Research Article - (2025) Volume 16, Issue 1
Pulmonary Tuberculosis and Diabetes Comorbidity is Associated with Systemic Raising of Th1, Th17 and Other Biochemical Parameters
Leonard Fonkeng Sama1*,
Thibau Flaurant Tchouangueu2,
Omer Bebe Ngouateu3,
Salomon Bonsi Tchuandom1,
Ousenu Karimo1 and
Christopher B Tume1,4
1Department of Biochemistry, University of Dschang, Research Unit of Microbiology and Antimicrobial Substances, P.O Box 96 Dschang, Cameroon
2Department of Microbiology, Haematology and Immunology, University of Dschang, P.O Box 96 Dschang, Cameroon
3Department of Animal Biology and Physiology, University of Yaoundé I, Cameroon
4Department of Biochemistry, University of Bamenda, P.O. Box 812-Yaoundé, Cameroon
*Correspondence:
Leonard Fonkeng Sama, Department of Biochemistry, University of Dschang, Research Unit of Microbiology and Antimicrobial Substances, P.O Box 96 Dschang,
Cameroon,
Email:
Received: 19-Jun-2024, Manuscript No. IPACM-24-14928;
Editor assigned: 21-Jun-2024, Pre QC No. IPACM-24-14928 (PQ);
Reviewed: 05-Jul-2024, QC No. IPACM-24-14928;
Revised: 08-Jan-2025, Manuscript No. IPACM-24-14928 (R);
Published:
15-Jan-2025
Abstract
Background: This study aims to assess the impact of type 2 diabetes on pulmonary tuberculosis immune response.
Methods: Diagnosis of tuberculosis was based on positivity on the sputum smear and culture, whereas type 2 diabetes was diagnosed based on fasting blood sugar, 2-h Plasma Glucose (PG), oxidative method and glycated haemoglobin. Standardized techniques were used to obtain liver enzymes and lipid profiles, whereas ELISA cytokine assay system was used to measured plasma cytokines levels.
Results: Significantly higher levels of fasting blood glucose (p<0.0001), 2-hPG (p=0.0097) and percentage of glycated haemoglobin (p<0.0001) were found in TB patients with diabetes as compared with TB patients without diabetes (p<0.05). Regarding lipid profile, higher total cholesterol (p=0.0093), serum triglycerides (p=0.0001) and low-density lipoprotein cholesterol (p=0.0086) were noted among TB patients with diabetes, whereas High Density Lipoprotein (HDL) cholesterol was found to be significantly elevated (p=0.0002) among TB patients without diabetes. Tuberculosis and diabetes were linked by increased concentration of Th1 (IFN-γ and TNF-α) and Th17 (IL-17A) cytokines. The increase levels of systemic cytokines were showed to be associated with the HbA1c levels among TB patients except for IL-6 where there was no association with HbA1c. A significant association (p=0.0001) was also found between IL-22 and IFN-γ plasmatic levels.
Conclusion: Our study shows an increase in cytokine response of TB diabetic patients, signalling that type 2 diabetes potentially participates in chronic inflammation that increases pathology and low control of tuberculosis.
