancer cell lines, even in cancer cells refractory to conventional radiotherapy and molecular targeted therapies [62]. Furthermore, deployment of coxsackie viruses into the local tumor environment induced productive cell spread and promoted immunogenic cytotoxicity following tumor oncolysis.
Reovirus
Reovirus, is a segmented double-stranded RNA virus, has been extensively tested in oncolytic virotherapy of human cancers over the past decade. While its clinical development has advanced into phase II and III clinical trials with human cancer patients, it has been hardly studied as a canine or feline oncolytic agent. A very recent study demonstrated for the first time that canine mast cell tumors (MCT) were highly susceptible to reovirus infection in vitro [63]. In addition, a single intratumoral reovirus injection significantly regressed canine mast cell tumor xenografts [63]. However, reovirus also infected normal canine mast cells raising safety concerns. Subsequent studies should address these concerns before using reovirus as an oncolytic agent for canine cancer therapy.
Possible mechanisms of oncolytic virusmediated tumor ablation
An oncolytic virus destroys tumors either by direct viral lysis of tumor cells [64,65], by the destruction of the tumor vasculature [65], by induction of host antitumoral immune responses [66,67] or most likely, a combination of these mechanisms [68] (Figure 3). An increased infiltration of neutrophils, macrophages and natural killer (NK) cells to the tumor site might be involved in the vaccinia virus (VACV)- mediated immune response in different canine cancer xenograft models [69,70]. The presence of such activated inflammatory cells in the tumor tissue may enhance the antitumoral effect by increasing the phagocytic or cytotoxic activities of these cells [71,72]. In addition, an increase in proinflammatory interferon-gamma (IFN-gamma), interleukin-2 (IL-2), interleukin-6 (IL-6), tumor necrosis factoralpha (TNF-alpha), interferon gamma-induced protein 10 (IP-10), macrophage inflammatory protein-1 alpha (MIP-1 alpha), macrophage inflammatory protein-1 beta (MIP-1 beta), monocyte chemotactic protein-1 (MCP-1), and monocyte chemotactic protein-3 (MCP-3) was observed in vaccinia virusinfected canine xenografted mice [73]. Many of these proteins stimulate innate immunity mediated by dendritic cells, neutrophils, macrophages and NK cells. OVs naturally prevent neoangiogenesis either by direct infection and destruction of tumor vasculature [74] or “vascular normalization” in tumor tissue, as described by Winker and colleagues [75].
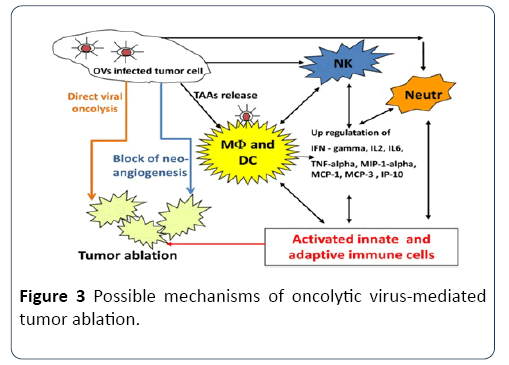
Figure 3: Possible mechanisms of oncolytic virus-mediated tumor ablation.
Moreover, oncolytic viruses can be additionally armed to enhance their natural antiangiogenic ability. Vascular Endothelial Growth Factor (VEGF) is a key regulator of tumor angiogenesis and several anti-VEGF strategies have been developed for the treatment of different cancers [76,77]. Vaccinia virus expressing anti-VEGF antibodies significantly decreased neoangiogenesis at the tumor site and inhibited tumor growth in canine and feline xenografts [78,79]. Thus, strategies employing virus-encoded and delivered anti-VEGF antibodies in combination with OV may be effective therapeutic approaches for pet cancer patients (Table 1).
