Keywords
Postmenopausal women; Protein microarray; Soluble biomarkers; Cytokines; Adhesion molecules; Cell populatiopns; Obesity; Bone status; Osteoporosis
Abbreviation
APCs: Antigen Presenting Cells; BMI: Body Mass Index; BMD: Bone Mineral Density; CD: Cluster Of Differentiation; DCs: Dendritic Cells; ICAM-1: Intercelluar Cell Adhesion Molecule; ICOS-L: Inducible Costimulator Ligand, IGF: Insulin-Like Growth Factor; IL: Interleukin; LFA: Lymphocyte Function Associated Antigen; MHC: Major Histocompatibility Complex; MCSF: Macrophage Colony-Stimulating Factor; OPG: Osteoprotegerin; RANK: Receptor Activator of Nuclear Factor-Kb; RANKL: Receptor Activator of Nuclear Factor-kB Ligand; sICAM-1: Soluble ICAM-1; TCR: T Cell Antigen-Specific Receptor; TGF: Transforming Growth Factor; TNF: Tumor Necrisis Factor; VCAM: Vascular Cell Adhesion Molecule
Introduction
Postmenopausal women are susceptible for environmental or genetic factors, and may experience a progression or initiation of diverse diseases such as obesity, osteoporosis and coronary heart disease, triggered by a systemic change in the balance of proinflammatory cytokine activity. The onset of menopause is generally associated with a hormone deficiency, which is a contributory factor for the increased incidence of osteoporosis, cardiovascular diseases, and vasomotor disturbances. There is a large evidence that the decline in ovarian function with menopause is associated with spontaneous increases in proinflammatory cytokines (e.g. IL-1, IL-6, and TNF-α), chemokines and colony-stimulating factors. The exact mechanisms by which estrogens interferes with cytokine activity are not completely known, potentially it could be the estrogen receptor interactions with other transcriptional regulators, modulation of nitric oxide activity, antioxidative effects, plasma membrane actions, and changes in immune cell function. The cytokine metabolism changes are affected by aging, general decline in immune responses contributes to increased susceptibility to infection. The significant differences between fertile women and their postmenopausal counterparts manifested in immunoregulatory molecules, such as anti-inflammatory cytokines (IL-4, IL-10) and growth hormones (e.g. IGF-1, insulin-like growth factor 1) [1-3].
During the last decades, obesity and osteoporosis have become important global health problems with an increasing prevalence and a high impact on both mortality and morbidity worldwide. Age and female sex increase the risk of developing both obesity and osteoporosis, which affect millions of women. The fat-derived mediators, which include resistin, leptin, and adiponectin, affect human energy homeostasis and are involved in bone metabolism, contributing to the complex relationship between adipose and bone tissue. Obesity is associated with chronic low-grade inflammation. Some proinflammatory cytokines (TNF-α and IL- 6) are the factors that negatively regulate bone metabolism in relation to obesity [4-7].
Experimental and clinical studies strongly support a link between the increased state of proinflammatory cytokine activity and postmenopausal bone loss. Bone metabolism is determined by a multitude of genetic and environmental influences. The pathogenesis of chronic disorders of these tissues is complex, but there is increasing support that the development of these disorders may be in part linked to an increased state of proinflammatory activity. The imbalance between bone formation and bone resorption is known to be responsible for postmenopausal bone loss. Clinical and molecular evidence indicates that estrogen-regulated cytokines exert regulatory effects on bone turnover implicating their role as being the primary mediators of the accelerated bone loss at menopause [8]. Proinflammatory cytokines are the most powerful stimulants of bone resorption. They directly and through the stimulation of other local factors intervene with osteoclastogenesis, from the proliferation and differentiation of the early osteoclast precursor cell to the resorption capacity and the lifespan of the mature osteoclast [9-11].
Peptides mediating energy homeostasis (i.e. leptin, adiponectin) may play an important role in the weight and body composition changes of postmenopausal women. Leptin and adiponectin play an important role in the regulation of body weight and energy homeostasis, and in postmenopausal women are partially determined by sexual hormones and inflammatory marker levels [12-15]. There was a strong association between elevated biomarkers of systemic inflammation and endothelial dysfunction among obese patients, insulin resistance and metabolic abnormalities. ICAM-1 (intercelluar cell adhesion molecule-1) and E-selectin expressions are generally upregulated in most inflammatory processes and represent important determinants for leukocyte recruitment [16,17]. In visceral adipose tissue, ICAM- 1 and VCAM-1 (vascular cell adhesion molecule-1) expression and protein levels positively correlated with body mass index (BMI). Obesity was associated with increased adhesion molecules mRNA expression and protein levels in visceral adipose tissue [18,19]. The purpose of the present study was to quantify serological biomarkers in fertile and postmenopausal women, namely adiponectin, leptin, E-selectin, ICAM-1, IGF-1, MCSF (macrophage colony-stimulationg factor), IL-6, IL-10. and IL-17A. The relationship of these parameters to obesity and women bone status has also been analysed. Finally, we evaluated the correlation between soluble biomarkers and cell populations, in fertile and postmenopausal women samples. The data and method for the detection of cell surface markers were introduced in our previous publication [20].
Materials and Methods
Samples
Peripheral blood was collected in 10-ml tubes and isolated sera were stored at -70°C. We analyzed fertile (n=96; age range 26- 52 years) and postmenopausal Slovak women (n=107, age range 48-79 years). Subjects were recruited via health practitioners in accordance with ethical committee requirements. Writen informed consent was obtained from all participants before entering the study (EC SMU 06102011). Criteria for exclusion were acute physical illness or unstable physical condition, pregnancy, daily smoking for over a one year, nephropathy with glomerular filtration rate (GFR<0.75 mL/s), endocrinopathy, diabetes mellitus, active hepatitis and liver cirrhosis, cancers, anemia, severe cardiovascular disease, malabsorption diseases or conditions after gastrectomy or any part of the intestine, alcohol abuse, drug addiction, treatment with glucocorticoids, hormone therapy or hormone replacement therapy, use of calcium, vitamin D (at a dose more than 400 IU), drugs affecting obesity, the presence of metal implants and pacemaker in the body. Obesity was estimated by calculating the BMI (kg/m2) and the whole body composition was measured using a total-body scanner (Lunar Prodigy Advance, USA). A bone densitometry measurement was done using dual energy X-ray absorptiometry (DXA). The results were evaluated according to the WHO expert criteria. Women were distributed into two subgroups, normal body weight (control group; BMI=20.0–29.9 kg/m2) and obese subjects (BMI=≥ 30.0 kg/m2). Postmenopausal women were at least five years since the beginning of menopause. Some of women were treated for hypertension (n=49), most of them postmenopausal (n=43). None of women were treated for osteopenia or osteoporosis in the past and they had no history of femur or vertebral fractures.