Keywords
Pulmonary tuberculosis; Serum cytokines; Type 2 diabetes; Glycated haemoglobin; Serum triglycerides
Introduction
Despite the improvement in Tuberculosis (TB) therapy, the disease remains responsible for 2-3 million deaths annually worldwide. Its association with type 2 diabetes mellitus is evident and has been recognized for centuries. It has been proven that, diabetes mellitus is a leading cause of tuberculosis and can affect the aspect and medical outcome of the disease [1]. Several authors have shown the relative risk of diabetic patients to develop tuberculosis ranging from 2.44 to 8.33 compared to patients without diabetes [1,2]. According to Dooley and Chaisson, as well as Jeon and Murray, the possibility of developing tuberculosis among type 2 diabetes patients has been recognized through clinical and epidemiological studies. It has been proven that type 2 diabetes is linked with the severity of tuberculosis, simultaneously affecting the disease manifestations and the response to medication. Despite all the clinical and epidemiological consequences of tuberculosis and diabetes, little data is available on the biochemical and immunological mechanisms explaining the susceptibility of TB to diabetes. The susceptibility of diabetic patients to tuberculosis has been imputed to factors among which macrophage and lymphocyte functions as well as hyperglycaemia and insulin resistance [3,4]. Previous studies in patients having both diabetes and tuberculosis have shown a reduction of proinflammatory cytokines [5,6]. A study conducted by Kumar and collaborators among children suffering from tuberculosis reported a suppressed level of T-helper type 1 (Th1), T helper type 2 (Th2) and type 17 (Th17) cytokine in active tuberculosis [7]. Kumar et al. [8] also reported a dysregulated cytokine response in pulmonary tuberculosis associated with prediabetes patients. Another study conducted by Kumar et al., on diabetic and non-diabetic patients with tuberculosis reported heightening systemic Th1, Th17, and other pro-inflammatory cytokines. The immune response orchestrated in TB infection with Th1, Th2 and Th17 cytokines family have been proven to be involved in protection against tuberculosis disease, whereas Th2 and anti-inflammatory cytokines and type 1 IFNs have been proved to be linked with elevated susceptibility to disease. This study aimed to evaluate the impact of type 2 diabetes on pulmonary TB. To achieve this, we assessed the plasma concentration of Th1 and Th17 and other cytokines among patients suffering simultaneously from tuberculosis and diabetes and compared them with those of patients suffering from pulmonary tuberculosis without diabetes.
Materials and Methods
Study population
Our study was based on a group of 42 active pulmonary Tuberculosis (TB) patients newly diagnosed at the tuberculosis reference laboratory in Bamenda, North West Region of Cameroon from November 2021 to May 2022.
Where included in this study all TB patients, whereas where excluded from the study TB patients under TB treatment, immunosuppressive treatment or under chemotherapies, all TB relapse patients but also Multiple Drugs Resistant (MDR) TB patients.
Among the 42 patients recruited in this study, 21 had diabetes and the other 21 were exempt of the disease. The diagnosis of tuberculosis was through sputum smear and culture positivity. The diagnosis of type 2 diabetes was based on American Diabetes Association (ADA guidelines of fasting blood sugar ≥ 126 mg/dl, 2h Plasma Glucose (PG) ≥ 200 mg/dl but also glycated Haemoglobin (HbA1c) cut-off point 6.5% [9]. All of these were further confirmed by the enzymatic method using glucose oxidase/peroxidase kit (Sino Biological Inc, India). Differences obtained in both groups regarding the smear grades was not significant, although the TB diabetes group presented a slightly elevated smear grade. Anthropometric parameters, among which, Body Mass Index (BMI) and waist circumference; and biochemical parameters including blood glucose level, lipid profile and liver enzymes were obtained by standardized techniques as detailed by the International Federation of Clinical Chemistry and Laboratory Medicine.
Determination of circulating cytokines levels
The concentration of plasma cytokines was determined using an ELISA cytokine assay system (Sino Biological Inc). Analysed parameters included IFN-γ, Tumor Necrosis Factor (TNF)-α, IL-1β, IL-12, IL-4, IL-6, IL-10, IL-12, IL-17A and IL-22.
Statistical analysis
Data were analysed using GraphPad Prism 8.0.2 Software, San Diego, California, USA. Continuous variables from subjects’ characteristics and cytokines plasma concentration were expressed as means (± Standard Deviation) for parametric data and medians (Inter Quartile Ranges (IQR)) for non-parametric data. Comparison between diabetic and non-diabetic sets of TB positive subjects was made using the t-test and non-parametric Mann-Whitney U test as appropriate for continuous variables. Correlation between glycated Haemoglobin (Hb1Ac) and cytokines levels was done using spearman “r” value. A p-value of (P<0.05) was considered statistically significant for all comparisons.
Ethical consideration
Ethical clearance was obtained from «Ethics Review and Consultancy Committee of Cameroon Bioethics Initiative (CAMBIN)» (CBI/294/ERCC/CAMBIN), and an informed written and signed consent was obtained from each participant.