| Virus species |
Virus strain |
Genetic/synthetic modifications |
Tumor selectivity |
Immune function |
References |
| Advantage |
Disadvantage |
Advantage |
Disadvantage |
|
| Adenovirus |
Ad5/Ad3 or Ad5/Ad35 |
Engineered expression of fiber-knob proteins |
Virus binds to tumor cells; avoids sequestration in liver Cells |
Limited tumor infection |
Ad as a single agent produces poor antitumor immunity; combined use of immunomodulatory agents enhances antitumor effect |
Antiviral immunity limits efficacy |
84-86 |
| Pseudo typed viruses (eg, Ad5 delta-24RGD) |
CAR/integrin-binding deleted Ad, added receptor tumor-targeting ligand |
Enhanced tumor specificity |
Attenuated viral spread |
|
| Vaccinia |
|
Coating with polyethylene glycine or other polymers; encapsulation with liposomes |
Avoid immune detection and viral clearance in the bloodstream |
Minimal inherent tumor selectivity; mild virus related infection |
Viral-mediated immunogenic cytotoxicity |
Potential for antiviral immunity |
87-90 |
| JX-594 |
TK-deletion GM-CSF (+) |
Productive replication in tumor cells; enhanced viral spread |
Mild virus-related infection |
Enhanced tumor infiltration of eosinophils, APC and CTLs |
Stimulates potent cellular and humoral immune response to transgene |
|
| Canine Distemper Virus |
CDV |
|
|
|
Natural tumor tropism as cellular receptor for entry CD46 is expressed on tumor cells. |
Only lymphoma therapy data |
53 |
| |
|
|
|
Good safety records as included in vaccine schedule of canines |
|
|
| Herpes simplex virus |
G207 |
ICP-6-deletion γ34.5-deletion |
Replicates only in cells with E1B-19K deletion (ie, anti-apoptotic factor) |
Latent infection is possible with native virus |
Co-treatment of virus with cyclophosphamide to abrogate host antiviral immunity |
Potential suppression of oncolytic virus-mediated antitumor immunity |
91-93 |
| Coxsackie-virus |
CVA13, CVA15, CVA18, CVA21, CVB3 |
Nonengineered |
Inherently tumor-selective strain Cancer-selective receptors ICAM-1, DAF, and CAR |
Potential for systemic toxicity |
Native virus induces strong immunity exposure |
Many patients have prior antiviral immunity |
94-98 |
| Reovirus |
Reolysin |
Nonengineered |
Inherently tumor-selective species, only replicates in cells with activated Ras-pathway and defective PKR |
Potential for mild toxicity |
Antigenicity generates immune response Antitumor response can be enhanced with chemotherapies |
Potential for antiviral immunity |
99–102 |
Table 1 Advantages and disadvantages of oncolytic viruses for cancer therapy.
For instance, we could envision administering OVs first by a series of intravenous infusions to ensure maximum distribution of the OV to all metastatic sites followed by multiple intratumoral in situ vaccine boosts. This type of administration regimen has already been piloted with an oncolytic vaccine [80]. The use of recombinant OVs as clinical biotherapies, it is important to determine whether viremia could be induced that could result in shedding of the OV. The use of Ad5-prime/MG1-booster vaccination as a promising, novel therapy for testing in the context of veterinary clinical trials [81-83].
Mä: Macrophages; NK: Natural Killer Cells; DC: Dendritic Cells; Neutr: Neutrophils; IFN-gamma: Interferon-gamma; IL-2: Interleukin-2: IL-6: Interleukin-6; TNF-alpha: Tumor Necrosis Factor Alpha: IP-10: Interferon Gamma-Induced Protein 10; MIP-1 Alpha: Macrophage Inflammatory Protein-1; MCP-1: Monocyte Chemotactic Protein-1; MCP-3: Monocyte Chemotactic Protein-3; TAA: Tumor-Associated Antigens
Limitations and prospects of cancer gene therapy
Despite some promising results in cancer gene therapy, there are many limitations to overcome [84-90]. Only a limited number of therapeutic genes can be used in clinical trials. Vectors are not efficient in vivo. Although the high transfection efficiency with adenovirus in vitro is well documented, it is still not clear whether adenoviral vectors are effective in vivo in solid tumor models [91-96].
