Research Article - (2022) Volume 16, Issue 7
Prevalence Of Polycystic Ovarian Syndrome (Pcos) In Women Of Child Bearing Age Within Port Harcourt Metropolis In Nigeria Using Sonographic Evaluation.
Keung Florence M
Nigerian Institute of Radiographers, a Division of the Radiographers Registration Board of Nigeria, Nigeria
1Department of Radiography and Radiation Science, Gregory University Uturu, Nigeria
2Department of Anatomy, University of Uyo, Uyo, Nigeria
*Correspondence:
Erondu Felix O, Nigerian Institute of Radiographers, a Division of the Radiographers Registration Board of Nigeria,
Nigeria,
Tel: +2348086667558,
Email:
Received: 14-Jun-2022, Manuscript No. Iphsj-22-12827;
Editor assigned: 16-Jun-2022, Pre QC No. PQ-12827;
Reviewed: 30-Jun-2022, QC No. QC No.Q-12807;
Revised: 04-Jul-2022, Manuscript No. Iphsj-22-12827 (R);
Published:
12-Jul-2022, DOI: 10.36648/1791-809X.16.7.959
Abstract
Polycystic ovarian syndrome (PCOS) in women of child-bearing age is one of the most
common causes of primary and secondary infertility, and may often be associated with
other health or social issues. The prevalence however, varies among age group and
geographical area. Ultrasound plays an important role in the initial evaluation and
follow-up treatment of patients with PCOS. This study was to evaluate the prevalence
of polycystic ovarian syndrome based on the sonographic survey of the ovaries of 200
women of childbearing ages of 13-51 years in Port Harcourt metropolis in Nigeria. This
was a retrospective study for a period of twelve months from two hospitals.
Results: A total of 33 women (16.5%) were diagnosed with PCOS. Ages 27-31 years
had the highest occurrence of polycystic ovarian syndrome (18 cases 9%). The most
common clinical presentations were primary and secondary infertility (24.2 and 21.2%,
respectively). In conclusion, polycystic ovarian syndrome was common among women
of child bearing age in Port Harcourt metropolis, with ages 27-31 years having the
highest occurrence, and primary infertility being the common clinical presentation.
Thus, women of child bearing age presenting with infertility should be examined with
ultrasound for early diagnosis and treatment of polycystic ovarian syndrome.
Keywords
Polycystic ovarian syndrome; Sonography; Infertility; PCOS
Introduction
Polycystic ovarian syndrome, also known as Stein-Leventhal
syndrome is an endocrine mediated disorder that affects the
ovaries in women of child bearing age (Encyclopaedia Britannica,
2017). Although the aetiology of polycystic ovarian syndrome is
not known, it is associated with numerous health risks such as
diabetes mellitus, hypertension, obesity, cardiovascular diseases,
and infertility problems such as anovulation, endometrial
hyperplasia and hyperandrogenism among others [1,2].
Psychological impairments including depression and other
mood disorders, as well as other patient observable symptoms
like irregular menstrual cycle, irregular ovulation, hirsutism and
acne, have all been documented [3]. Recent studies appear to
establish an increasing susceptibility to coronavirus disease 2019
(COVID-19) among patients with PCOS especially in the presence
of comorbidities of obesity, Type 2 diabetes mellitus (T2DM),
fatty liver, and androgen excess [4]. It is also a fact that Insulin
resistance which may occur in PCOS may be linked to low-grade
chronic inflammation as well as androgen excess which has a
direct impact on adipocyte biology and metabolism. Accordingly
PCOS is also associated with comorbidities such as non-alcoholic
fatty liver disease (NAFLD), obesity, metabolic syndrome as well
as the alterations in the gut micro biome, further worsening the
potential for COVID-19 infection [4].
Different types of polycystic ovarian syndrome have been reported
to include insulin-resistant, inflammatory, hidden-cause, and pillinduced.
The insulin-resistant polycystic ovary syndrome is the
most common type, and is caused by smoking, pollution, sugar
and trans fat, while, the pill-induced polycystic ovary syndrome
comes second and is caused mostly by birth control pills [5].
A positive ultrasound diagnosis of PCOS, is one of the three
conditions to be met in the Rotterdam criteria for diagnosis. A
more inclusive diagnostic criterion for diagnosis of PCOS has been
evaluated in Nigerian women using three-model criteria such as
the 1990 National Institutes of Health (NIH), the 2003 Rotterdam
and 2006 Androgen Excess Society (AES) criteria [6].