Results
Characteristics of the study population
Characteristics of the study population such as anthropometric, clinical and biochemical features examined during the study are presented in Table 1. No significant difference was observed among anthropometric parameters (Age, sex, BMI, and waist circumference between TB patients with and without diabetes. Clinical parameters were found to be significantly elevated in TB patients with diabetes) fasting blood glucose (5.70 ± 0.1721 vs. 5.70 ± 0.1721 mg/dl; p<0.0001), 2hPG (11.30 ± 1.815 vs. 6.88 ± 0.3133 mg/dl; p=0.0097) and glycated haemoglobin% (12.42 ± 1.678 vs. 4.265 ± 2.221 mg/dl; p<0.0001) compared to TB patients without diabetes. While, regarding biochemical features, total cholesterol (157(132–198) vs. 131(109–198 mg/dl), p=0.0093), serum triglycerides (143(56– 398) vs. 48 (26–317 mg/dl), p=0.0001) and Low Density Lipoprotein (LDL)-cholesterol (109 (51–162) vs. 92 (42–136 mg/ dl), p=0.0086), were significantly higher among TB patients with diabetes, whereas HDL-cholesterol levels were found to be significantly reduced among TB patients without diabetes (39 (32– 63) vs. 49 (28–70 mg/dl), p=0.0002). Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Gamma-glutamyl Transferase (δGT) and Alkaline Phosphatase (ALP) showed no significant differences between the two study populations.
| Characteristics |
TB-DM (n=21) |
TB-NDM (n=21) |
P-value |
| Age (year) |
46.76 ± 2.789 |
46.95 ± 2.816 |
0.9619 |
| Sex, M/F |
10/11 |
11/10 |
0.9999 |
| BMI, kg/m2 |
19.81 ± 0.9611 |
20.19 ± 1.027 |
0.7882 |
| Waist circumference |
79.29 ± 2.014 |
80.29 ± 2.240 |
0.7417 |
| Fasting blood glucose (mg/dl) |
10.52 ± 0.9521 |
5.70 ± 0.1721 |
<0.0001 |
| 2h-PG (mg/dl) |
11.30 ± 1.815 |
6.88 ± 0.3133 |
0.0097 |
| HbA1c (%) |
12.42 ± 1.678 |
4.265 ± 2.221 |
<0.0001 |
| Total cholesterol (mg/dl) |
157 (132-198) |
131 (109-198) |
0.0093 |
| Serum triglycerides (mg/dl) |
143 (56-398) |
48 (26-317) |
0.0001 |
| HDL cholesterol (mg/dl) |
39 (32-63) |
49 (28-70) |
0.0002 |
| LDL cholesterol (mg/dl) |
109 (51-162) |
92 (42-136) |
0.0086 |
| ASAT (U/l) |
247.6 (7.2-73,5) |
239.8 (3.3-30.4) |
0.8813 |
| ALAT (U/l) |
379.8 (43.8-486,9) |
325.5 (108.1-633,5) |
0.5499 |
| A³-GT (U/l) |
379.8 (43.8-486,9) |
325.5 (108.1-633.5) |
0.8178 |
| ALP (U/l) |
64.79 (221.8-616) |
31.71 (177.2-691.3) |
0.2403 |
| Note: 2h-PG: 2 hours plasma glucose; ALP: Alkaline phosphatase; ALAT: Alanine aminotransferase; ASAT: Aspartate aminotransferase; BMI: Body mass index; ÃÂ?³-GT: Gamma glutamyl transferase; M/F: Male/Female; HbA1c: Glycated hemoglobin; HDL: High density lipoprotein; LDL: Low Density Lipoprotein; OGTT: Oral Glucose Tolerant Test; TBâ??DM: Tuberculosis with Diabetes Mellitus; TBNDM: Tuberculosis without Diabetes Mellitus. |
Table 1: Anthropometric and biochemical parameters of TB-DM and TB without DM.
