Keywords
Quality control circle, Surgical instrument, Security management
Introduction
Quality control circle (QCC) is a group of workers who do the same or similar work, whom meet regularly to identify, analyze and solve work-related problems. Normally small in size, the group is usually led by a supervisor or manager and presents its solutions to management; where possible, workers implement the solutions themselves in order to improve the performance of the organization and motivate employees. Quality circles were at their most popular during the 1980s, but continue to exist in the form of similar worker participation schemes.
At our hospital, a quality management system was developed according to the ISO 9001. Additionally, several quality circles and an external quality control system with three tracer diagnoses were carried out and two studies were performed to detect the internal and external acceptance of the hospital. All strategies induce an increase in the quality of management and of the patients' outcome.
The rapid development of new and improved surgical techniques has led to more types of surgical instruments being used in medicine. In the operating room, the performance and integrity of surgical instruments can directly influence patients' safety. Therefore, the security management of surgical instruments can improve surgical patients' safety. QCC is a quality management approach where the staff volunteers to work as a team actively promoting security management of surgical instruments [1]. Our operating room set up a team named "Safe Circle" in January 2015 that allowed our staff to analyze and improve existing problems of security management of tracking surgical instruments.
Materials and Methods
Objective
QCC is made up of seven individuals that included four chief nurses and three senior nurses. One chief nurse was designated team leader who was responsible for overseeing the other nurses and did statistical analysis of the data. First, deficiencies existing in the security management of surgical instrument traceability from August to December in 2014 was determined and recorded. Second, QCC method was put into practice. Finally, they collected the deficiencies in surgical instrument traceability again from January to May in 2015 and analyzed the result.
Methods
Figure 1 shows the design for the “Safe Circle” emblem. The name and the design was developed to illustrate the importance of excellent security management of surgical instruments can directly contribute to patient safety. The "SSS" stands for Service, Safety, and Satisfaction describing our goal of providing patients with safe and satisfactory service.

Figure 1: Circle emblem
Put forward more practicable themes via QCC brainstorming and filtered out one topic through voting to reduce the deficiency rates in surgical instruments traceability.
Data collection: Two time periods were selected for this comparative study and surgical instrument deficiency report were collected for each period of time: The first one was from August to December of 2014, when the QCC method was not applied. The second period was from January 2015 to the end of May 2015 when the QCC method were implemented and managed by the “Safe Circle” team. The deficiencies in surgical instrument traceability were identified and recorded monthly for these two different time periods and a comparative analysis was performed.
Goals
Set a target value: Reduce deficiencies due to the damage of instrument performance, target value=status value-(the status value×cumulative percentage×the members' capacity)=269- (269×22.6%×80%)=220.
Set a second target value: reduce deficiencies resulting from instruments placed in wrong position, with a target value is 116.
Set a third target value: reduce deficiencies due to the damage of instrument integrity; with a target value is 91.
Strategy and implementation: Take pictures of all the surgical instruments to make a photo album and training nurses to identify each particular instruments performance, usage and be familiar with the instruments processing. Regularly check and replace damaged surgical instruments.
Build graphical processes on how to handle instruments after surgery, and following the standardized procedures: first, scrub nurses call the surgical instrument recycling staff before checking the instruments with recycling staff, then package and transfer surgical instruments; In the next step, the recycling staff will transfer the instruments to the workers in the decontamination area through the supply elevators, which allows the cleaning workers to take the instruments directly from the elevator and count them carefully. Next, they will scan each name and the receiver's work license; then place them in the automatic cleaning machines for cleaning and disinfection. Next, packing workers will scan the instruments' names, where the computer traceability system will automatically display package types, quantities and pictures of the corresponding instrument. The instruments will not be placed into rigid containers until their types and quantities are double checked by the workers. Afterwards, sterilization workers will scan the bar codes outside rigid containers before the containers are placed into the autoclave sterilizer; after the above steps, they will be uploaded to the sterile storage room.
The training staff will recycle, clean, pack, sterilize, store and distribute the instruments. Every step is recorded automatically in the computer, so that any mistakes can be traced to the person responsible.
There is a need to systematically train staff to master the instrument traceability system processes.
Standardize the handling processes of exotic instruments by tracking management and confirming the correct types and quantities, by attaching package lists.
The special instruments for specialized management, such as making a notebook for specialized instruments in which includes the names of specialized microsurgical instruments, precision ones, precious ones and their corresponding pictures. Consequently, it is important that staff is able to identify instrument integrity clearly (Figure 2).