Protein microarray
We have used a protein microarray for the detection of soluble proteins in biological samples. The simultaneous quantitative measurement of multiple biomarkers, e.g. cytokines, growth factors, adhesion molecules and others, was performed with the multiplexed ELISA, Quantibody Human array kit. The panel of capture antibodies was printed in multiple identical arrays on a standard slide. After a blocking step, samples were incubated with the arrays, according to manufacturer´s instructions (RayBiotech, Inc., USA). Nonspecific proteins were washed off, and the arrays were incubated with a cocktail of biotinylated antibodies, directed toward 9 different parameters, followed by a streptavidin-labeled Cy3 equivalent dye. Signals were then visualized using a fluorescence laser scanner (Innoscan 900AL, Innopsys, France) at 532 nm and the data analysed using the Mapix software (Innopsys) and Quantibody Q-Analyzer software (RayBiotech, Inc.). Briefly, the median local background was subtracted from the median fluorescence of each spot and the corrected fluorescence was used to calculate the average fluorescence signal as well as the standard deviation.
Statistical analysis
Analysis of variables was carried out using the Mann-Whitney test with SPSS statistical software package (SPSS Inc., Chicago, IL, USA). A value of p<0.05 was considered statistically significant. A multiple linear regression model was used for the evaluation of the potential confounders, such as women age, BMI, bone mineral density (BMD), waist size and tissue fat. Each variable was entered in sequence and assessed by the retain criteria in the model. We also estimated the correlation between serological biomarkers and cell surface receptors in women samples. Pearson´s correlation coefficient was significant at the 0.05 level. The statistical analysis of the cell surface molecules expression and cell populations determination in whole blood samples, from fertile and postmenopausal women, was reported by Horváthová et al. [20].
Results
Our experiments demonstrated protein microarray analysis of 9 serological biomarkers in two groups, fertile and postmenopausal women. The Table 1 show characteristic of parameters (mean and percentiles) from all women. We have found statistically significantly higher level of ICAM-1 in postmenopausal women (Figure 1).
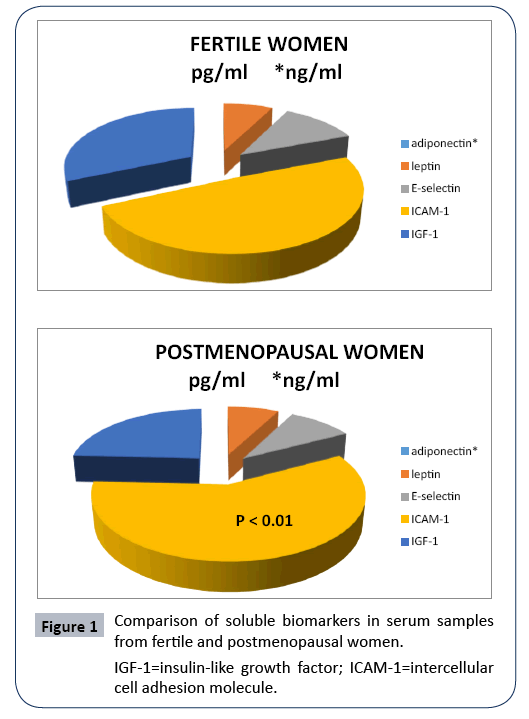
Figure 1: Comparison of soluble biomarkers in serum samples from fertile and postmenopausal women.
IGF-1=insulin-like growth factor; ICAM-1=intercellular cell adhesion molecule.
Table 1: Total soluble biomarkers in women serum samples. *adiponectin [ng/ml]. 1Below the Limit of Detection. IGF-1=insulin-like growth factor; MCSF=macrophage-colony stimulating factor; ICAM-1=intercellular cell adhesion molecule; IL=interleukin.
| Serological Markers |
Mean [pg/ml] |
Percentiles 25 |
Percentiles 75 |
| Adiponectin |
17 764,95* |
16 513,80* |
20 639,90* |
| Leptin |
33,218.33 |
20 233, 30 |
42,328.90 |
| E-selectin |
46,447.04 |
27,891.60 |
57,624.70 |
| ICAM-1 |
2,40.518.75 |
1,06,053.30 |
3,37,421.80 |
| IGF-1 |
1,19,311.89 |
23,732.90 |
1,64,270.70 |
| MCSF |
576.99 |
0.00 1 |
839.40 |
| IL-6 |
58.64 |
7.40 |
82.10 |
| IL-10 |
21.98 |
2.20 |
30.10 |
| IL-17 |
21.80 |
0.00 1 |
22.20 |
We have analysed level of biomarkers in women divided into four groups according to the fertility/postmenopausal status and BMI parameters: fertile obese, fertile control, postmenopausal obese and postmenopausal control. Adipopnectin was up-regulated in postmenopausal control compared to fertile obese women (p<0.05). ICAM-1 was increased in postmenopausal obese women when compared to fertile obese women (p<0.05) (Table 2).
Table 2: Comparison of biomarkers in serum samples from fertile and postmenopausal women. •The comparison of the groups with a significant difference. The higher levels were in postmenopausal women. ICAM-1=intercellular cell adhesion molecule
Biomarkers |
Fertile women |
Postmenopausal |
Significance |
| Fertile obese |
Fertile control |
Postmenopausal obese |
Postmenopausal control |
| adiponectin |
• |
|
|
• |
P<0.05 |
| ICAM-1 |
• |
|
• |
|
P<0.05 |
The significant changes of biomarkers in the women with different BMD are shown in Figure 2a and 2b. The soluble ICAM-1 (sICAM-1) level was higher in women with osteopenia (2. group, p<0.01). IL-6 was decreased in women affected by osteoporosis (3. goup, p<0.05).
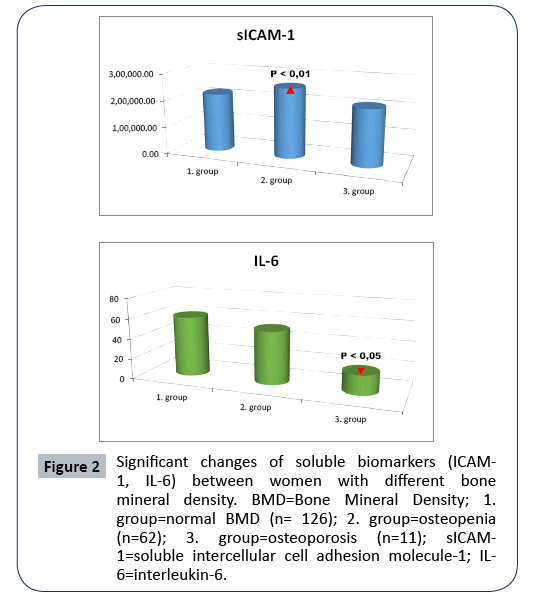
Figure 2: Significant changes of soluble biomarkers (ICAM- 1, IL-6) between women with different bone mineral density. BMD=Bone Mineral Density; 1. group=normal BMD (n= 126); 2. group=osteopenia (n=62); 3. group=osteoporosis (n=11); sICAM- 1=soluble intercellular cell adhesion molecule-1; IL- 6=interleukin-6.
In the total study group we identified association between soluble biomarkers levels and confounding variables: BMI, BMD, tissue fat, waist size, and age. Significant negative correlation was between IL-6 and age (r=-0.177, p<0.05).