Conclusion
The significant incidence and mortality associated with cancers continue to challenge modern medicine to develop more reliable therapies. One of the most promising novels cancer therapies is oncolytic virotherapy. This method is based on the capability of OVs to preferentially infect and lyse cancer cells and to initiate tumor-specific immunity. Several oncolytic viruses including human and canine adenoviruses, canine distemper virus (CDV), reovirus and vaccinia virus strains have been tested with convincing results in preclinical studies. As for oncolytic virotherapy of human cancers, the most important challenges for the successful clinical use of OVs in veterinary practice are a reduction of viral toxicity, optimization of virus delivery to the tumor, and enhancement of viral spread throughout the tumor mass. Recently, the first clinical studies with vaccinia and adenovirus for canine cancer therapy are underway and we look forward to the forthcoming demonstrations of clinical utility.
Declarations
Availability of data and materials
The findings were declared from available data source. All possible required information are attached and included in the manuscript. Moreover, raw data is available in the hand of the corresponding author. All coauthors gave full responsibility for corresponding author to share or discus with editors and reviewers in review process.
Acknowledgments
None of the authors of this article has a financial or personal relationship with other people or organizations that could appropriately influence or bias the content of the article. There are no funding sources for this study.
23108
References
- Merlo DF, Rossi L, Pellegrino C, Ceppi M, Cardellino U, et al. (2008) Cancer incidence in pet dogs: Findings of the animal tumor registry of Genoa, Italy. J Vet Intern Med 22: 976-984.
- Gobar GM, Case JT, Kass PH (1998) Program for surveillance of causes of death of dogs, using the Internet to survey small animal veterinarians. J Am Vet Med Assoc 213: 251-256.
- Animal Health Survey in Companion Animal News, Englewood Colorado. Morris Animal Foundation; 2005.
- Hansen K, Khanna C (2004) Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer 40: 858-880.
- Kelsey JL, Moore AS, Glickman LT (1998) Epidemiologic studies of risk factors for cancer in pet dogs. Epidemiol Rev 20: 204-217.
- Patil SS, Gentschev I, Nolte I, Ogilvie G, Szalay AA (2012) Oncolytic virotherapy in veterinary medicine: Current status and future prospects for canine patients. J Transl Med 10: 3.
- Jourdier TM, Moste C, Bonnet MC, Delisle F, Tafani JP, et al. (2003) Local immunotherapy of spontaneous feline fibrosarcomas using recombinant poxviruses expressing interleukin 2 (IL2). Gene Ther 10: 2126-2132.
- Paoloni M, Khanna C (2008) Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer 8: 147-156.
- Tang J, Le S, Sun L, Yan X, Zhang M, et al. (2010) Copy number abnormalities in sporadic canine colorectal cancers. Genome Res 20: 341-350.
- Bell J, McFadden G (2014) Viruses for tumor therapy. Cell Host Microbe 15: 260-265.
- Russell SJ, Peng KW, Bell JC (2012) Oncolytic virotherapy. Nat. Biotechnol 30: 658-670.
- Moore AE (1949) The destructive effect of the virus of Russian Far East encephalitis on the transplantable mouse sarcoma 180. Cancer 2: 525-534.
- Mulligan RC (1993) The basic science of gene therapy. Sci 260: 926-932.
- Sherr CJ, McCormick F (2002) The RB and p53 pathways in cancer. Cancer Cell 2: 103-112.
- Martinez LA, Naguibneva I, Lehrmann H, Vervisch A, Tchenio T, et al. (2002) Synthetic small inhibiting RNAs: efficient tools to inactivate oncogenic mutations and restore p53 pathways. Proc Natl Acad Sci 99: 14849-14854.
- Nicholas TW, Read SB, Burrows FJ, Kruse CA (2003) Suicide gene therapy with Herpes simplex virus thymidine kinase and ganciclovir is enhanced with connexins to improve gap junctions and bystander effects. Histol Histopathol 18: 495-507.
- Sinkovics J, Horvarth J (1993) New developments in the virus therapy of cancer: a historical review. Intervirology 36: 193-214.