Impact of diabetes on the serum circulating level of th1, th2, th17, il1 family, proinflammatory cytokine and regulatory cytokines
To evaluate the impact of type 2 diabetes on Th1, Th2, Th17, IL1 Family, Pro-inflammatory and Regulatory cytokines on tuberculosis, we determined the plasma concentration of Interferon-gamma (IFN-γ), Tumor Necrosis Factor (TNF)-α, Interleukins (IL) namely: IL-1β, IL-12, IL-4, IL-6, IL-10, IL-17A and IL-22 in TB patients with and without diabetes (Figure 1). Figure 1 shows the geometric mean of circulating concentration of Th1 cytokines, namely IFN-γ (794.5 ± 326.2 vs. 147.8 ± 130.5 pg/ml) and TNF-α (800.9 ± 372.7 vs. 500.6 ± 324.9 pg/ml) to be highly important among diabetics compared to patients without diabetes. The plasma concentration of regulatory cytokine IL-10 was also found to highly increased in patients having diabetes compared to those without (160.7 ± 81.03 pg/ml vs. 68.2 ± 67.72 pg/ml). Furthermore, the plasma concentration of Th17 cytokine IL-17A, was also found to be significantly raised among diabetes compared to non-diabetic patients with tuberculosis (229.0 ± 187.4 vs. 105.7 ± 108.4 pg/ml). Contrary to IL-22, the concentration was significantly lower in diabetic compared to non-diabetic patients (7.09 ± 3.538 vs. 22.720 ± 2.278 pg/ml). Thus, co-morbid tuberculosis patients are linked with high plasma concentration of IFN-γ, TNF-α, IL-17A and IL-10 cytokines.
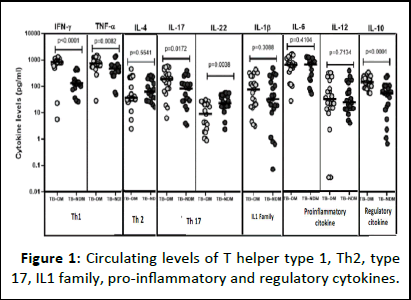
Figure 1: Circulating levels of T helper type 1, Th2, type 17, IL1 family, pro-inflammatory and regulatory cytokines.
Note: TB–DM: Tuberculosis with Diabetes Mellitus; TB–NDM: Tuberculosis without Diabetes Mellitus; IFN-γ: Interferon gamma; TNF-α: Tumor Necrosis Factor alpha; Ilβ: Interleukine-1 Beta; IL12: Interleukine-12; IL4: Interleukine-4; IL6: Interleukine-6; IL10: Interleukine-10; IL17: Interleukine-17; IL22: Interleukine-22; Th1: Type 1 helper T-cells; Th2: Type 2 helper Tcells; Th17: Type 17 helper T-cells.
Correlation between plasma cytokines and Hba1c concentration
To assess the relationship between the plasma concentrations of Th1, Th17, pro-inflammatory cytokines, and the level of diabetic complication, we determined the link between IFN-γ, TNF-α, IL-6, IL-17A, and IL-22 with HbA1c concentration. Figure 2 presents plasma concentration of IFN-γ (r=0.700; p<0.0001), TNF-α (r=0.5263, p=0.0003), IL-17A (r=0.3931; p=0.0100) and IL-22 (r=0.6490; p=0.0001), each shows an important positive association with the HbA1c concentration among TB patients. This was not the case with IL-6 (r=0.2251; p=0.1517) where there was no association with glycated haemoglobin.
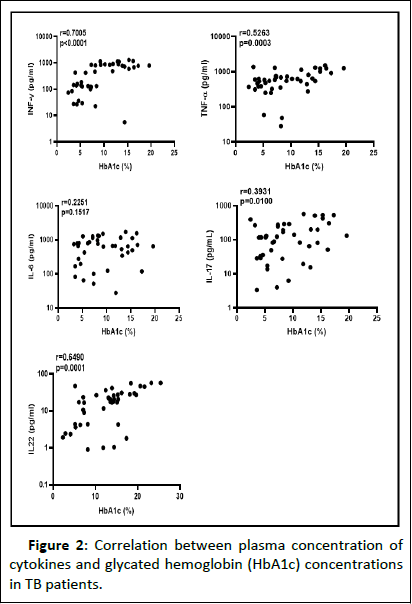
Figure 2: Correlation between plasma concentration of cytokines and glycated hemoglobin (HbA1c) concentrations in TB patients.