Finally, we tested the correlation between serological biomarkers, cell surface receptors and cell populations, in women samples. We identified several statistically significant positive association, namely IGF-1 and B-lymphocytes, IL-6 and myeloid dendritic cells (DCs), IL-10 and plasmacytoid DCs, and others. A negative correlation was found between ICAM-1 and memory effector T cells, IL-17A and naive CD3+ T-lymphocytes (Table 3). The results from the flow cytomteric study of cell surface markers and cell populations were reported in our previous publication [20].
Table 3: The correlation between biomarkers and cell surface receptors and cell populations in women samples. Each cell surface receptor of interest was analyzed by multi-color immunophenotyping using monoclonal antibodies, and using a flow cytometer. Data were reported by Horváthová et al. [20]. IGF-1=insulin-like growth factor; MCSF=macrophage-colony stimulating factor; sICAM-1=soluble intercellular cell adhesion molecule; sE-selectin=soluble E-selectin; DCs=dendritic cells; IL=interleukin; RANK=receptor activator of nuclear factor-kB.
| |
IL-6 |
IL-10 |
IL-17 |
IGF-1 |
MCSF |
ICAM-1 |
E-selectin |
| CD19+ B-lymphocytes |
|
|
|
r=0.144 P<0.05 |
|
|
|
| CD19+HLADR+ HLADR+ B-lymphocytes |
|
|
|
r=0.152 P<0.05 |
|
|
|
| CD4+CD127+CD25- Memory efector T cells |
|
|
|
|
|
r=-0.193 P<0.01 |
|
| CD4+CD127-CD25+ Natural regulatory T cells |
|
|
r=0.169 P<0.05 |
|
|
|
|
| CD3+CD45RO-CD45RA+ Naive CD3+ T-lymphocytes |
|
|
r=-0.193 P<0.01 |
r=0.143 P<0.05 |
|
|
|
| CD3+CD8+CD45RO-CD45RA+ Naive CD8+ T-lymphocytes |
|
r=0.161 P<0.05 |
|
r=0.146 P<0.05 |
|
|
|
| CD19-HLADR+CD11c+ Dendritic cells |
|
|
|
|
r=0.143 P<0.05 |
r=0.183 P<0.05 |
r=0.154 P<0.05 |
| CD19-HLADR+CD11c+CD123- Myeloid Dendritic cells |
r=0.170 P<0.05 |
|
|
|
r=0.163 P<0.05 |
|
|
| CD19-HLADR+CD11c-CD123+ Plasmacytoid Dendritic cells |
|
r=0.201 P<0.01 |
|
|
|
|
|
| CD265+CD19-HLADR+CD11c+CD123- RANK on Myeloid Dendritic cells |
|
|
|
|
r=0.171 P<0.05 |
|
|
Discussion
Recent studies have established that the onset of menopause is associated with a low systemic inflammatory status, an inflammation manifested by increased serum levels of the key proinflammatory cytokines (IL-1, IL-6 or TNF-α). The crosstalk between fat and bone involve effects of bone metabolism on energy homeostasis. The relationship between obesity and bone metabolism is complex and includes several factors [21]. The positive association between body weight and bone density has been established in numerous laboratory and clinical studies, a number of cytokines and hormones contribute to the positive association between adipose and bone tissue, acting either locally in sites where cells of the two tissues are adjacent to each other or systemically through the circulation [1,22]. The effect of obesityinduced metabolic abnormalities on BMD and osteoporosis is well established [23]. The positive influence of adipose tissue on bone tissue may be a consequence of the boosting the bone tissue load, leading to increased bone anabolism. It may also be connected with changes frequently occurring in postmenopausal women in the formation of some osteotropic factors (e.g. estrogens, androgens, leptin). The system of receptor activator of nuclear factor-kB, its ligand and osteoprotegerin (RANKL/ RANK/OPG) is the principal signaling pathway through which osteoblasts regulate the rate of the activated osteoclast pool. This effect may be achieved through a direct effect on the expressions OPG and/or RANKL in osteoblasts and marrow stroma cells and/ or indirectly through cytokines (IL-1, IL-6, TNF-α, M-CSF, TNF-β). Malutan et al. [24] found that serum levels of IL-4, IL-10 and IL- 17 are significantly lower in patients with natural or surgically induced menopause compared with patients of childbearing age or in premenopause. Significantly higher levels of IL-4 and IL-10 represent a chronic inflammatory pathology.
IL-6 has potent antiapoptotic properties on osteoblasts and may affect osteoclast development, both of which could lead to osteoporosis. Human studies show that IL-6 and sIL-6r levels are negatively associated with BMD, and the IL-6 gene is an independent predictor of BMD and peak bone mass [25]. Proinflammatory cytokines are frequently regulated in cascades, and the specificity for cytokines response is provided by unique cytokine receptors. Al-Daghri et al. [26] investigated the relationship between osteoporosis and inflammation, and reported that proinflammatory cytokines (IL-1b and IL-6) were significantly elevated in patients than controls. Significantly higher secretion level of IL-6 was observed in osteoporotic bone marrow mesenchymal stem cells compared with normal control [27].
It is known that IL-6 has pro- and anti-inflammatory properties, and only few cells express the IL-6 receptor and respond to IL-6 (classic signaling). All cells can be stimulated via a soluble IL-6 receptor (trans-signaling) since gp130 is ubiquitously expressed [28]. IL-6 is a pleiotropic cytokine that possesses activities that may enhance or suppress inflammatory bone destruction, the anti-inflammatory properties of IL-6 predominate in inflammatory responses. Although the mechanisms of action still need to be defined, these may involve the direct suppression of IL-1 or the induction of endogenous antagonists or inhibitors of IL-1 such as IL-1ra and IL-10 [29]. Evidence from animal and in vitro studies suggests that increases in these cytokines promote bone resorption through several mechanisms, including increasing osteoclast differentiation, activation, and survival, enhancing RANKL expression and inhibiting osteoblast survival [30]. There is evidence that IL-6 levels tend to rise during the ageing process [25,31].
In contrast to other studies, we found that IL-6 was decreased in women affected by osteoporosis, and we reported a negative correlation between IL-6 and age. This may be due to the antihypertensive therapy in a large number of postmenopausal and/or elderly osteoporotic women. Several authors demonstrate that antihypertensive treatment significantly decreased circulating levels of selected proinflammatory mediators, e.g. IL-6 [32,33]. It is also known that osteoporosis is most common among older women. Some authors decsribed that IL-6 may directly inhibit RANKL signaling in osteoclast precursor cells, and decrease osteoclast formation [34-36]. IL-6 inhibitors prevent bone loss and cartilage degeneration, IL-6 may be an important factor associated with osteoporosis, and was identified as a promising target for osteoporosis therapy [27,37,38].