- Smith RR, Huebner J, Rowe WP (1956) Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer, pp: 1211-1218.
- Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, et al. (2002) Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of auto reactivity in mice. Cancer Immun 2: 1.
- Mullen JT, Tanabe KK (2003) Viral oncolysis for malignant liver tumors. Ann Surg Oncol 10: 596-605.
- Roberts MS, Lorence RM, Groene WS Bamat MK (2006) Naturally oncolytic viruses. Curr Opin Mol Ther 8: 314-321.
- Kelly E, Russell SJ (2007) History of oncolytic viruses: genesis to genetic engineering. Mol Ther 15: 651-659.
- Stewart PL, Burnett RM, Cyrklaff M, Fuller SD (1991) Image reconstruction reveals the complex molecular organization of adenovirus. Cell 67: 145-154.
- Mathias P, Wickham T, Moore M, Nemerow G (1994) Multiple adenovirus serotypes use alpha v integrins for infection. J Virol 68: 6811-6814.
- Roelvink PW, Lee GM, Einfeld DA, Kovesdi I, Wickham TJ (1999) Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286: 1568-1571.
- Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG (1953) Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 84: 570-573.
- Davison AJ, Benko M, Harrach B (2003) Genetic content and evolution of adenoviruses. J Gen Virol 84: 2895-2908.
- Chen WW, Nie WM, Xu W, Xie YX, Tu B, et al. (2014) Cross-sectional study of the relationship of peripheral blood cell profiles with severity of infection by adenovirus type 55. BMC Infect Dis 14: 147.
- Rosen L (1960) A hemagglutination-inhibition technique for typing adenoviruses. Am J Hyg 71: 120-128.
- Berk T (2007) Adenoviridae: the viruses and their replication. Fields Virology (5th edn.) pp: 2355-2394.
- Kunz AN, Ottolini M (2010) The role of adenovirus in respiratory tract infections. Curr Infect Dis Rep 12: 81-87.
- Smith BF, Curiel DT, Ternovoi VV, Borovjagin AV, Baker HJ, et al. (2006) Administration of a conditionally replicative oncolytic canine adenovirus in normal dogs. Cancer Biother Radiopharm 21: 601-606.
- Le LP, Rivera AA, Glasgow JN, Ternovoi VV, Wu H, et al. (2006) Infectivity enhancement for adenoviral transduction of canine osteosarcoma cells. Gene Ther 13: 389-399.
- Westberg S, Sadeghi A, Svensson E, Segall T, Dimopoulou M, et al. (2013) Treatment efficacy and immune stimulation by AdCD40L gene therapy of spontaneous canine malignant melanoma. J. Immunother 36: 350-358.
- Moss B (2001) Poxviridae: the viruses and their replication. Fields Virology. Philadelphia, Lippincott Williams & Wilkins.
- Upton C, Slack S, Hunter AL, Ehlers A, Roper RL (2003) Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J Virol 77: 7590-7600.
- Smith GL (1993) Vaccinia virus glycoproteins and immune evasion. The sixteenth Fleming Lecture. J Gen Virol 74: 1725-1740.
- Carter GC, Law M, Hollinshead M, Smith GL (2005) Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J Gen Virol 86: 1279-1290.
- Senkevich TG, Ojeda S, Townsley A, Nelson GE, Moss B (2005) Poxvirus multiprotein entry-fusion complex. Proc Natl Acad Sci 102: 18572-18577.
- Moss B (2012) Poxvirus cell entry: how many proteins does it take? Viruses 4: 688-707.
- Lakhani S (1992) Early clinical pathologists: Edward Jenner (1749-1823). J Clin Pathol 45: 756-758.
- Theves C, Biagini P, Crubezy E (2014) The rediscovery of smallpox. Clin Microbiol Infect 20: 210-218.
- Halsell JS, Riddle JR, Atwood JE, Gardner P, Shope R, et al. (2003) Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 289: 3283-3289.
- Verardi PH, Titong A, Hagen CJ (2012) A vaccinia virus renaissance: new vaccine and immunotherapeutic uses after smallpox eradication. Hum Vaccin Immunother 8: 961-970.