The prevalence of polycystic ovarian syndrome varies according
to diagnostic consensus used, with estimates ranging from 6%
(National Institutes of Health consensus) to 18% (Rotterdam
consensus) of reproductive-aged women of different ethnicity
(Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop
Group, 2004; The morphological features of the ovaries in
women with polycystic ovary syndrome have been well described
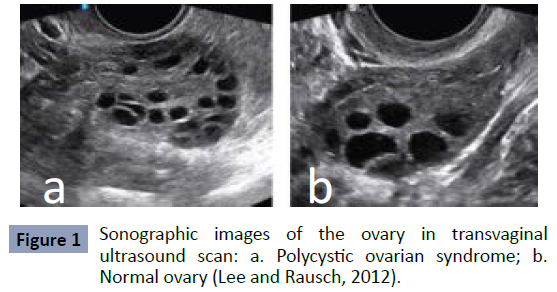
by ultrasound imaging technology; enlarged ovary size, with
multiple follicles, increased ovarian stroma and echogenicity are
some of the features (Figure 1).
The most basic form of treatment for women with polycystic ovary
syndrome is the initiation of an exercise regimen combined with
proper nutrition to encourage weight loss and improve insulin
sensitivity. Suppressing excess androgen production may be the
focus if the syndrome is severe. Polycystic ovarian syndrome
induced infertility is often treated with drugs that induce
ovulation; however, oral contraceptives can be administered in
women who may desire not to become pregnant. In extreme
cases, laparoscopic surgery can be used to remove portions
of the ovary and thus, reduce the production of androgens. In
addition, anti-diabetic drugs have been reported with positive
outcome [7].
Ultrasound identification of the presence of polycystic ovarian
morphology is recognized as a component of its diagnosis.
Transvaginal ultrasound is considered the gold standard due to
the optimal visualization it provides of the internal structure of
the ovary [8]. There is, however, a paucity of data for the ovarian
morphology in normal and polycystic ovary syndrome in women
of child bearing age in our environment. This study therefore
aimed to evaluate the prevalence of polycystic ovarian syndrome
based on the sonographic survey of the ovaries in women of child
bearing ages of 13-51 years in Port Harcourt metropolis in Nigeria
(Figure 1).
Figure 1: Sonographic images of the ovary in transvaginal
ultrasound scan: a. Polycystic ovarian syndrome; b.
Normal ovary (Lee and Rausch, 2012).
Materials and Methods
A retrospective study of polycystic ovarian syndrome in women
of child bearing age, 13-51 years of age who undertook
transvaginal ultrasound examination of the pelvic for a period of
twelve months from fifth day of January, 2019 to the twentieth
day of December, 2019 from two ultrasonography centres in port
Harcourt Metropolis, Rivers, Nigeria.
The study was carried out on all women of child bearing age 13-
51 years who were clinically suspected to have polycystic ovarian syndrome, and referred for pelvic ultrasound examination within
a period of 12 months, from January 2, 2019 to December 20,
2019. Approval for this study was obtained from the Ethical
Committee of the Rivers State University Teaching Hospital, as
well as the administration of Image Diagnostic Centre.
A total of hundred ultrasound reports were obtained from each
of the two health facilities. Ultrasound images were Assessed
based on pelvic ultrasound examination, diagnosis or impression
of the scan images. Information on the sex, age and the clinical
indications were obtained from patient request and report forms
and patient’s registration log books, respectively.
Results
A total of 200 pelvic sonographic reports were analysed for
sonographic diagnosis of PCOS. A total of 33 (16.5%) had
diagnosis, while 167 (83.5%) were negative. The result is
presented in Table 1. Out of the 100 patients for each facility, 12
cases were identified in Facility 1 and 21 cases in facility 2, giving
a total of 33 positive cases (Table 1).
| Hospitals |
Total Number of Women |
Total Females With PCOS |
Percentage (%) |
Total Female Without PCOS |
Percentage (%) |
| Image Diagnostic Centre |
100 |
21 |
21 |
79 |
79 |
| RSUTH |
100 |
12 |
12 |
88 |
88 |
| Total |
200 |
33 |
33 |
167 |
16 |
Table 1.The number and percentage distribution of the positive and negative cases of PCOS in RSUTH and Image Diagnostic Centre.
Percentage Distribution of Polycystic Ovarian
Syndrome
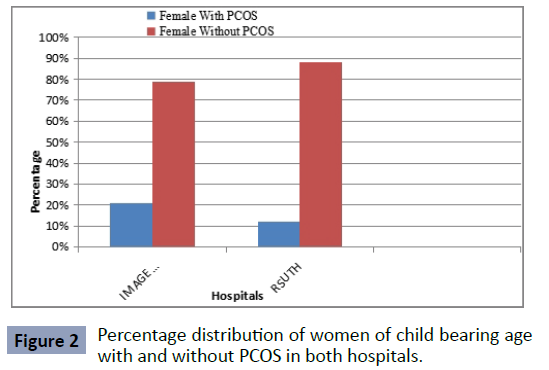
Twenty one per cent of the cases at Image Diagnostic Centre
were positive for polycystic ovarian syndrome, while 12% of the
cases at RSUTH were positive for polycystic ovarian syndrome
(Figure 2).