Adiponectin has an important role in metabolism, primarily through reducing insulin resistance, and it has been shown to exert actions in the female reproductive system, including the hypothalamic–pituitary–ovarian axis and the endometrium. The expression of receptors (AdipoR1, AdipoR2) has been reported in the brain, ovaries, endometrium, and the placenta [39]. A study of Diwan et al. [40] showed that serum adiponectin was lower in obese participants compared to non-obese participants, and adiponectin is inversely associated with BMI and waist circumference. Circulating adiponectin concentrations increase with age in normal-weight middle-aged and older women [41,42]. The serum adiponectin levels in non-obese women were significantly higher than in the women with obesity or overweight [43]. We have confirmed the effect of age and obesity according to other studies, our results shown that adiponectin was increased in postmenopausal control compared to fertile obese women. Matsui et al. [44] find that serum adiponectin level in late postmenopausal women was significantly higher than that in early postmenopausal women. The literature reports indicating a link between plasma levels of adiponectin and body fat, BMD, sex hormones, and peri- and postmenopausal changes, draw attention to the possible use of adiponectin as an indicator of osteoporotic changes, suggesting that adiponectin may also modulate bone metabolism. Although several studies have shown that adiponectin has an adverse effect on bone mass, mainly by intensifying resorption, there are some authors demonstrating that this peptide may increase the proliferation and differentiation of osteoblasts, inhibit the activity of osteoclasts, and reduce bone resorption [45].
The cell adhesion molecules expression (ICAM-1, VCAM-1 and E-selectin) on endothelial cells greatly increases upon cytokine stimulation and their main function is to bind ligands present on leukocytes to promote leukocyte attachment and transendothelial migration. These molecules can be cleaved and shed from the cell surface, releasing soluble forms. The biological significance of circulating cell adhesion molecules may be manifold, including the competitive inhibition of their binding receptors located on leukocytes, the reduction of endothelial binding sites as well as signaling functions. The soluble forms of cell adhesion molecules are increasingly released from endothelial cells under inflammatory conditions and endothelial cell stimulation [46]. Obesity may induce endothelial activation or increased shedding of cell surface E-selectin that leads to subsequent increase in soluble E-selectin levels. The high serum concentrations of E-selectin closely correlated with increased total fat volume, but not with regional fat distribution [47]. In our study we noticed that ICAM-1 increased in postmenopausal obese women when compared to fertile obese women. The ICAM-1 level was higher in women with osteopenia, but not with osteoporisis, in comparison to the control group. In bone metabolism, ICAM-1 exerts important osteotropic effects by mediating cell–cell adhesion of osteoblasts and osteoclast precursors, thereby facilitating osteoclast differentiation and bone resorption. Furthermore, it has been shown that osteoblasts adhere to opposing cells through adhesion molecules, resulting in the activation of intracellular signals and leading to the production of bone-resorbing cytokines, such as TNFα, IL-1β and IL-6 [48]. Liu et al. [49] identify ICAM-1 as a regulator in the bone marrow niche. The two major processes of bone metabolism - bone formation and resorption - are regulated by cellular interactions. Osteoblasts and osteoclasts play a significant role in bone metabolism, which is known to be regulated by local soluble factors and systemic hormones. Bone is a heterogeneous tissue comprised of osteogenic and hematopoietic cells. Cellular adhesion by which osteoblasts communicate with opposing cells in bone milieu is involved in the osteoblast activation. Tanaka et al. [50] resulted that osteoblasts adhere to opposing cells through particular adhesion molecules on their surface and that the adhesion molecules on the osteoblasts not only function as glue with opposing partners but transduce activation signals that facilitate the production of boneâ€ÂÂÂÂresorbing cytokines. Cellular adhesion of osteoblasts as well as soluble factors is significant for the regulation of bone metabolism.
Bone diseases such as osteoporosis, osteoarthritis and rheumatoid arthritis affect a great proportion of individuals, with debilitating consequences in terms of pain and progressive limitation of function. Existing treatment of these pathologies has been unable to alter the natural evolution of the disease and, as such, a clearer understanding of the pathophysiology is necessary in order to generate new treatment alternatives. One therapeutic strategy could involve the targeting of ICAM-1. In bone, ICAM-1 is expressed at the surface of osteoblasts and its counter-receptor, LFA-1 (lymphocyte function associated antigen), at the surface of osteoclast precursors. ICAM-1 blockade between the osteoblast and the pre-osteoclast results in an inhibition of osteoclast recruitment and a modulation of inflammation, which could potentially help in controlling disease activity in bone pathologies [51].
IGF-1 and B-lymphocytes and naive T cells correlation
IGF-1 produced by bone marrow stromal cells in the hematopoietic microenvironment plays a key role in regulating primary B lymphopoiesis [52]. IGF-1 enhances diverse aspects of bone marrow function, including lymphocyte maturation [53]. In bone marrow, administration of IGF-1 promotes the production of mature B cells [54]. IGF-1 regulates diverse aspects of T-cell, B-cell and monocyte function through its interactions with IGF-1 receptor (IGF-1R). Nearly all cells of the immune system including T- and B-lymphocytes, NK cells express IGF-1R and are therefore susceptible to the effects of IGF-1 [55]. IGF-1 levels have been shown to exhibit a positive relationship with thymopoiesis. IGF-1 play important roles in hemopoietic cell growth and differentiation and normal immune function. It promotes T cell proliferation during early activation and inhibits apoptosis of both immature and mature T cells. Most naive CD3+ and CD8+CD45RA+ T cells, display IGF-1R. IGF-1 may directly promote the survival or expansion of antigen-specific T cells through their interaction with IGF-1R [56]. We have described a significant association between IGF-1 and and B-lymphocytes, naive CD3+ and CD8+ T cells. Our results are consistent with Chen et al. [57] who found the trend of positive correlation in IGF-1 levels and percentage of naive CD8+ T cells, even if not significant.
IL-6 and DCs correlation
Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and DCs substes [58]. IL-6 regulates DCs differentiation in vivo [59]. After spontaneous differentiation in culture, the DCs up-regulated the cytokine levels, such as IL- 6, IL-15, etc. [60]. In opposite to Zhang et al. [61] we found a positive correlation between IL-6 and the level of myeloid DCs. IL-6 production by the antigen presenting cells (APCs) is involved in the priming of naïve CD8+ T cells and formation of memory CD8+ T cells [62]. It was, therefore, concluded that obesity was a positive modulator of IL-6R and IL-6 expression in the adipose tissue which might be a contributory mechanism to induce metabolic inflammation [63].
IL-10 and DCs and naive T cells correlation
DCs are key regulators of adaptive immunity with the potential to induce T cell activation/immunity or T cell suppression/tolerance [64]. IL-10 is limiting and terminating excessive T-cell responses to microbial pathogens to prevent chronic inflammation and tissue damage [65,66]. T cell activation requires at least two signals from an APCs to become fully activated. These signals (TCR/MHC, CD28/CD80. CD86) are transmitted to the nucleus of T cells, which results in the expression of activation markers at the cell surface, induction of cytokine secretion or cytotoxic function, cell proliferation, and differentiation into effector cells. In adipose tissue, similar activation steps occur with interactions between T cells and adipose tissue-resident DCs. There is strong evidence that T cell activation is induced by adipose tissue components [67]. Maturing plasmacytoid DCs rapidly and strongly up-regulate inducible costimulator ligand (ICOS-L) and specifically drive the generation of IL-10–producing T regulatory cells regardless of the activation pathway [68]. Plasmacytoid DCs have the potential to prime CD4+ T-cells to differentiate into IL-10-producing T regulatory cells through preferential expression of ICOS-L. We have found a positive correlation of IL-10 to naive CD8+ T cells and plasmacytoid DCs. Smith et al. [69] identified a regulatory loop in which IL-10 directly restricts CD8+T cell activation and function through modification of cell surface glycosylation allowing the establishment of chronic infection. Cytotoxic CD8 T cells may regulate their inflammatory effects during acute infections by producing IL-10. and thereby minimize immunopathological disease [70].