Increased correlation of IFN-γ and IL-22 among diabetic patients with active pulmonary tuberculosis
In the goal of discriminating diabetic from non-diabetic TB positive individuals, the correlation between certain plasmatic cytokine levels were determined as shown in Figure 3. These data showed a significantly increased association between IL-22 and IFN-γ plasmatic levels (r=0.5150; p=0.0005).
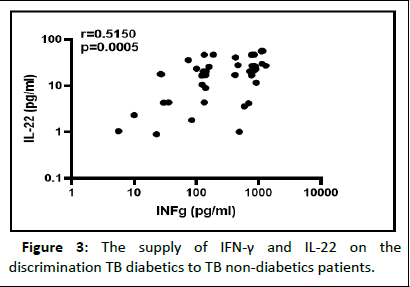
Figure 3: The supply of IFN-γ and IL-22 on the discrimination TB diabetics to TB non-diabetics patients.
Discussion
The immune components are denatured in diabetes mellitus. Changes include specific cytokines, activation process and state of immune cell subsets, as well as an increase of tissue apoptosis and fibrosis [1,10]. This may be due to inflammation involved in the diabetes pathogenesis, but this anti-genetic response remains unclear. It has been proven that a diabetic patient is 3.59 times at risk of developing tuberculosis compared to nondiabetic patient. The immunological basis for this susceptibility is not yet well understood. One possible explanation is dysglycaemia in which both diabetic and pre-diabetes that impairs the immune function and, in consequence, permits the primary infection or the reactivation of latent TB [3,11,12]. According to Kumar and Babu, immune responses against microbial pathogens in patients with diabetes are impaired, especially in those with continuous hyperglycaemia. The application of this to tuberculosis infection is not welldocumented [4,13]. The infection outcome of host defence against mycobacterial infections is mediated mostly by cytokines among which IFN-γ and TNF-α, of major importance for body defence to fight foreign intracellular infective pathogens [5,14].
Newly recognized Th17 cells have provided new observation on important mechanisms in antimicrobial host defence including M. tuberculosis [7,15,16], although the main function of IL-17 against Mycobacterium tuberculosis is not clear. Our results on concomitant tuberculosis and diabetes patients showed an important type of cytokine expression. Firstly, the expression of cytokines implicated in the protection against infection, namely Interferon-gamma, Tumor necrosis factoralpha, Interleukin-6, Interleukin-10 and Interleukin-17A, are all expressed at higher concentration in diabetic's patients. Secondly, those not having a role in resistance, including Interleukin-22, are found at extremely lower concentrations. The increase of Th17 cells led in a high production of inflammatory cytokines produced by Th17/Th22 cells [14] and T cells. Interleukin-17A induce mature granuloma formation which is very important in preventing Mycobacterium tuberculosis infection [17-19]. IL-22 stimulates the secretion of antibacterial peptides component that destroy infective pathogen but also activate macrophages that induce the production of TNF-α. Our study, therefore, suggests that in TB-diabetes patients, the regulation of IL-17A is different compared to IL-22. However, cell-mediated immune responses are distorted in patients having resistance to insulin, which may point out that T-cell factors are associated with insulin sensitivity imbalance. T cells having CD4+ markers also known as TCD4+ cells, probably increase Interferongamma and Interleukin-17A production thus promoting inflammation and increase of Th1 or Th17 cytokines leading to insulin resistance [1,20]. Moreover, type 2 diabetes has also been proven to lower frequencies of regulatory T cells, demonstrating pro-inflammatory imbalance cytokines in patients with diabetes [2,21]. This underlying the need of self-diabetes controlled that could lead to raising of cytokines in TB diabetic patients which are linked with high plasma concentration of the interleukin-1 family and other cytokines having proinflammatory activities. Resistance to infection is mostly associate with IL-1α, whereas IL-12 appeared to produce a protective action against Mycobacterium tuberculosis [22]. In response to inflammatory stimuli, Interleukin-6 (IL-6) produced will induce cells proliferation, differentiation and apoptosis [4,23]. It has been proven to intermediate in the inhibition of disease advancement. The increasing baseline concentration of pro-inflammatory cytokines in TB patients co-morbid to diabetes could decrease plasma concentration of regulatory cytokines as shown by the decrease of plasma concentration of IL-10. Elsewhere, regulation of Th2 cytokines increases susceptibility of infection. Nevertheless, this study suggests that host protective cytokines do not expose to altered homeostasis production but are currently present in higher concentrations in TB diabetes patients and are not linked to the decreased production of host protective proinflammatory cytokines.