Figure 2: Percentage distribution of women of child bearing age
with and without PCOS in both hospitals.
Age Distribution of Positive Polycystic Ovarian
Syndrome
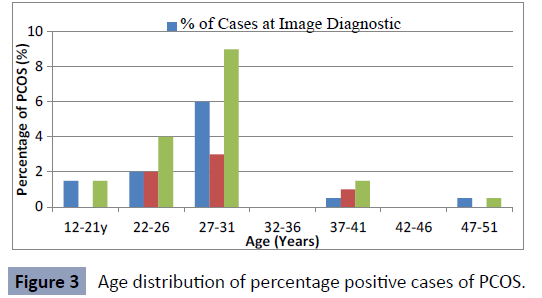
The Age distribution of positive polycystic ovarian syndrome at Image Diagnostic Centre and Rivers State University Teaching
Hospital presented in (Figure 3).
Figure 3: Age distribution of percentage positive cases of PCOS.
Clinical Presentations
Primary infertility was the commonest clinical indication follow
by secondary infertility, chronic anovulation, amenorrhea,
oligomenorrhea, sexual transmitted disease, diabetes, obesity
and metabolic syndrome (Table 2).
Clinical Presentation |
Frequency |
Percentage (%) |
| Primary Infertility |
8 |
24.2 |
| Secondary Infertility |
7 |
21.2 |
| Chronic Anovulation |
5 |
15.2 |
| Amenorrhea |
4 |
12.1 |
| Oligomenorrhea |
4 |
12.1 |
| Sexual Transmitted Disease |
3 |
9.2 |
| Diabetes |
1 |
3 |
| Obesity and Metabolic Syndrome |
1 |
3 |
| Hirsutism |
0 |
0 |
| Total |
33 |
100 |
Table 2. Clinical presentation of polycystic ovarian syndrome from the two hospitals.
Discussion
this study aimed to evaluate the prevalence of polycystic ovarian
syndrome based on the sonographic survey of the ovaries in
women of child bearing ages of 13-51 years in Port Harcourt metropolis in Nigeria. Out of a sample population of 200 women
of child bearing age 13-51 years, only 33 (16.5%) were positive
for polycystic ovarian syndrome. It was observed that polycystic
ovarian syndrome was minimal between ages 12-21 and 32-51
years and increased in the age groups 22-26 and 27-31 years.
Where there are larger population of ovarian follicles, the more
likelihood for the diagnosis of polycystic ovarian syndrome [9- 12]. Hudecova et al. (2009) reported that ultrasonography is a
reliable tool for the detection of polycystic ovarian syndrome in
women of reproductive age, which may have accounted for the
high incidence in age groups 22-26 and 27-31 years of the present
study.
The decrease in age distribution in the 12-21 years group may
be attributed to premature ovarian failure or primary ovarian
insufficiency or signs and symptoms that characterize polycystic
ovarian syndrome overlap with normal [13]. However, Bronstein
et al. (2011) reported a high diagnosis among adolescents (13-
18 years), although his focus was among children less than 18
years. The decrease in age distribution from 32-51 years may be
attributed to perimenopause/menopause as the incidence of
polycystic ovarian syndrome tends to be less (Karjula et al., 2017).
Perimenopause or menopause transition can begin eight to ten
years before menopause, when the ovaries gradually produce
less oestrogen. It usually starts in a woman‘s 40s but can start in
the 30s as well [14].
Based on clinical presentation, the study showed that primary
infertility was the most common indication, followed by secondary
infertility, chronic anovulation, amenorrhea, oligomenorrhea,
sexual transmitted disease, diabetes and obesity. The present
result is similar to a previous report where the commonest clinical
presentations were primary and secondary infertility [15-21].
Conclusion
In a sample population of 200 and 33 (16.5%) positive case of
polycystic ovarian syndrome, it can be concluded that polycystic
ovarian syndrome is common in women of child bearing age in
Port Harcourt Metropolis in the South-South region of Nigeria.
The affected ages were 22 to 26 years, with highest population
within ages 27 to 31 years and most common clinical presentations
being primary and secondary infertility.
REFERENCES
- Akpata C B, Uadia P O, Okonofua F E (2018) Prevalence of Polycystic Ovary syndrome in Nigerian women with infertility: A prospective study with Three assessment criteria. OJOG 8.