IL-17A and regulatory cells and naive T cells correlation
The effector T-cell lineage shows great plasticity. Th17 cells are acknowledged to be instrumental in the response against microbial infection, but are also associated with autoimmune inflammatory processes. Human regulatory T cells can differentiate into IL-17– producing cells, when stimulated by allogeneic antigen-presenting cell [71]. We observed positive relationship between IL-17A and natural regulatory cells production. Conventional T regulatory cells exert their suppressive effect via cell-cell contact-dependent and contact-independent mechanisms, and they produce antiinflammatory cytokines (IL-10. TGF-β, IL-35) [72]. Regulatory T cells are effectively recruited at sites of inflammation, it is possible that may have undesirable effects through their ability to differentiate into pathogenic Th17 in the presence of IL-6 and/or IL-23 [73]. IL-17A-producing cells may be "inflammatory" regulatory T cells (Foxp3+ Treg) in the pathological microenvironments, and may contribute to the pathogenesis of inflammatory disease through inducing inflammatory cytokines and inhibiting local T cell immunity, and in turn may mechanistically link human chronic inflammation to tumor development [74,75]. The efficient differentiation of natural regulatory T cells to Th17 occurs after in vitro stimulation of circulating naive CD4+ T cells in the presence of Th17 polarizing factors [76]. Consistent with this our data point to the reduced circulatory naive T cells and increased production of IL-17, most likely due to activation and differentiation of naive CD4 T cells to Th17 cells.
MCSF and DCS and RANK correlation
DCs may have an important role in bone resorption associated with various inflammatory diseases, they involve ability to produce cytokines (e.g. IL-1, IL-6, TNF-α), stimulate T cell to express RANK-L, a major differentiation factor for osteoclast precursors, and could play a role in osteoclastogenesis. Several in vitro studies support the notion that immature DCs are dependent on RANK-L and MCSF, and can differentiate into osteoclast-like cells. It is possible that additional cytokines or growth factors play a role during this process [77]. We detected an association between MCSF, DCs and RANK. MCSF is able to promotes the development of DCs in vitro and in vivo [78]. MCSF signaling is indispensable for commitment of monocyte differentiation into osteoclasts, allowing subsequent induction of osteoclastogenesis by RANKL. Modulation of MCSF receptor by TNF-α converting enzyme plays a critical role in the regulation of bifurcated differentiation of monocytes into DCs or osteoclasts [79]. Once activated by MCSF, osteoclast precursor cells then express RANK, receptor for the pro-osteoclastogenesis cytokine RANKL which is expressed on the surface of the osteoblast cells, and binding of RANKL to the RANK is required for the commitment and differentiation of the preosteoclast [80].
sICAM-1 and memory effector CD4+ T cells and DCs correlation
ICAM-1 is expressed predominantly by epithelial and endothelial cells, macrophages, monocytes, B and T-lymphocytes, fibroblasts and DCs, etc. ICAM-1 might be responsible for endothelial adhesion or transmigration of DCs. Immature DCs continuously communicate with T cells in an antigen-independent manner, and this might be the mechanism used by DCs to preparing T cells for antigen encounter. These phenomena require both the ICAM- 1/LFA-1 interaction and DC-released chemokines. Protective immune responses depend on the formation of immune synapses between T cells and APCs, ICAM-1 is abundantly expressed by mature DCs and is the primary ligand of the T cell integrin LFA-1 [81-84]. ICAM-1/LFA-1 interaction may influence early events in the priming of naive T cells by facilitating T cell- APCs conjugate formation and maturation of the immunological synapse, inducing T cell adhesion and movement, T cell activation and proliferation. sICAM-1 is produced either by proteolytic cleavage of extracellular cell membrane portion or directly by cells. The shedding of surface ICAM-1 is regarded as a protective mechanism to prevent from excessive leukocyte and monocyte attachment, it is part of a negative feedback loop, sICAM-1 fails to co-stimulate T cell priming [85,86].
Our results confirm a positive correlation of sICAM-1 with DCs, and a negative correlation with memory effector CD4+ T cells. Parameswaran et al. [87] report that signals provided to T cells by ICAM-1 on APCs promote their differentiation into long-lived, proliferation-competent, central memory T cells. T cells primed on ICAM-1-null APCs differentiate preferentially into an effector memory T population. ICAM-1 expression is necessary for stable T cells-APCs synapses that enhance CD69 expression, proliferation, IFN-γ secretion, and memory cell formation [88]. Memory CD4 cells may provide protection to the host via an enhanced effector cytokine response that directs other immune cells. Thus, inhibition of proliferation may be a direct and inevitable consequence of the effector functions of the memory cells; somehow during effective immune responses CD4 memory cells must strike the right balance between expansion and effector function [89,90]. We support the opinion that elevated serum levels of ICAM-1 may be associated with lowered intercellular interactions, activation and proliferation of memory effector cells [87]. The manipulation and changes in interactions governed by ICAM-1 could influence priming and potentially bolster the development and maintenance of effector-memory populations. This process may curtail the development of highly proliferative memory cells, and ICAM-1 may also enhance the antigen-driven functional exhaustion and deletion of T cells. ICAM-1 expression on T cells may permit the activated cells to cluster and receive paracrine IL-2 signals, which may push the terminal differentiation of T cells that cannot subsequently be stably maintained. Another possibility is that the lack of ICAM-1 interactions leads to inefficient separation of memory and effector cell properties as the activated cells divide [91,92].
E-selectin and DCs correlation
E-selectin is important for cell trafficking to sites of inflammation in humans, and it plays a critical role in the recruitment of immune effectors cells to target inflammatory sites. Efficient extravasation of DCs to inflamed tissues is crucial in facilitating an effective immune response, but also fuels the immunopathology of several inflammatory disorder. Our results show a positive correlation of E-selectin to DCs. E-selectin is constitutively expressed on the surfaces of endothelial cells, and seems to be involved in adhesion process of blood DCs during inflammation. DCs, like other leukocytes, become activated and secrete many potent inflammatory mediators, including proinflammatory cytokines (e.g., TNF-α and IL-6). As professional APCs, DCs are the main orchestrators of the immune response, they patrol the body to capture antigens and migrate to the secondary lymphoid organs, while the internalized antigen is processed and presented to other immune cells [93-95].
Conclusions
We demonstrated that sICAM-1 and adiponectin, differed between fertile and postmenopausal obese women, and fertile obese and postmenopausal control women, respectively. The significant changes of IL-6 and ICAM-1 serum levels were dependent on the different BMD status. We noticed a correlation between soluble biomarkers and some blood cell populations: a) IGF-1 and B-lymphocytes, b) IL-6, IL-10. E-selectin, sICAM-1, MCSF and DCs, c) IL-10. IL-17 and naive T cells.