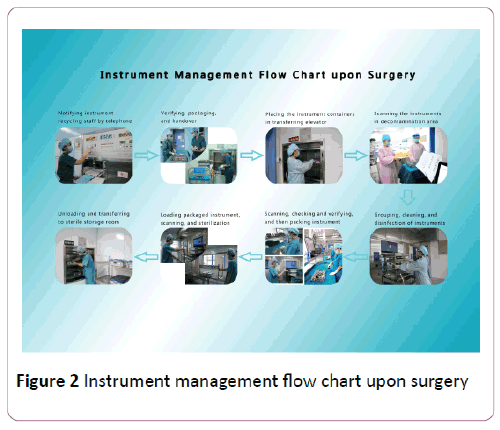
Figure 2: Instrument management flow chart upon surgery
Data analysis
Statistical analysis was performed using SPSS software for windows (Version 20.0 Armonk, NY: IBM Corp) for data processing. Comparative analysis has revealed the significant difference among surgical instrument deficiencies between before and after QCC management. The significant decrease of the surgical instrument deficiencies were detected for the observation test group. Chi-square tests were used for this comparative study and the significance level was set at p<0.05.
Results
In order to establish an intervention for improved instrument management, all the reports of surgical instrument damages were collected from different parts of operating rooms monthly for five months (control group). Analysis of these collected data enabled us to identify nine different types of surgical instrument damage or deficiency problems (Table 1).
| No |
Major problems |
2014-8 |
2014-9 |
2014-10 |
2014-11 |
2014-12 |
Total |
Percentage
(%) |
Cumulative (%) |
| 1 |
The damage of instrument performance |
59 |
56 |
48 |
52 |
54 |
269 |
22.6 |
22.6 |
| 2 |
Instruments placed in the wrong position |
48 |
44 |
28 |
25 |
20 |
165 |
13.9 |
36.5 |
| 3 |
The damage of instrument integrity |
45 |
28 |
23 |
30 |
25 |
151 |
12.7 |
49.2 |
| 4 |
Instrument packages are expired |
36 |
28 |
31 |
26 |
25 |
146 |
12.3 |
61.5 |
| 5 |
Receiver of instruments does not sign |
24 |
32 |
30 |
24 |
22 |
132 |
11.1 |
72.6 |
| 6 |
Instrument packages arebroken |
26 |
30 |
25 |
23 |
16 |
120 |
10.1 |
82.7 |
| 7 |
The loss of instruments |
20 |
25 |
20 |
21 |
17 |
103 |
8.7 |
91.4 |
| 8 |
The sign of instruments is wrong or unable to identify |
18 |
12 |
15 |
10 |
8 |
63 |
5.3 |
96.7 |
| 9 |
The date of validity is wrong |
11 |
13 |
5 |
5 |
6 |
40 |
3.3 |
100.0 |
| 10 |
Total |
287 |
268 |
225 |
216 |
193 |
1189 |
|
|
Table 1: The deficiency data about surgical instrument traceability management from August to December in 2014.
Comparative analysis also indicated that among the total of 1,189 cases of reported instrument damages for the period time, the top three causes which were accounted for nearly 50% of totally reported damages were due to:
1) Physical damage of instruments (269/1189): This is mainly due to the staff being unfamiliar with the surgical instruments leading to using the instruments improperly and improper maintenance of the precision instruments.
2) Instruments placed in the wrong position (165/1189): This is attributed to poor accountability of the staff who does return unused instruments in the operating room promptly to the sterile storage room.
3) The damage of instrument integrity (151/1189): This is due to instruments are not being properly checked during procedures and the instrument package cloth is damaged or improperly packaged (Table 1).
This initial study and analysis allowed us to identify the main problems associated with current instrument management and to focus our intervention attempt on these different areas. Following the establishment and implementation of QCC system, reports of instrument damage were also collected monthly for another five months between January and May of 2015 (test group). The reported cases of instrument damage have significantly reduced from 1189-693 for the test period time (p<0.05) even with the largely increased numbers of instrument packs (20,071).
In particular, the deficiencies due to the damage of instrument performance, instruments placed in the wrong position, and the damage of instrument integrity the mentioned three areas were reduced from 269, 165, and 151 to 209, 115, and 89, respectively for the second time period with the QCC intervention. These data have met the designed respective target values (220, 116, and 91, respectively) for these three deficiency areas, showing a prominent reduction in the deficiency of instrument management. In addition, significant improvement in instrument management has also been achieved in the other six areas.
Before starting QCC, a total of 1,189 cases of surgical instrument traceability deficiencies occurred during the use of 14,645 packaged instruments over the initial time period (control group).
After QCC was implemented, the number of deficiencies dropped to 693 between January to May in 2015 with the use of 20,071 packaged surgery instruments over the same period (test group), indicating that the application of the QCC method significantly decreased the total surgical instrument failure rate from 8.12% (first time period) to 3.45% (second time period) (χ2=359.54, P <0.001).
Comparison between the percentage of deficiencies before and after QCC is shown in Table 2.
| Group |
The number of damaged surgical instruments
(pack) |
The number of all the instruments used over the same time(pack) |
Percentage (%) |
| Control group |
1189 |
14645 |
8.12 |
| Test group |
693 |
20071 |
3.45 |
| χ2=359.54; P<0.0015 |
Table 2: The percentage of deficiencies before and after QCC.
Discussion
A well-managed instrument traceability system allows to ensure the safe use of medical devices during surgeries directly leading to protecting our patient’s health. In addition, by tracing the history of medical devices it ensures the proper management of high-risk medical devices.