- Beineke A, Puff C, Seehusen F, Baumgartner W (2009) Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet Immunol Immunopathol 127: 1-18
- Sayed-Ahmed M (2011) Influence of paramyxovirus infection on phenotypical and functional characteristics of canine histiocytic sarcoma cells (DH82). Thesis, (Dr. Med. Vet.), University of Veterinary Medicine Foundation, Hannover, Germany, pp: 1-203.
- Suter SE, Chein MB, von Messling V, Yip B, Cattaneo R, et al. (2005) In vitro canine distemper virus infection of canine lymphoid cells: a prelude to oncolytic therapy for lymphoma. Clin Cancer Res 11: 1579-1587.
- Pfankuche VM, Sayed-Ahmed M, Contioso VB, Spitzbarth I, Rohn K, et al. (2016) Persistent morbillivirus infection leads to altered cortactin distribution in histiocytic sarcoma cells with decreased cellular migration capacity. PLoS ONE 11: e0167517.
- Sato H, Yoneda M, Honda T, Kai C (2012) Morbillivirus receptors and tropism: Multiple pathways for infection. Front Microbiol 3: 75.
- Bluming AZ, Ziegler JL (1971) Regression of Burkitt’s lymphoma in association with measles infection. Lancet 2: 105-107.
- Russell SJ, Peng KW (2009) Measles virus for cancer therapy. Curr Top Microbiol Immunol 330: 213-241.
- Tatsuo H, Ono N, Tanaka K, Yanagi Y (2000) SLAM (CD150) is a cellular receptor for measles virus. Nature 406: 893-897.
- Buckland FE, Bynoe ML, Tyrrell DA (1965) Experiments on the spread of colds. II. Studies in volunteers with coxsackievirus A21. J Hyg (Lond) 63: 327-343.
- Couch RB, Cate TR, Gerone PJ (1965) Production of illness with a small-particle aerosol of coxsackie A21. J Clin Invest 44: 535-542.
- Spickard A, Evans H, Knight V, Johnson K (1963) Acute respiratory disease in normal volunteers associated with Coxsackie A-21 viral infection. III. Response to nasopharyngeal and enteric inoculation. J Clin Invest 42: 840-852.
- Miyamoto S, Inoue H, Nakamura T (2012) Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res 72 : 2609-2621.
- Hwang CC, Umeki S, Kubo M, Hayashi T, Shimoda H, et al. (2013) Oncolytic reovirus in canine mast cell tumor. PLoS One 8: e73555.
- Chu RL, Post DE, Khuri FR, van Meir EG (2004) Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clin Cancer Res 10: 5299-5312.
- Weibel S, Raab V, Yu YA, Worschech A, Wang E, et al. Viral-mediated oncolysis is the most critical factor in the late-phase of the tumor regression process upon vaccinia virus infection. BMC Cancer 11: 68.
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, et al. (2007) Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow Mol Ther 15: 1686-1693.
- Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, et al. (2009) The case of oncolytic viruses versus the immune system: Waiting on the judgment of Solomon. Hum Gene Ther 20: 1119-1132.
- Toda M, Rabkin SD, Kojima H, Martuza RL (1999) Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum. Gene Ther 10: 385-393.
- Atherton MJ, Lichty BD (2013) Evolution of oncolytic viruses: Novel strategies for cancer treatment. Immunotherapy 5: 1191-1206.
- Gentschev I, Adelfinger M, Josupeit R, Rudolph S, Ehrig K, et al. (2012) Preclinical evaluation of oncolytic vaccinia virus for therapy of canine soft tissue sarcoma. PLoS One 7: e37239.
- Gentschev I, Patil SS, Adelfinger M, Weibel S, Geissinger U, et al. (2013) Characterization and evaluation of a new oncolytic vaccinia virus strain LIVP6.1.1 for canine cancer therapy. Bioengineered 4: 84-89.
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, et al. (2009) Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16: 183-194.