Highlights
• Adiponectin enhancement in postmenopausal control women - simultaneous effect of age and the impact of obesity when compared to fertile obese group.
• sICAM-1 increase in postmenopausal women, and in women with osteopenia.
• IL-6 decline in osteoporosis group.
• Inverse asociation between (1) IL-6 and age, (2) IL-17A and naive T cells, (3) sICAM-1 and memory effector CD4+ T cells.
• Positive correlation between (1) IL-6 and myeloid DCs, (2) IGF-1 and B cells, and naive T cells, (3) IL-10 and naive CD8+ T cells, and plasmacytoid DCs, (4) IL-17A and regulatory cells, (5) MCSF and DCs and RANK, (6) sICAM-1 and DCs.
Acknowledgements
This article was created by the realisation of the project ITMS No.26240120033, based on the supporting operational Research and development program financed from the European Regional Development Fund.
Author Contributions
Mira Horvathova designed the protein microarray analyses, interpreted the results and wrote the manuscript. Mira Horvathova, Michaela Szabova, Aurelia Liskova and Jana Tulinska performed immunological experiments and data analysis. Zora Krivosikova was involved in clinical biochemical data analysis. Kornelia Stefikova and Viera Spustova were responsible for clinical studies planning, densitometry and coordination of biochemical clinical analysis. Kornelia Stefikova, Michaela Szabova, Zora Krivosikova, Jana Tulinska, Viera Spustova, Aurelia liskova and Martin Gajdos critically reviewed the manuscript and approved the final version. Martin Gajdos is the guarantor of this work.
Competing Interests
The authors declare that they have no competing interests.
26498
References
- Malutan AM, Mihu D, Costin N, Mihu C (2014) Proinflammatory and anti-inflammatory cytokine changes related to menopause. Prz Menopauzalny 13: 162-168.
- Sivro A, Lajoie J, Kimani J, Jaoko W, Plummer FA, et al. (2013) Age and menopause affect the expression of specific cytokines/chemokines in plasma and cervical lavage samples from female sex workers in Nairobi, Kenya. Immunity Ageing 10: 42.
- Cioffi M, Esposito C, Vietri MT, Gazzerro P, D'Auria A, et al. (2002) Cytokine pattern in postmenopause. Maturitas 41: 187-192.
- Wang T, He C (2018) Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev 44: 38-50.
- Wang T, He C, Yu X (2017) Pro-Inflammatory Cytokines: New Potential Therapeutic Targets for Obesity-Related Bone Disorders. Curr Drug Targets 18: 1664-1675.
- Greco EA, Lenzi A, Migliaccio S (2015) The obesity of bone. Ther Adv Endocrinol Metab 6: 273-286.
- Yasui T, Maegawa M, Tomita J, Miyatani Y, Yamada M, et al. (2007) Changes in serum cytokine concentrations during the menopausal transition. Maturitas 56: 396-403.
- Brincat SD, Borg M, Camilleri G, Calleja-Agius J (2014) The role of cytokines in postmenopausal osteoporosis. Minerva Ginecol 66: 391-407.
- Azizieh FY, Shehab D, Jarallah KA, Mojiminiyi O, Gupta R, et al. (2019) Circulatory pattern of cytokines, adipokines and bone markers in postmenopausal women with low BMD. J Inflamm Res 12: 99-108.
- Yang DH, Yang MY (2019) The Role of Macrophage in the Pathogenesis of Osteoporosis. Int J Mol Sci 20.
- Pfeilschifter J, KOditz R, Pfohl M, Schatz H (2002) Changes in Proinflammatory Cytokine Activity after Menopause. Endocr Rev 23: 90-119.
- Gupta V, Mishra S, Mishra S, Kumar S, Gupta V (2018) Association of Leptin: Adiponectin ratio and metabolic risk markers in postmenopausal women. Immunology Letters 196: 63-67.
- Soni AC, Conroy MB, Mackey RH, Kuller LH (2011) Ghrelin, Leptin, Adiponectin, and Insulin Levels and Concurrent and Future Weight Change in Overweight Postmenopausal Women. Menopause 18: 296-301.
- Gerges AG, Mallat S, Matar CHF, Zerbe R, Filfili E, et al. (2008) Adiponectin and Inflammation in Health and Disease: An Update. Open Medicine J 5: 20-32.
- Rolland YM, Perry HM, Patrick P, Banks WA, Morley JE (2006) Leptin and adiponectin levels in middle-aged postmenopausal women: associations with lifestyle habits, hormones, and inflammatory markers--a cross-sectional study. Metabolism 55: 1630-1636.
- Nirmalkar K, Murugesan S, Pizano-Zárate ML, Villalobos-Flores LE, García-González C, et al. (2018) Gut Microbiota and Endothelial Dysfunction Markers in Obese Mexican Children and Adolescents. Nutrients 10.
- Al-Dahr MHS (2017) Inflammatory Biomarkers and Endothelial Dysfunction among Obese Patients with Type 2 Diabetes: A Correlational Study. Austin J Gastroenterol 4: 1080.
- Bosanská L, Michalský D, Lacinová Z, Dostálová I, Bártlová M, et al. (2010) The influence of obesity and different fat depots on adipose tissue gene expression and protein levels of cell adhesion molecules. Physiol Res 59: 79-88.
- Song Y, Manson JE, Tinker L, Rifai N, Cook NR, et al. (2007) Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes 56: 1898-1904.
- Horváthová M, Ilavská S, Štefíková K, Szabová M, Krivošíková Z, et al. (2017) The Cell Surface Markers Expression in Postmenopausal Women and Relation to Obesity and Bone Status. Int J Environ Res Public Health 14: 751-765.
- Savvidis C, Tournis S, Dede AD (2018) Obesity and bone metabolism. Hormones 17: 205–217.
- Naot D, Cornish J (2014) Cytokines and hormones that contribute to the positive association between fat and bone. Front Endocrinol (Lausanne) 5: 70.
- Chen YY, Fang WH, Wang CC, Kao TW, Chang YW, et al. (2018) Body fat has stronger associations with bone mass density than body mass index in metabolically healthy obesity. PLoS One 13: e0206812.
- Malutan AM, Costin N, Ciortea R, Mihu D (2013) Variation of Anti-inflammatory Cytokines in Relationship with Menopause. Appl Med Inform 32: 30-38.
- Maggio M, Guralnik JM, Longo DL, Ferrucci L (2006) Interleukin-6 in Aging and Chronic Disease: A Magnificent Pathway. J Gerontol A Biol Sci Med Sci 61: 575-584.
- Al-Daghri NM, Yakout S, Al-Shehri E, Al-Fawaz HA, Aljohani N, et al. (2014) Inflammatory and bone turnover markers in relation to PTH and vitamin D status among saudi postmenopausal women with and without osteoporosis. Int J Clin Exp Med 7: 3528-3535.