The QCC method has been gradually applied to areas in the medical and healthcare fields worldwide [2]. The main objective of QCC application is to increase the morale of medical workers by improving their awareness of spotting and solving medical problems, improve medical working environments, and to eventually increase the quality of medical care, reduce the costs of medical management, and increase the efficiency of medical services [2].
However, even with the best safety practices in place, you will still see issues with surgical equipment such as decreased instruments performance, destruction of instrument integrity, and missing or expired instruments. In many of these instances, human error plays a part which emphasizes the need to optimize the safety management of surgical instruments.
The QCC team takes 10 implementation strategies which are problems exploration, activity plan, situation grasp, goal setting, factor analysis, measures formulation, measures implementation, effect confirmation, standards development, and the daily management of the setting goals [3]. A previous study showed that adopting scientific management methods were able to reduce the deficiency rate of surgical instruments, ensure the safety of surgical patients while mobilizing team members work enthusiasm [4]. Our findings of positive effect of QCC method on the enhanced management of surgical instruments are in agreement with the results reported from several other research teams [5-10]. The improvement of instrument management is known to have a positive effect on the safety of surgical patients since it enables to mobilize the enthusiasm of surgical team members to make a positive effect on patient care [5]. By developing and harnessing team members’ enthusiasm, it may lead to team members working more independently, promoting their creativity, and taking initiative to solve problems [11].
This study outlines the development and implementation of the QCC method to improve security management of surgical instrument traceability. Safe management of surgical instruments is multifaceted and subjective to many factors, including resource availability, hospital policies and procedures, and individual nurse’s action. We have developed the QCC system with the consideration and addressing important factors and procedure vulnerable to error. It is vitally important to consider that all management procedures to be simplified and friendly structured in addition to build harmonious atmosphere and helping relationship among team members. The findings from this single-time period study in our operating rooms of university hospital setting can be important and interesting to our nurse readers and possibly other healthcare and hospital settings since the positive impact of the QCC demonstrated in this study is consistent with other reports. Future research will focus on further investigation of QCC mediated improvement of safety management of surgical instrument in a longer period of time.
Limitation
By the 1980s, QCC had morphed from the manufacturing industry to Fortune 500 companies, which also formed QCC to address issues concerning employee relations among other operational issues.
QCC was shifted as a management tool to the employees on the production line, rather than waiting until production was complete and inspected by the managers and engineers. This, in turn, ensured the quality of the product during the manufacturing process when adjustments could be made, instead of waiting until completion when it was too late.
QCC theories were tested and enlisted key line employees as members of quality circles. These employees met with upper management and engineers to discuss any problems with quality they saw on the line during the manufacturing process. This gave management and engineering the ability to tackle production issues at the source and streamline manufacturing to ensure all products would pass quality control standards upon final inspection.
Some organizations still use them or a variation of their concept and invite key employees to participate in quality circles as a form of quality control method. Despite the employer and employees' best efforts, quality circles come with disadvantages. Consider those disadvantages before implementing this concept in your organization.
QCC was utilized by our organization as useful and highefficiency management method. It can invite key employee to participate in team as a form of quality control. Despite the employer and employees’ best effort, quality circle come with disadvantage, that is QCC can solve the problem of 80% and the remaining20% of the questions have yet to be solved.
9391
References
- Liang M, Liu T, Dong S (2012) QCC application in the continuous improvement of the medical quality. Chinese Hospital Management 32: 37-39.
- Wang LR, Wang Y, Lou Y, Zhang XG (2013) The role of quality control circles in sustained improvement of medical quality. Springerplus 2: 141.
- Xu C, Ke Y, Chen R (2012) QCC in surgical pathology specimens Security Management. Chinese Nursing Management 12:20-23.
- Hong S, Hu W (2011) QCC application for the disposable medical supplies management in the surgery. Journal of Nursing Training 26: 1560-1561.
- Hong SJ, Hu WL (2011) QCC application for the disposable medical supplies management in the surgery. Journal of Nursing Training 26: 1560-1561.
- Wensing M, Broge B, Riens B, Kaufmann-kolle, Akkermans R (2009) Quality circles to improve prescribing of primary care Physicians. Three comparative studies. Pharmacoepidemiol Drug saf 18: 763-769.
- Xu CY, Ke YJ, Chen RM, Feng FF, Cai Q (2012) QCC in surgical pathology specimens Security Management. Chinese Nursing Management 12: 20-23.
- Zhang XG, Zhao QW, Li Y (2009) The exploration and practive of QCC in the hospital pharmacy management. Prac Pharm Clin Rem 12: 233-235.
- Zhang Y, Li Y, Xu LF (2010) Improving management quality of outpatient dispensary. Chinese Health Qual Manag 17: 14-16.
- Zhong RH, Ming YF, Xiong ML (2002) Improving work quality of operating room nursing through QCC activity. Acta Academiae Medicinae Zunyi 25: 582–583.
- Dai X, Zhang M, Zheng X (2012) QCC application in the Operating Room and CSSD integration model [J]. Journal of Hospital Infection 22: 2631-2633.