Conclusion
The baseline description of anthropometric parameters shows no significant difference between TB patients with and without diabetes. Clinical parameters were found to be significantly higher in TB diabetic patients compared to non-diabetic TB, while some biochemical features except for HDL cholesterol were found to be significantly higher among TB patients without diabetes, others were found to be significantly higher among TB patients with diabetes. Pulmonary TB with diabetes is linked with a high concentration of Th1 (IFN-γ and TNF-α), Th17 (IL-17A) and regulatory cytokines (IL-10). The plasma concentration of IFN-γ, TNF-α, IL-17A, and IL-22 exhibited each an important positive association with the HbA1c concentration in TB patients except with IL-6 where there is no association with glycated haemoglobin. A significantly high association was found between IL-22 and IFN-γ plasmatic levels.
Acknowledgments
We wish to thank all participants who sacrificed their time and donated blood and sputum samples for this study. We also thank all technicians of tuberculosis reference laboratory in Bamenda, North West Region of Cameroon for their contributions.
Disclosure
The authors declare that they have no competing interests.
Author’s Contributions
LFS and CBT designed the study. LSF and IMA and OK performed the field data collection, TFT and OBN analysed the data, LSF, STB draft the manuscript; all the authors read and approved the final manuscript.
Funding
The authors received no financial support for the research and writing of this manuscript.
Competing Interests
None declared.
Provenance and Peer Review
Not commissioned; externally peer reviewed.
Data Availability Statement
No additional data that support the finding of this study is available.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the methods section for further details.
Author Information
• LSF is a PhD in the Department of Biochemistry of theUniversity of Dschang, Cameroon.
• TFT is a lecturer in the Department of Microbiology, Haematology and Immunology of the University of Dschang,Cameroon.
• OBN is a Lecturer in the Department of Biology of the University of Yaounde
• STB is a PhD in the Department of Biochemistry of the University of Dschang, Cameroon and Public School of Medical Laboratory Technicians, Yaounde, Cameroon
• OK is a PhD student in the Department of Biochemistry of the University of Dschang, Cameroon.
• CBT is an Associate Professor of Immunology and Molecular Biology in the Department of Biochemistry of the University of Dschang in Cameroon and Head of Department of Biochemistry of the University of Bamenda, Cameroun.