- Li X, Zhou Z, Zhang Y, Yang H (2016) IL-6 Contributes to the Defective Osteogenesis of Bone Marrow Stromal Cells from the Vertebral Body of the Glucocorticoid-Induced Osteoporotic Mouse. PLoS One 11.
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878-888.
- Balto K, Sasaki H, Stashenko P (2001) Interleukin-6 Deficiency Increases Inflammatory Bone Destruction. Infect Immun 69: 744-750.
- Al-Daghri NM, Aziz I, Yakout S, Aljohani NJ, Al-Saleh Y, et al. (2017) Inflammation as a contributing factor among postmenopausal Saudi women with osteoporosis, Medicine 94: 1-6.
- Puzianowska-KuÃÂÃÂÃÂâÂÂÃÂâââÂÂìÃÂæÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂúnicka M, Owczarz M, Wieczorowska-Tobis K, Nadrowski P, Chudek J, et al. (2016) Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing 13: 21.
- Toledo J, Moraes C, Souza V, Tonet-Furioso A, Afonso L, et al. (2015) Tailored antihypertensive drug therapy prescribed to older women attenuates circulating levels of interleukin-6 and tumor necrosis factor-α. Clin Interv Aging 10: 209 -215.
- Andreeva AA (2013) Dynamics the level of visfatin and markers of immune inflammation in hypertensive patients with abdominal obesity using the combination of antihypertensive therapy. Georgian Med News 225: 72-77.
- Lorenzo J (2016) The effect of immune cell products (cytokines and hematopoietic cell growth factors) on bone cells. Chapter 9 in Osteoimmunology: Interactions of the Immune and Skeletal Systems. Editors: Lorenzo J, Horowitz M, Choi Y, Takayanagi H, Schett G. Academic Press, Second Edition, pp: 143-168.
- Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, et al. (2010 ) Effect of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25: 72-81.
- Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, et al. (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double blind study. J Clin Oncol 28: 5132-51329.
- Harmer D, Falank C, Reagan MR (2018) Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front Endocrinol 9: 788.
- Edwards CJ, Williams E (2010) The role of interleukin-6 in rheumatoid arthritis-associated osteoporosis. Osteoporosis Int 21: 1287–1293.
- Angelidis G, Dafopoulos K, Messini CI, Valotassiou V, Tsikouras P, et al. (2013)The Emerging Roles of Adiponectin in Female Reproductive System-Associated Disorders and Pregnancy. Reproductive Sciences 20: 872-881.
- Diwan AG, Kuvalekar AA, Dharamsi S, Vora AM, Nikam VA, et al. (2018) Correlation of serum adiponectin and leptin levels in obesity and Type 2 diabetes mellitus. Indian J Endocr Metab 22: 93-99.
- Silvestris E, de Pergola G, Rosania R, Loverro G (2018) Obesity as disruptor of the female fertility. Reprod Biol Endocrin 16: 22.
- Jürimäe J, Jürimäe T (2007) Plasma adiponectin concentration in healthy pre- and postmenopausal women: relationship with body composition, bone mineral, and metabolic variables. Am J Physiol Endocrinol Metab 293: E42-7.
- Milewicz A, Jedrzejuk D, Dunajska K, Lwow F (2010) Waist circumference and serum adiponectin levels in obese and non-obese postmenopausal women. Maturitas 65: 272-5.
- Matsui S, Yasui T, Keyama K, Tani A, Kato T, et al. (2014) High adiponectin level in late postmenopausal women with normal renal function. Clin Chim Acta 430: 104-108.
- Lubkowska, Dobek A, Mieszkowski J, Garczynski W, Chlubek D (2014) Adiponectin as a Biomarker of Osteoporosis in Postmenopausal Women: Controversies. Disease Markers 2014: 975178.
- Díaz-Pérez FI, Hiden U, Gauster M, Lang I, Konya V, et al. (2016) Post-transcriptional down regulation of ICAM-1 in feto-placental endothelium in GDM. Cell Adh Migr 10: 18–27.
- Matsumoto K, Sera Y, Abe Y, Tominaga T, Horikami K, et al. (2002) High serum concentrations of soluble E-selectin correlate with obesity but not fat distribution in patients with type 2 diabetes mellitus. Metabolism 51: 932-934.
- Shi Q, Benderdour M, Lavigne P, Ranger P, Fernandes JC (2007) Evidence for two distinct pathways in TNFα-induced membrane and soluble forms of ICAM-1 in human osteoblast-like cells isolated from osteoarthritic patients. Osteoarthr Cartil 15: 300-308.
- Liu Y, Zhang S, Chen Y, Shi K, Zou B, et al. (2018) ICAM-1 Deficiency in the Bone Marrow Niche Impairs Quiescence and Repopulation of Hematopoietic Stem Cells. Stem Cell Reports 11: 258–273.
- Tanaka Y, Morimoto I, Nakano Y, Okada Y, Hirota S, et al. (1995) Osteoblasts are regulated by the cellular adhesion through ICAMÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂ1 and VCAMÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂ1. JBMR 10: 1462-1469.
- Lavigne P, Benderdour M, Shi Q, Lajeunesse D, Fernandes JC (2005) Involvement of ICAM-1 in bone metabolism: a potential target in the treatment of bone diseases? Expert Opin Biol Th 5: 313-320.
- Landreth KS, Narayanan R, Dorshkind K (1992) Insulin-like growth factor-I regulates pro-B cell differentiation. Blood 80: 1207-12.
- Smith TJ (2010) Insulin-Like Growth Factor-I Regulation of Immune Function: A Potential Therapeutic Target in Autoimmune Diseases? Pharmacol Rev 62: 199-236.
- Skarlis C, Nezos A, Mavragani CP, Koutsilieris M (2019)The role of insulin growth factors in autoimmune diseases. Ann Res Hosp 3: 10.
- Ni F, Sun R, Fu B, Wang F, Guo C, et al. (2013) IGF-1 promotes the development and cytotoxic activity of human NK cells. Nat Commun 4: 1479.
- Douglas RS, Gianoukakis AG, Kamat S, Smith TJ (2007) Aberrant Expression of the Insulin-Like Growth Factor-1 Receptor by T Cells from Patients with Graves’ Disease May Carry Functional Consequences for Disease Pathogenesis. J Immunol 178: 3281-3287.
- Chen J, Li J, Lim FC, Wu Q, Douek DC, et al. (2010) Maintenance of naïve CD8 T cells in nonagenarians by leptin, IGFBP3 and T3, Mech. Ageing Dev 131: 29-37.
- Macdougall CE, Wood EG1, Loschko J2, Scagliotti V1, Cassidy FC, et al. (2018) Visceral Adipose Tissue Immune Homeostasis IsRegulated by the Crosstalk between Adipocytes and Dendritic Cell Subsets. Cell Metab 27: 588-601.
- Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, et al. (2004) IL-6 Regulates In Vivo Dendritic Cell Differentiation through STAT3 Activation. J Immunol 173: 3844-3854.
- Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, et al. (2001) Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood 98: 1512-1523.
- Zhang W, Li M, Xiong S, Wang H, Xiong Y, et al. (2017) Decreased myeloid dendritic cells indicate a poor prognosis in patients with severe fever with thrombocytopenia syndrome. Int J Infect Dis 54: 113-120.