Indexed at, Google Scholar, Crossref
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven J S E (2016) Polycystic ovary syndrome. Nat Rev Dis Primers 2:16057.
Indexed at, Google Scholar, Crossref
- Bronstein J, Tawdekar S, Liu Y, Pawelczak M, David R (2011) Age of onset of polycystic ovarian syndrome in girls may be earlier than previously thought. J Pediatr Adolesc Gynecol 24:15-20.
Indexed at, Google ScholarGoogle scholar, Crossref
- Brutocao C, Zaiem F, Alsawas M, Morrow A S, Murad M H et al. (2018) Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine 62:318-325.
Indexed at, Google Scholar, Crossref
- Carmina E, Oberfield S E, Lobo R A (2010) the diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 203:201-205.
Indexed at, Google Scholar, Crossref
- Cleveland Clinic (2019) Menopause Perimenopause and Post menopause Accessed on 6.
Google Scholar
- Ding C, Luo L, Gu F, Jie H, Zhao Q (2017) early miscarriage rate in lean polycystic ovary syndrome women after euploid embryo transfer a matched-pair study. Reproductive Bio Medicine Online, 35:576-582.
Indexed at, Google Scholar, Crossref
- Encyclopaedia Britannica (2017) Stein-Leventhal syndrome. Encyclopaedia Britannica Inc.
Google Scholar
- Escobar Morreale H F (2018) polycystic ovary syndrome definition, aetiology diagnosis and treatment. Nature Reviews. Endocrin14:270-284.
Indexed at, Google Scholar, Crossref
- Ge J J, Wang D J, Song W, Shen S M, Ge W H (2021) The effectiveness and safety of liraglutide in treating overweight/obese patients with polycystic ovary syndrome: a meta-analysis. J Endocrinol Investig.
Indexed at, Google Scholar
- Hudecova M, Holte J, Olovsson M, Sundstrm Poromaa I (2009) Long-term follow-up of patients with polycystic ovary syndrome: reproductive outcome and ovarian reserve. Human Reproduction 24:1176-1183.
Indexed at, Google Scholar, Crossref
- Karjula S, Morin-Papunen L, Auvinen J, Ruokonen A, Puukka K et al. (2017) Psychological distress is more prevalent in fertile age and premenopausal women with PCOS symptoms 15-year follow-up. J Clin Endocr 102:1861-1869.
Indexed at, Google Scholar, Crossref
- Joshi SR (2021) Polycystic Ovary Syndrome and COVID-19 An Emerging Risk Factor. J Hum Reprod Sci 14:211-212.
Indexed at, Google Scholar, Crossref
- Lee T T, Rausch M E (2012) polycystic ovarian syndrome: Role of imaging in diagnosis. Radio Graphics 32:1643-1657.
Indexed at, Google Scholar, Crossref
- Patel S (2018) polycystic ovary syndrome (PCOS) an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol 182:27-36.
Indexed at, Google Scholar, Crossref
- Peng Q, Karvonen-Gutierrez C A, Randolph J F, Nan B, McConnell D et al. (2019) Age at onset of metabolic syndrome among women with and without polycystic ovary syndrome like status. The J Clin Endocr 104:1429-1439.
Indexed at, Google Scholar, Crossref
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility 81:19-25.
Indexed at, Google Scholar, Crossref
- Ruman U, Chowdhury T A, Chowdhury T S, Mahmud N, Hasan I (2015) TSH & prolactin versus LH & FSH: a dialemma in hormone assay for patients with primary or secondary infertility in a tertiary care hospital in Bangladesh. Int J Gynecol Obstet 2:212-214.
Google Scholar
- Subramanian A, Anand A, Adderley NJ, Okoth K, Toulis KA et al. (2021) Increased COVID-19 infections in women with polycystic ovary syndrome: A population-based study. Eur J Endocrinol 184:637-645.
Google Scholar, Crossref
- Welt C k, Carmina E (2013) Lifecycle of polycystic ovary syndrome (PCOS): From in utero to menopause. J Clin Endocr 98:4629-4638.
Indexed at, Google Scholar, Crossref
- Zhu R Y, Wong Y C, Yong E L (2016) Sonographic evaluation of polycystic ovaries. Best Practice & Research. Clinical Obstetrics & Gynaecology 37:25-37.
Indexed at, Google Scholar, Crossref
Citation: Felix OE, Florence MK, Moses BE
(2022) Prevalence of Polycystic Ovarian
Syndrome (Pcos) In Women of Child Bearing
Age Within Port Harcourt Metropolis in
Nigeria Using Sonographic Evaluation.
Health Sci J. Vol. 16 No. 7: 959.