References
- Dooley KE, Chaisson RE (2009) Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 9:737-746
[Crossref] [Google Scholar] [PubMed]
- Jeon CY, Murray MB (2008) Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 5:e152
[Crossref] [Google Scholar] [PubMed]
- Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Fay MP, et al. (2013) Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc 10:441-449
[Crossref] [Google Scholar] [PubMed]
- Martinez N, Kornfeld H (2014) Diabetes and immunity to tuberculosis. Eur J Immunol 44:617-626
[Crossref] [Google Scholar] [PubMed]
- Vallerskog T, Martens GW, Kornfeld H (2010) Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol 184:6275-6282 [Crossref] [Google Scholar] [PubMed]
- Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, et al. (2008) Tuberculosis in poorly controlled type 2 diabetes: Altered cytokine expression in peripheral white blood cells. Clin Infect Dis 47:634-641
[Crossref] [Google Scholar] [PubMed]
- Kumar NP, Anuradha R, Suresh R, Ganesh R, Shankar J, et al. (2011) Suppressed type 1, type 2, and type 17 cytokine responses in active tuberculosis in children. Clin Vaccine Immunol 18:1856-1864
[Crossref] [Google Scholar] [PubMed]
- Kumar NP, Banurekha VV, Nair D, Sridhar R, Kornfeld H, et al. (2014) Coincident pre-diabetes is associated with dysregulated cytokine responses in pulmonary tuberculosis. PLoS One 9:e112108
[Crossref] [Google Scholar] [PubMed]
- Rodriguez-Saldana J (2019) The diabetes textbook: Clinical principles, patient management and public health issues. 1st Edition. Springer International Publishing. Phra Nakhon, Bangkok. 2740
[Google Scholar]
- Kumar Nathella P, Babu S (2017) Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 152:13-24
[Crossref] [Google Scholar] [PubMed]
- Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, et al. (2004) Tuberculosis and diabetes in southern Mexico. Diabetes Care 27:1584-1590
[Crossref] [Google Scholar] [PubMed]
- Ayelign B, Negash M, Genetu M, Wondmagegn T, Shibabaw T (2019) Immunological Impacts of Diabetes on the Susceptibility of Mycobacterium tuberculosis. J Immunol Res 2019:6196532
[Crossref] [Google Scholar] [PubMed]
- Al-Attiyah RJ, Mustafa AS (2009) Mycobacterial antigen-induced T helper type 1 (Th1) and Th2 reactivity of peripheral blood mononuclear cells from diabetic and non-diabetic tuberculosis patients and Mycobacterium bovis Bacilli Calmette-Guerin (BCG)-vaccinated healthy subjects. Clin Exp Immunol 158:64-73
[Crossref] [Google Scholar] [PubMed]
- Li Y, Wei C, Xu H, Jia J, Wei Z, et al. (2018) The Immunoregulation of Th17 in Host against Intracellular Bacterial Infection. Mediators Inflamm 2018:6587296
[Crossref] [Google Scholar] [PubMed]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123-1132
[Crossref] [Google Scholar] [PubMed]
- Stockinger B, Veldhoen M, Martin B (2007) Th17 T cells: linking innate and adaptive immunity. Semin Immunol 19:353-361
[Crossref] [Google Scholar] [PubMed]
- Shen H, Chen ZW (2018) The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell Mol Immunol 15:216-225
[Crossref] [Google Scholar] [PubMed]
- Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien RL, Ikuta K, et al. (2010) Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol 184:4414-4422
[Crossref] [Google Scholar] [PubMed]
- Guillon A, Jouan Y, Brea D, Gueugnon F, Dalloneau E, et al. (2015) Neutrophil proteases alter the interleukin-22-receptor-dependent lung antimicrobial defence. Eur Respir J 46:771-782
[Crossref] [Google Scholar] [PubMed]
- Xia C, Rao X, Zhong J (2017) Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J Diabetes Res 2017:6494795
[Crossref] [Google Scholar] [PubMed]
- Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, et al. (2013) Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis 208:739-748
[Crossref] [Google Scholar] [PubMed]
- Martinez AN, Mehra S, Kaushal D (2013) Role of interleukin 6 in innate immunity to Mycobacterium tuberculosis infection. J Infect Dis 207:1253-1261
[Crossref] [Google Scholar] [PubMed]
- Domingo-Gonzalez R, Prince O, Cooper A, Khader SA (2016) Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol Spectr 4:738-749
[Crossref] [Google Scholar] [PubMed]
Citation: Sama LF, Tchouangueu TF, Ngouateu OB, Tchuandom SB, Karimo O, et al. (2025) Pulmonary Tuberculosis and Diabetes Comorbidity is Associated with Systemic Raising of Th1, Th17 and Other Biochemical Parameters. Arch Clin Microbiol Vol:16 No:1
Copyright: © Copyright it Medical Team | This article is available from: https://www.itmedicalteam.pl/archives-of-clinical-microbiology.html