- Daudelin JF, Mathieu M, Boulet S, Labrecque N (2013) IL-6 Production by Dendritic Cells Is Dispensable for CD8+ Memory T-Cell Generation. BioMed Res Int 2013: 1-12.
- Sindhu S, Thomas R, Shihab P, Sriraman D, Behbehani K, et al. (2015) Obesity Is a Positive Modulator of IL-6R and IL-6 Expression in the Subcutaneous Adipose Tissue: Significance for Metabolic Inflammation. PLoS ONE 10: 1-17.
- Jiang HR, Muckersie E, Robertson M, Xu H, Liversidge J, et al. (2002) Secretion of interleukin-10 or interleukin-12 by LPS-activated dendritic cells is critically dependent on time of stimulus relative to initiation of purified DC culture. J Leukoc Biol 72: 978-85.
- Schülke S (2018) Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses. Front Immunol 9: 1-19.
- Haase C, Jørgensen TN, Michelsen BK (2002) Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived dendritic cells in vitro and strongly influences T-cell priming in vivo. Immunology 107: 489-499.
- Morin S, Poggi M, Alessi MC, Landrier JF, Nunes J (2017)Modulation of T Cell Activation in Obesity. Antioxidants and Redox Signaling. Mary Ann Liebert 26: 489-500.
- Ogata M, Ito T, Yang M, Wang Y, Lande R, et al. (2007) Plasmacytoid dendritic cells prime IL-10–producing T regulatory cells by inducible costimulator ligand. Exp Med 204: 105-115.
- Smith LK, Boukhaled GM, Condotta SA, Mazouz S, Guthmiller JJ, et al. (2018) Interleukin-10 Directly Inhibits CD8+T Cell Function by Enhancing N-Glycan Branching to Decrease Antigen Sensitivity. Immunity 48: 299-231. Trandem K, Zhao J, Fleming E, Perlman S (2011) Highly Activated Cytotoxic CD8 T Cells Express Protective IL-10 at the Peak of Coronavirus-Induced Encephalitis. J Immunol 186: 3642-3652.
- Koenen HJPM, Smeets RL, Vink PM, van Rijssen E, Boots AMH, et al. (2008) Human CD25highFoxp3pos regulatory T cells differentiate into IL-17–producing cells. Blood 112: 2340-2352.
- Jung MK, Kwak J, Shin EC (2017) IL-17A-Producing Foxp3+ Regulatory T Cells and Hum. Dis Immune Netw 17: 276-286.
- Kitani A, Xu L (2008) Regulatory T cells and the induction of IL-17. Mucosal Immunol Suppl 1: S43-46.
- Swanson SM, Harper J, Zisman TL (2018) Obesity and inflammatory bowel disease: diagnostic and therapeutic implications. Curr Opin Gastroent 34: 112-119.
- Kryczek I, Wu K, Zhao E, Wei S, Vatan L, et al. (2011) IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 186: 4388-4395.
- Ayyoub M, Raffin C, Valmori D (2012) Generation of Th17 from human naive CD4+ T cells preferentially occurs from FOXP3+ Tregs upon costimulation via CD28 or CD5. Blood 119: 4810-4812.
- Maitra R, Follenzi A, Yaghoobian A, Montagna C, Merlin S, et al. (2010) Dendriric cell-mediated in vivo bone resorption. J Immunol 185: 1485-1491.
- Fancke B, Suter M, Hochrein H, O'Keeffe M (2008) M-CSF: a novel plasmacytoid and conventional dendritic cell poietin. Blood 111: 150-159.
- Hiasa M, Abe M, Nakano A, Oda A, Amou H, et al. (2009)Receptor Activator of NF-κB (RANK) Signaling. Blood 114: 4517-26.
- Sher RB, Cox GA, Ackert-Bicknell C (2012) Development and Disease of Mouse Muscular and Skeletal Systems. Laboratory Mouse (Second Edition) 2012: 209-239.
- Fotis L, Giannakopoulos D, Stamogiannou L, Xatzipsalti M (2012) Intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 in children. Do they play a role in the progression of atherosclerosis? Hormones 11: 140-146.
- Weis M, Schlichting CL, Engleman EG, Cooke JP (2002) Endothelial Determinants of Dendritic Cell Adhesion and Migration. New Implications for Vascular Diseases. Arterioscler Thromb Vasc Biol 22: 1817-1823.
- Real E, Kaiser A, Raposo G, Amara A, Nardin A, et al. (2004) Immature Dendritic Cells (DCs) Use Chemokines and Intercellular Adhesion Molecule (ICAM)-1, But Not DC-Specific ICAM-3-Grabbing Nonintegrin, to Stimulate CD4+ T Cells in the Absence of Exogenous Antigen. J Immunol 173: 50-60.
- Feigelson SW, Solomon A, Biram A, Hatzav M, Lichtenstein M, et al. (2018) ICAMs Are Not Obligatory for FunctionalImmune Synapses between Naive CD4 T Cells and Lymph Node DCs. Cell Reports 22: 849-859.
- Stanciu LA, Djukanovic R (1998) The role of ICAM-1 on T-cells in the pathogenesis of asthma. Eur Respir J 11: 949-957.
- Comrie WA, Li S, Boyle S, Burkhardt JK (2015) The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining ICAM-1 mobility. J Cell Biol 208: 457.
- Parameswaran N, Suresh R, Bal V, Rath S, George A (2005) Lack of ICAM-1 on APCs during T Cell Priming Leads to Poor Generation of Central Memory Cells. J Immunol 175: 2201-2211.
- Engelhardt JJ, Krummel MF (2008) The Importance of Prolonged Binding to Antigen-Presenting Cells for T Cell Fate Decisions. Immunity 28: 143-145.
- MacLeod MKL, McKee A, Crawford F, White J, Kappler J, et al. (2008) CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. PNAS 105: 14521-14526.
- Mueller SN, Gebhardt T, Carbone FR, Heath WR (2013) Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Ann Rev Immunol 31: 137-161.
- Cox MA, Barnum SR, Bullard DC, Zajac AJ (2013) ICAM-1–dependent tuning of memory CD8 T-cell responses following acute infection. Proc Natl Acad Sci USA 110: 1416–1421.
- Cox M, Bullard D, Zajac A (2012) ICAM-1 interactions enhance effector cell contraction and memory T cell function following acute infection. J Immunol 188: 1416–1421.
- Silva M, Videira PA, Sackstein R (2017) E-Selectin Ligands in the Human Mononuclear Phagocyte System: Implications for Infection, Inflammation, and Immunotherapy. Front Immunol 8: 1878.
- Srinivas U, Larsson M, Lundblad A, Forsum U (1993) EÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂSelectin Involvement in In Vitro Adhesion of Blood Dendritic Cells to Human Umbilical Cord Endothelial Cells. Scand J Immunol 38: 273-278.
- Ye ZS, Huang RC (2018) Selectins modify dendritic cells during atherosclerosis. Chronic Dis Transl Med 4: 205-210.