- Moehler MH, Zeidler M, Wilsberg V, Cornelis JJ, Woelfel T, et al. (2005) Parvovirus H-1-induced tumor cell death enhances human immune response in vitro via increased phagocytosis, maturation, and cross-presentation by dendritic cells. Hum. Gene Ther 16: 996-1005.
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, et al. (2007) Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther 15: 1686-1693.
- Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, et al. (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6: 553-563.
- Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9: 669-676.
- Millanta F, Lazzeri G, Vannozzi I, Viacava P, Poli A (2002) Correlation of vascular endothelial growth factor expression to overall survival in feline invasive mammary carcinomas. Vet Pathol 39: 690-696.
- Patil SS, Gentschev I, Adelfinger M, Donat, U, Hess M, et al. (2012) Virotherapy of canine tumors with oncolytic vaccinia virus GLV-1h109 expressing an anti-VEGF single-chain antibody. PLoS One 7: e47472.
- Adelfinger M, Gentschev I, Grimm de Guibert J, Weibel S, Härtl B, et al. (2014) Evaluation of a new recombinant oncolytic vaccinia virus strain GLV-5b451 for feline mammary carcinoma therapy. PLoS One; 9: e104337.
- Breitbach CJ, Lichty BD, Bell JC (2016) Oncolytic Viruses: therapeutics with an identity crisis. Ebio Medicine 9: 31-36.
- Humme J, Bienzle D, Morrison A, Cieplak M, Tephenson K, et al. (2017) Maraba virus-vectored cancer vaccines represent a safe and novel therapeutic option for cats Scientific Reports 7: 15738.
- Roth JA, Cristiano RJ (1997) Gene therapy for cancer: what have we done and where are we going?. J Natl Cancer Inst 89: 21-39.
- Lee SG, Yoon SJ, Kim CD, Kim K, Lim DS, et al. (2000) Enhancement of adenoviral transduction with polycationic liposomes in vivo. Cancer Gene Ther 7: 1329-1335.
- Yamamoto M, Davydova J, Wang M (2003) Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology 125: 1203-1218.
- Green NK, Herbert CW, Hale SJ (2004) Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther 11: 1256-1263.
- Doronin K, Shashkova EV, May SM, Hofherr SE, Barry MA (2009) Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum Gene Ther 20: 975-988.
- Thorne SH, Hwang TH, O’Gorman WE (2007) Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest 117: 3350-3358.
- Thorne SH, Hwang TH, Kirn DH (2005) Vaccinia virus and oncolytic virotherapy of cancer. Curr Opin Mol Ther 7: 359-365.
- Kim JH, Oh JY, Park BH (2006) Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther 14: 361-370.
- Parato KA, Breitbach CJ, Le Boeuf F (2012) The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther 20: 749-758.
- Ikeda K, Ichikawa T, Wakimoto H (1999) Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med 5: 881-887.
- Cantin C, Holguera J, Ferreira L, Villar E, Munoz-Barroso I (2007) Newcastle disease virus may enter cells by caveolae-mediated endocytosis. J Gen Virol 88: 559-569.
- Ockert D, Schirrmacher V, Beck N (1996) Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin Cancer Res 2: 21-28.
- Benencia F, Courreges MC, Conejo-Garcia JR (2005) HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol Ther 12: 789-802.
- Zhang Y, Chirmule N, Gao GP (2001) Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther 3: 697-707.
- Pichlmair A, Reis e Sousa C (2007) Innate recognition of viruses. Immunity 27: 370-383.
- Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S (2010) Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest 120: 1151-1164.
- Prestwich RJ, Errington F, Ilett EJ (2008) Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res 14: 7358-7366.
- Norman KL, Hirasawa K, Yang AD, Shields MA, Lee PW (2004) Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc Natl Acad Sci 101: 11099-11104.
- Errington F, Steele L, Prestwich R (2008) Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol 180: 6018-6026.
- Connolly JL, Rodgers SE, Clarke P (2000) Reovirus-induced apoptosis requires activation of transcription factor NF-kappaB. J Virol 74: 2981-2989.








